Abstract
Paneth cell–derived enteric antimicrobial peptides provide protection from intestinal infection and maintenance of enteric homeostasis. Paneth cells, however, evolve only after the neonatal period, and the antimicrobial mechanisms that protect the newborn intestine are ill defined. Using quantitative reverse transcription–polymerase chain reaction, immunohistology, reverse-phase high-performance liquid chromatography, and mass spectrometry, we analyzed the antimicrobial repertoire in intestinal epithelial cells during postnatal development. Surprisingly, constitutive expression of the cathelin-related antimicrobial peptide (CRAMP) was observed, and the processed, antimicrobially active form was identified in neonatal epithelium. Peptide synthesis was limited to the first two weeks after birth and gradually disappeared with the onset of increased stem cell proliferation and epithelial cell migration along the crypt–villus axis. CRAMP conferred significant protection from intestinal bacterial growth of the newborn enteric pathogen Listeria monocytogenes. Thus, we describe the first example of a complete developmental switch in innate immune effector expression and anatomical distribution. Epithelial CRAMP expression might contribute to bacterial colonization and the establishment of gut homeostasis, and provide protection from enteric infection during the postnatal period.
The innate immune system provides efficient protection from microbial infection of vulnerable body surfaces through the expression of small cationic peptide antibiotics named antimicrobial peptides. In the small intestine, they are produced in large quantities by Paneth cells situated at the lower end of intestinal crypts (1). The importance of Paneth cell–derived antimicrobial peptides for antibacterial protection and the maintenance of intestinal homeostasis has been previously demonstrated (2, 3). Metalloproteinase (MMP)-7–deficient mice, which are unable to activate antimicrobial peptides via proteolytic cleavage, show enhanced susceptibility to Salmonella infection (2). Also, transgenic mice expressing human α-defensin 5 in Paneth cells show enhanced resistance against bacterial challenge (3). Finally, impaired synthesis of antimicrobial peptides has recently been shown in patients with inflammatory bowel disease, and a causal link has been proposed (4).
Significant physiological and anatomical changes are observed in the gastrointestinal tract during the postnatal period. Although neonates are fed solely with maternal milk, they soon start to ingest additional nutrients and finally rely on solid food. These changes induce a significant increase and diversification of the bacterial microflora within the intestinal tract (5). Also, the spectrum of pathogens that gain entrance via the oral route is markedly altered. Exposure to important neonate pathogens such as Streptococcus agalactiae (group B streptococci [GBS]), Escherichia coli K1, or Listeria monocytogenes is largely limited to the intense contact with the mother's intestinal or vaginal microflora during the passage through the birth canal (6). These pathogens are taken up through the oral route, gain entrance through gastrointestinal mucosal membranes, and potentially lead to systemic disease during the postnatal period.
In mice, Paneth cells and significant expression of enteric antimicrobial peptides appear only ∼2 wk after birth accompanying the development of intestinal crypts (7–9). However, the innate host defense effector molecules that protect the neonate intestine in the absence of Paneth cells and Paneth cell–derived antimicrobial peptides are ill defined. In this paper, we describe a systematic analysis of the expression of antimicrobial peptides by intestinal epithelial cells (IECs) during the postnatal period. We confirm absent or marginal synthesis of known enteric antimicrobial peptides during the postnatal period. Strikingly, we demonstrate strong expression of the cathelicidin cathelin-related antimicrobial peptide (CRAMP) in IECs and restriction of CRAMP production to the neonatal period. Thus, this study for the first time describes a complete developmental switch in the expression of antimicrobial peptides and their anatomical distribution. Our findings highlight the variability of the innate immune defense mechanisms to combat the changing microbial challenge encountered by the developing organism.
RESULTS
Quantitative expression analysis of antimicrobial peptides in IECs
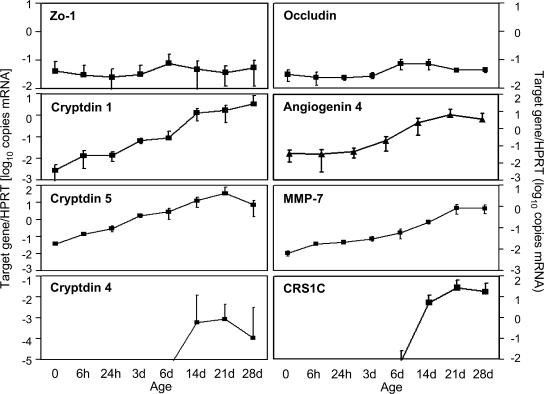
To investigate gene expression of antimicrobial peptides during postnatal intestinal development, we quantitatively examined messenger RNA (mRNA) levels in highly enriched primary IECs prepared according to a recently established protocol (10). Total RNA of IECs from mouse small intestinal tissue was isolated at the time of birth or at the ages of 6 and 24 h, or 3, 6, 14, 21, and 28 d and analyzed by real-time PCR. Gene expression was quantified using a standard curve obtained with a known concentration of the target gene and normalized to the expression level of the housekeeping gene hypoxanthine guanine phosphoribosyl transferase 1 (Hprt1). A gradual up-regulation of Cryptdin 1 and 5, Angiogenin 4, and the peptide-processing enzyme Mmp-7 over 2.5–3 log during the 4 wk after birth was observed (Fig. 1). Detection of Cryptdin 4 and the Cryptdin-related-sequence (Crs) peptide 1C expression was restricted to IECs isolated from mice at or older than the age of 14 d. The highest expression levels in adult IECs (day 28) were observed for Crs1C as well as Cryptdin 1 and 5, with expression levels reaching Hprt1 expression. Moderate levels were measured for Angiogenin 4 and Mmp7, whereas only marginal expression of Cryptdin 4 was noted. No mouse β-defensin (Mbd) expression (including Mbd 1, 2, 4, and 14) was detected at any time point in primary IECs (not depicted). As controls, genes encoding Occludin, Zonula occludens protein 1 (Zo-1), and E-cadherin, all involved in the formation of epithelial tight junctions, as well as Angiogenin 1, which has not been associated with antimicrobial function, were included in the analysis. No significant changes in the expression level of these genes over the observed time period were noted (Angiogenin 1 and E-cadherin [not depicted]). Thus, significant alterations of intestinal epithelial antimicrobial peptide expression were detected with a marked up-regulation from day 14 and onwards.
Figure 1.
Postnatal expression of antimicrobial peptides in IECs. Quantitative mRNA expression analysis for the indicated antimicrobial peptides and the peptide-processing enzyme MMP7 in primary IECs isolated from the small intestinal tissue of fetal, 6- and 24-h-, and 3-, 6-, 14-, 21-, and 28-d-old mice. Occludin and Zo-1 are involved in the formation of epithelial tight junction and were included as controls. Values represent the mean ± SD of gene expression determined in total IECs from three individual mice and indicate the target/housekeeping (Hprt1) gene expression ratio.
Analysis of antibacterial activity and immunodetection of antimicrobial peptides in the developing intestine
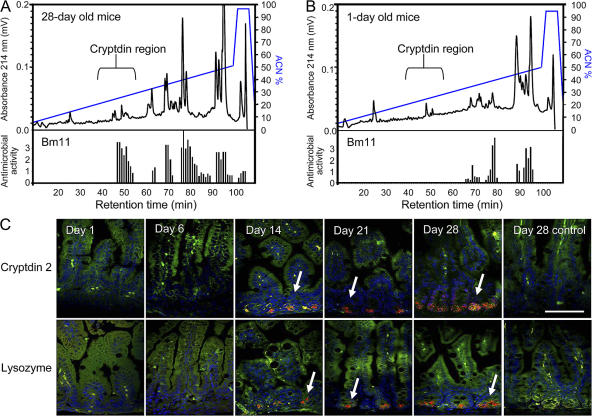
To verify the developmental changes of intestinal antimicrobial peptide expression, detection at the protein level and quantification of antibacterial activity were performed. IECs isolated from newborn (day 1) or adult (day 28) small intestinal tissue were homogenized, extracted, and fractionated by reverse-phase HPLC. All fractions were analyzed for antimicrobial activity against Bacillus megaterium (strain Bm11) using a standardized agar diffusion assay (11). In addition, mass spectrometry was performed on positive fractions to identify antimicrobially active substances. The presence of a large variety of cryptdins and CRS peptide homo- and heterodimers was confirmed by mass spectrometric analysis in fractions 45–55 from adult IECs (day 28) associated with significant antibacterial activity, as recently reported (Fig. 2 A) (11, 12).
Figure 2.
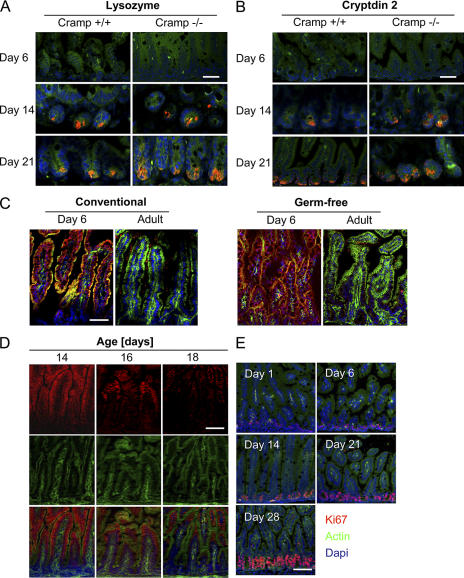
Comparison of antibacterial activity and antimicrobial peptide expression between neonate and adult IECs. Cationic antimicrobial peptides from 28-d-old adult (A) and 1-d-old neonate (B) mice were extracted from isolated IECs and fractionated by reverse-phase HPLC. (top) Components eluting between 10 and 50% acetonitrile (ACN). Absorbance was measured at λ = 214 nm (A214). (bottom) Fractions were tested for antibacterial activity using B. megaterium Bm11 as the indicator strain in an agar diffusion assay. The killing zone diameter is indicated the figure. (C) Immunostaining for cryptdin 2 (red, top) and the Paneth cell marker lysozyme (red, bottom) in the small intestinal tissue of 1-, 6-, 14-, 21-, and 28-d-old mice. MFP488 phalloidin (green) and DAPI (blue) were used as counterstaining. Arrows indicate the cryptdin 2– and lysozyme-positive Paneth cells at the bottom of the intestinal crypts. Bar, 75 μm.
In contrast, the corresponding fractions obtained from neonate IECs completely lacked antimicrobial activity and were devoid of known enteric antimicrobial peptides (Fig. 2 B). Antimicrobial activity in fractions 65–95 was associated with the presence of ribosomal proteins, known cationic molecules with antibacterial activity expected to be found in both neonate and adult tissue. Additional antimicrobial activity eluting in fractions 80–82 from day 28 mice correlated with the elution position of a Paneth cell–derived lysozyme. To confirm these findings, intestinal tissue of newborn and 6–28-d-old mice was stained for cryptdin 2, a prominent member of the family of Paneth cell–derived peptides expressed in mouse small intestinal tissue (Fig. 2 C). Again, cryptdin 2 expression was completely absent in neonate (1- and 6-d-old) mice, but increasing staining was found in 14- and 21-d-old mice, reaching maximum expression only in adult (day 28) animals. The clear restriction of cryptdin 2 to intestinal crypt Paneth cells prompted us to include staining for the Paneth cell marker lysozyme (Fig. 2 C). Indeed, lysozyme staining revealed a very similar picture confirming a close association of postnatal Paneth cell differentiation with enteric antimicrobial peptide expression. These data suggest that the established enteric antimicrobial peptides are absent in neonatal intestinal epithelium.
Detection of the cathelicidin CRAMP in neonate intestinal tissue
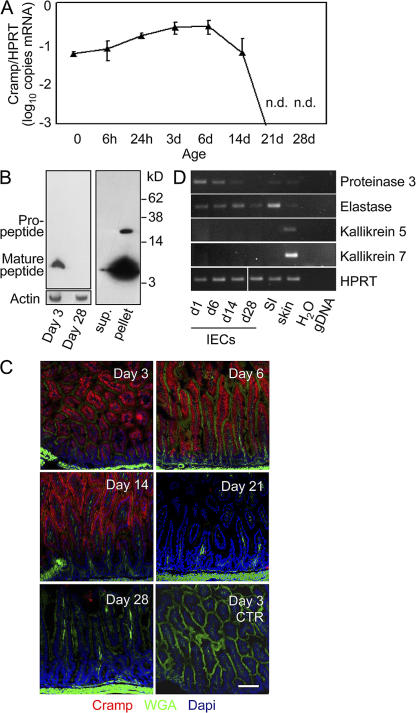
To identify protective antibacterial molecules in mouse neonate intestine, gene expression analysis for the mouse Cramp was included. Strikingly, significant expression of Cramp in primary IECs was found during the early postnatal developmental stages but completely disappeared between days 14 and 21, concomitant with the appearance of Paneth cell–derived peptides (Fig. 3 A). Similar results were obtained using a commercial hybridization probe–based assay for quantitative determination of Cramp mRNA (not depicted). Also, primary IECs isolated from older healthy individuals did not reveal any detectable constitutive Cramp expression (Fig. S1A, available at http://www.jem.org/cgi/content/full/jem.20071022/DC1). Immunoblotting of neonate (day 3) and adult (day 28) IECs confirmed the restriction of CRAMP expression to newborn epithelium and revealed the presence of the processed mature form of CRAMP in neonate IECs (Fig. 3 B, left). Although the cathelin propeptide was exclusively found in cell lysate, the processed mature form was additionally seen in cell supernatant, suggesting intracellular cleavage before secretion of the biologically active peptide by IECs (Fig. 3 B, right). Restriction of CRAMP expression to the neonate period was also noted by immunostaining of intestinal tissue of 3-, 6-, 14-, 21-, and 28-d-old mice (Fig. 3 C). Enzymatic processing could be performed by pancreatic elastase or proteinase 3, both of which are expressed in primary neonatal intestinal epithelium (Fig. 3 D). Alternatively, trypsin activity has recently been identified in mouse small intestinal tissue (13). Thus, constitutive expression and production of the processed mature form of CRAMP was detected in small intestinal epithelium restricted to the neonatal period.
Figure 3.
CRAMP expression in IECs during postnatal development. (A) Quantitative analysis of CRAMP mRNA expression in primary IECs isolated from the small intestinal tissue of fetal, 6- and 24-h-, and 3-, 6-, 14-, 21-, and 28-d-old mice. Values represent the mean ± SD of gene expression determined in total IECs from three individual mice and indicate the target/housekeeping (Hprt1) gene expression ratio. n.d., not detectable. (B) Immunoblot for CRAMP in cell lysate of isolated IECs from 3- and 28-d-old mice (left) as well as in culture supernatant and cell pellet of isolated IECs from 3-d-old mice (right). Actin staining was included to demonstrate equal protein loading. (C) Immunostaining for CRAMP (red) in the small intestinal tissue of 3-, 6-, 14-, 21-, and 28-d-old mice. The FITC-conjugated lectin wheat germ agglutinin (WGA; green) binding to the mucus surface, and DAPI (blue) was used to delineate the anatomical structures of the mucoid surface and cell nuclei, respectively. Bar, 75 μm. (D) RT-PCR expression analysis for the known CRAMP-cleaving enzymes proteinase 3, pancreatic elastase, and kallikrein 5 and 7 in primary IECs of 1-, 6-, 14-, and 28-d-old mice, as well total small intestinal (SI) and skin tissue as positive control. H2O and genomic DNA were included as negative controls for the intron-spanning primers.
m-ICcl2 cells derived from E20 fetal tissue express CRAMP
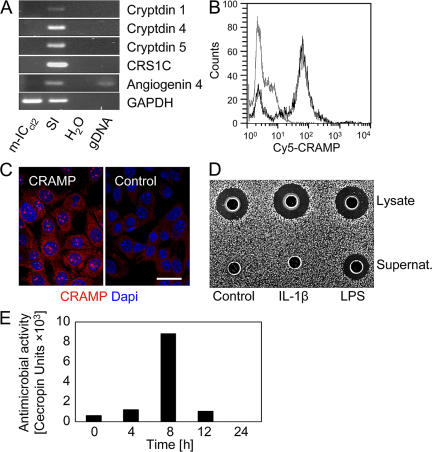
Bens et al. have recently described a highly differentiated and polarized IEC line derived from embryonic day 20 fetuses of transgenic mice carrying the large T antigen of the simian virus 40 under the control of the 5′ regulatory sequence from the L-pyruvate kinase gene (14). Although none of the established enteric antimicrobial peptides, such as α-defensins, CRS peptides, or angiogenin 4, were detectable by RT-PCR (Fig. 4 A) or mass spectrometry (not depicted), the synthesis of CRAMP was confirmed by FACS analysis and immunohistology (Fig. 4, B and C). Evaluation of m-ICcl2 cells cultured under differentiating and polarizing conditions revealed a significant and inducible antibacterial activity in cell lysate and culture supernatant (Fig. 4, D and E). In addition, m-ICcl2 cells express pancreatic elastase and proteinase 3 (not depicted) and, thus, confirm the phenotype observed in primary neonate IECs isolated from newborn mice.
Figure 4.
Intestinal epithelial m-ICc12 cells derived from embryonic day 20 mouse fetuses exhibit antimicrobial activity and express CRAMP. (A) Absence of enteric antimicrobial peptide expression in fetus-derived m-ICcl2 cells measured by RT-PCR. SI, total small intestine. (B) FACS analysis and (C) immunostaining of CRAMP expression by m-ICcl2 cells. Normal rabbit polyclonal serum was used as control. Bar, 10 μm. (D) Agar diffusion assay demonstrating antibacterial activity against the indicator strain B. megaterium Bm11 in cell lysate and supernatant of unstimulated and IL-1β– and LPS-stimulated (25 and 100 ng/ml, respectively) m-ICcl2 cells. (E) Time course of antibacterial activity in m-ICcl2 cell supernatant after stimulation with 100 ng/ml LPS. Activity is given as cecropin units, with one unit corresponding to the activity of 1 ng of cecropin.
Loss of intestinal epithelial CRAMP expression is accompanied by increased proliferation
Cathelicidins have been reported to promote cell differentiation and angiogenesis, and enhance cell proliferation and migration (15, 16). Neonatal CRAMP expression might thereby contribute to postnatal organ development and intestinal cell differentiation. Therefore, the postnatal development of the intestinal mucosa and the appearance of intestinal Paneth cells were examined during the postnatal period of Cramp+/+ and Cramp−/− mice. However, Paneth cells and Paneth cell–derived effector molecules were similarly detected in Cramp+/+ and Cramp−/− mice at 14 d after birth (Fig. 5, A and B). Also, recombinant CRAMP had no significant effect on intestinal epithelial proliferation or cell viability (Fig. S1, B and C). Although CRAMP significantly inhibited LPS-mediated stimulation of naive epithelial cells similar to other antimicrobial peptides, it showed no effect on endotoxin-tolerant epithelial cells (Fig. S1, D and E) (12, 17).
Figure 5.
Loss of CRAMP expression is associated with enhanced epithelial cell proliferation during postnatal development. Immunostaining for (A) lysozyme and (B) cryptdin 2 (red) in small intestinal tissue of 6-, 14-, and 21-d-old Cramp+/+ and Cramp−/− mice to demonstrate similar organ development. Bars, 25 μm. (C) Immunostaining for CRAMP (red) in the small intestinal tissue of 6-d-old as well as adult conventionally bred (left) and germ-free–bred (right) mice. Bar, 75 μm. (D) Immunostaining for CRAMP (red) in small intestinal tissue of 14-, 16-, and 18-d-old mice. Bar, 75 μm. (E) Immunostaining for the cell division marker Ki67 (red) in the small intestinal tissue of 1-, 6-, 14-, 21-, and 28-d-old mice illustrating the significant enhancement of epithelial proliferation in the intestinal crypts during the third week of life. MFP488 phalloidin (green) and DAPI (blue) were used as counterstaining. Bar, 75 μm.
Mucosal colonization of the intestinal tract starts immediately after birth, leading to an increasingly dense and complex microbial flora during the first weeks of life (5). To examine a possible effect of microbial colonization on the disappearance of CRAMP expression, conventionally bred and germ-free–bred animals were compared. However, epithelial CRAMP expression was restricted to the neonatal period irrespective of colonization by the microbial flora (Fig. 5 C).
Strikingly, careful analysis of epithelial CRAMP expression during the third week after birth suggested that disappearance of CRAMP after the postnatal period was not caused by down-regulation of transcriptional activity but rather occurred in an anatomically compartmentalized fashion. Reduced expression was first noticed at the level of the crypts and lower villi and reached the tip of the villi some days later (Fig. 5 D). Loss of CRAMP expression was accompanied by an increase of epithelial cell proliferation and the formation of intestinal crypts (Fig. 5 E and Fig. 2C). These data are in accordance with a recent report on low Wnt signaling activity in the intervillus region directly after birth and increased activity in the crypt area of adult individuals (18). They further suggest that CRAMP expression is limited to the neonate epithelium and lost during the developmental changes that occur in the postnatal period associated with increased IEC proliferation and differentiation.
CRAMP-mediated antibacterial activity against commensal and pathogenic enteric bacteria
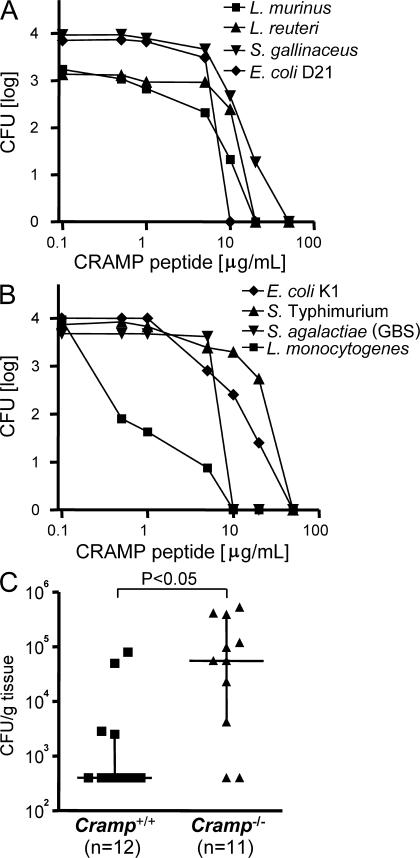
To establish a possible role in enteric host protection, we next tested the CRAMP-mediated antibacterial activity against gut commensal bacteria, as well as defined pathogenic bacteria known to be transmitted to the neonate organism during passage through the birth canal (6). Rapid bacterial killing resulting in a 3–4 log decrease of viable bacteria after 2 h was noted against selected Gram-positive and -negative intestinal commensal bacteria of the small intestine such as S. gallinaceus, Lactobacillus murinus, L. reuteri, and the nonpathogenic E. coli strain D21 at peptide concentrations between 10 and 50 μg/ml (Fig. 6 A). Similarly, perinatally transmitted pathogens associated with systemic disease in neonates such as S. agalactiae (GBS), capsule-positive E. coli K1, or Salmonella enterica serovar Typhimurium (S. Typhimurium) were susceptible toward the bacterial killing activity of CRAMP (Fig. 6 B).L. monocytogenes, a major cause of neonatal meningitis after oral ingestion during birth (described as late onset disease in contrast to the early onset disease after transplacental transmission) demonstrated particular susceptibility, with 99% killing within 2 h by <1 μg/ml CRAMP (Fig. 6 B).
Figure 6.
CRAMP rapidly kills commensal and pathogenic bacteria and controls the growth of L. monocytogenes in neonate small intestine in vivo. Antibacterial activity of recombinant CRAMP against Gram-positive and -negative (A) commensal bacteria such as L. murinus, L. reuteri, S. gallinaceus, and E. coli D21, and (B) pathogenic bacteria such as L. monocytogenes, S. Typhimurium, S. agalactiae, and capsule-positive E. coli K1. Viable bacteria were determined after 2 h in the presence of the indicated peptide concentration. (C) 5-d-old Cramp+/+ (squares) and CRAMP−/− (triangles) newborn mice were orally infected with 106 L. monocytogenes in a volume of 5 μl, and the number of viable bacteria in small intestinal tissue was counted 24 h after infection. Squares and triangles indicate the bacterial load in each individual animal. Horizontal bars indicate the median ± interquartile range.
Finally, a neonate oral infection model with L. monocytogenes was chosen to verify the protective activity of intestinal epithelial CRAMP expression in vivo. Because of structural differences of the epithelial receptor E-cadherin between mice and men, the invasion rate of L. monocytogenes after oral infection of mice is very low and results in only a marginal local inflammatory reaction (19, 20). This allows application of significant bacterial numbers, minimizes the possible effect of infiltrating granulocytes, and, thus, favors the analysis of the intraluminal intestinal host defense. 5-d-old wild-type (Cramp+/+) and Cramp-deficient (Cramp−/−) neonate mice were orally challenged with 106 CFU of L. monocytogenes. Indeed, the number of viable L. monocytogenes in intestinal tissue 24 h after oral infection of Cramp+/+ mice was significantly diminished (>2 log). In contrast, no decrease of viable bacteria was noted in Cramp−/− mice, resulting in a significant difference between both groups of animals (P < 0.05; Fig. 6 C). Analysis of spleen and liver tissue 24 h after oral infection confirmed the low invasion rate with viable L. monocytogenes in none of the Cramp+/+ mice and only 2 out of 11 Cramp−/− mice (not depicted). Notably, these results illustrate that the experimental setting mainly reflects the intraluminal host defense. Thus, intestinal CRAMP harbors significant antibacterial activity against commensal and pathogenic microorganisms and might contribute to protect the intestinal mucosa against pathogenic bacteria and restrict growth or shape the composition of the commensal microflora.
DISCUSSION
The present work demonstrates an unexpected and unprecedented complete change in the small intestinal antimicrobial peptide repertoire and anatomical distribution from the postnatal period to weaning. Although the important role of Paneth cell–derived antimicrobial peptides in adult individuals has been well established, less is known about the antimicrobial protection and particular susceptibility toward bacterial infections during the neonatal period. Importantly, low expression of Paneth cell–derived antimicrobial peptides has also been noted in human neonates (21). Besides infection via the transplacental route during pregnancy, several microbial organisms are transmitted from the mother to the newborn during passage through the birth canal, gain entrance via the intestinal tract, and may evoke systemic disease in neonates. Examples are GBS (S. agalactiae), E. coli K1, S. Typhimurium, Ureaplasma urealyticum, or L. monocytogenes, which all colonize the urogenital or lower intestinal tract of the mother and cause significant morbidity in newborns during the postnatal period. Thus, expression of CRAMP in neonate intestine and the documented developmental change in the enteric antibacterial peptide repertoire might reflect the age-related spectrum of orally ingested pathogens. Similarly, CRAMP expression has been detected in vernix caseosa and neonate skin (22–24). Together with our results, these data suggest that CRAMP plays a prominent role in the protection of the newborn.
Cathelicidins are released by proteolytic cleavage from their precursor molecules. Expression of the precursor peptide has been noted in myeloid cells, keratinocytes, and epithelial cells from the skin, lung, stomach, colon, meninges, and eye, as well as endothelial cells (25–28). Although they represent a large and important group of antimicrobial peptides in a variety of mammals, only one member, LL-37 and CRAMP, is encoded in humans and mice, respectively. Significant cathelicidin expression has previously been noted in the gastrointestinal tract, such as human gastric or colonic tissue, with higher expression levels in individuals with persistent bacterial infection or chronic inflammation (27, 29). Although our results obtained using highly purified primary IECs indicated the absence of constitutive Cramp expression in adult individuals, Cramp mRNA was recently noted in total adult small intestinal tissue (30). The different results might reflect expression by resident tissue myeloid cells. However, we cannot rule out the reappearance of small intestinal epithelial Cramp expression in adult individuals during conditions such as tumorigenesis or inflammatory processes.
Importantly, lack of cathelicidin synthesis was associated with clinical disease, such as periodontitis, in human individuals with congenital neutropenia (Kostmann syndrome) and enhanced susceptibility to recurrent urinary tract infection (26, 31). CRAMP expression also conferred protection from invasive skin infection by group A streptococci and Citrobacter rodentium–induced colitis in mice (25, 32). The clinical importance of CRAMP was further underlined by the description of mechanisms to gain resistance against peptide-mediated killing by several human pathogenic bacteria (33, 34). In addition to the broad and potent antibacterial activity, cathelicidin-derived antimicrobial peptides have also been noted to exert other biological activities so as to promote cell differentiation and to stimulate angiogenesis and epithelial cell proliferation (15, 16). Although our results do not support an involvement of epithelial CRAMP in the postnatal intestinal organ development, other CRAMP-mediated biological effects on neonate gut homeostasis cannot be excluded.
The finding that germ-free mice, similar to conventionally bred mice, completely lost intestinal epithelial CRAMP expression precludes an active role of the enteric microflora in the down-regulation of intestinal CRAMP expression. Similar results have been found for cryptdin expression in Paneth cells (11). The close temporal association between the loss of CRAMP expression and developmental changes such as the increase of epithelial proliferation, the emergence of crypts, and the appearance of mature Paneth cells instead indicate a developmentally controlled process. Indeed, cathelicidin expression has previously been linked to cell differentiation and exposure to differentiation-promoting agents (35, 36). Developmental regulation is also supported by the recently identified differential expression profile of the Wnt transcriptional effectors Tcf3 and Tcf4 during the development of the mouse intestinal epithelium (18). The identified increase in epithelial proliferation during the postnatal development is paralleled by enhanced Wnt signaling in the area of cellular proliferation (18). The restriction of CRAMP expression to neonate epithelium and the gradual loss of CRAMP-positive cells along the crypt–villus axis might therefore result from a combined developmental program of cell differentiation and proliferation.
In conclusion, we describe a complete change of the antimicrobial peptide repertoire in the intestinal epithelium during the postnatal period. This switch of the antimicrobial peptide expression highlights the variability of the innate host defense system to encounter the changing spectrum of microbial challenge. Neonate enteric CRAMP expression might provide protection from pathogens and contribute to the establishment of a stable host–microbe homeostasis. The establishment of the physiological microflora in combination with an increase in epithelial cell turnover in adult individuals might later facilitate restriction of antimicrobial peptide production to Paneth cells at the bottom of intestinal crypts to form a more dynamic barrier that is efficient against bacterial pathogens but allows the existence of a bacterial microflora.
MATERIALS AND METHODS
Isolation of primary IECs.
C57BL/6 mice were purchased from Charles River Laboratories, housed under specific pathogen-free conditions, and treated in accordance with the local animal protection legislation (Regierungspräsidium Stuttgart). Germ-free– as well as conventionally bred mice were obtained from the Gnotobiotic Facility (Karolinska Institutet). Mice were killed by cervical dislocation, and the small intestine was removed. Primary IECs were isolated from the intestinal tissue at different ages based on a published protocol (10). All tissue, except that from 0 h mice, was obtained from spontaneously delivered newborns. 0-h-old IECs were isolated from mice born by caesarean section after natural birth of the first sibling to ensure maturity of the obtained neonate tissue.
Quantitative real-time PCR analysis.
Total RNA was obtained using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. Complementary DNA (cDNA) was synthesized from 1 μg of total mRNA using oligo(dT)18 primer from the first-strand cDNA synthesis AMV kit (Roche). The following primers were used for cDNA amplification: Angiogenin 1 (forward, 5′-AGGCCCGTTGTTCTTGATCT-3′; reverse, 5′-TGAGGTTAGGCTTCTTCTCTTCA-3′), Angiogenin 4 (forward, 5′-GACAATGAGCCCATGTCCTT-3′; reverse, 5′-TTTGGCTTGGCATCATAGTG-3′), Cramp (forward, 5′-CCGAGCTGTGGATGACTTCAAML-3′; reverse,5′-CTGCCCCCATACACTGCTTCAC-3′), Crs1-C (forward, 5′-GTCTCCTTTGGAGGCACAGA-3′; reverse, 5′-GCTTGGGTGGTGATAGCAGT-3′), E-cadherin (forward, 5′-GGCTTCAGTTCCGAGGTCTA-3′;reverse, 5′-CGAAAAGAAGGCTGTCCTTG-3′), Elastase (forward, 5′-TGTGGACACAGTACCGAGGA -3′; reverse, 5′-GGTCATCACCCAGTTGCTTC-3′), Kallikrein 5 (forward, 5′-CAATGGCTACCCTGATCACA-3′; reverse, 5′-GTCCTCCCCAGACTTGGAAT-3′), Kallikrein 7(forward, 5′-CAGGGAGTGCAAGAAGGTGT-3′; reverse, 5′-TAGGTACCCCAGGACACCAG-3′), Mmp-7 (forward, 5′-TTTGATGGGCCAGGGAACACTCTA-3′; reverse, 5′-ATGGGTGGCAGCAAACAGGAAGT-3′),Occludin (forward, 5′-AGCGCTATCCTGGGCATCAT-3′; reverse, 5′-ATCCATCTTTCTTCGGGTTTTCAC-3′), Proteinase 3 (forward, 5′-ACGGTGGTCACCTTCCTATG-3′; reverse, 5′-GCGAAGAAATCAGGGAACTG-3′), and Zo-1 (forward, 5′-TCTGAGGGGAAGGCGGATGGTGCT-3′; reverse, 5′-TTGTGGCTGCGCTTGTGGTGAGTAA-3′). The primers for Cryptin 1 (forward [Defcrp130], 5′-AAGAGACTAAAACTGAGGAGCAGC-3′; reverse, 5′-CGCAGCAGAGCGTGTA-3′), Cryptin 4(forward [Defcrp130], 5′-AAGAGACTAAAACTGAGGAGCAGC-3′; reverse, 5′-CGGCGGGGGCAGCAGTA-3′), and Cryptin 5 (forward [Defcrp130], 5′-AAGAGACTAAAACTGAGGAGCAGC-3′; reverse, 5′-GCAGCAGAATACGAAAGT-3′) were as previously described (9). Hprt1 (forward, 5′-TGATCAGTCAACGGGGGACA-3′; reverse, 5′-TTCGAGAGGTCCTTTTCACCA-3′) and β2-microglobulin (b2M; forward, 5′-TGGTGCTTGTCTCACTGAML-3′; reverse, 5′-CCGTTCTTCAGCATTTGGAT-3′) were used as endogenous controls to normalize gene expression levels. Normalization to both Hprt1 and b2M revealed very similar results; the data shown in the figures were obtained after normalization to Hprt1. All primers used in this study—except for Angiogenin 1 and 4, as well as Mbd 1, 2, 4, and 14—were intron spanning, as demonstrated by the absence of amplification from genomic DNA. Non–intron-spanning PCR amplification was preceded by an RQ1 RNase-free DNase (Promega) digestion step, including the appropriate controls. SYBR green–based real-time PCRs for cDNA quantification were performed in 20-μl volumes by using both standard curves and the comparative CT method on a LightCycler instrument (Roche) and a sequence detector (ABI Prism 7900; Applied Biosystems).
Additionally, the comparative CT method was used to confirm alterations in Cramp gene expression by using Quantitect assays QT01195922 (Cramp) and QT00166768 (Hprt1; both from QIAGEN) and, additionally, by using TaqMan gene expression assays Mm00438285_m1 (Cramp) and Mm00446968_m1 (Hprt1; both from Applied Biosystems), according to the manufacturers' instructions. Quantitect assays were run on the LightCycler instrument, and Taqman assays were performed with the ABI Prism 7900 sequence detector. Quantitative analysis was performed according to the manufacturer's manuals and tutorials (available at http://www.appliedbiosystems.com). All PCRs were run in triplicate.
HPLC fractionation and mass spectrometric analysis.
Cationic antimicrobial peptides were extracted from isolated IECs of newborn and adult C57BL/6 mice or m-ICcl2 cell lysate, according to a published protocol (12). The antibacterial activity of HPLC fractions was analyzed using a zone inhibition assay using B. megaterium strain 11 (11). Cell-culture supernatant was purified using Sep-Pak Light tC18 cartridges (Waters Corp.) before antibacterial testing and mass spectrometric analysis. Matrix-assisted laser desorption-ionization time-of-flight mass spectrometry analysis was performed with a Reflex III instrument (Bruker Daltronics), as recently described (11).
Immunohistology, flow cytometry, and immunoblotting.
Immunostaining was performed on small intestines from newborn and 1-, 3-, 6-, 14-, 16-, 18-, 21-, and 28-d-old mice. Paraformaldehyde-fixed slides were deparaffinized and heated at 120°C for 3 min in 10 mM Na-citrate, pH 6, to improve antigen accessibility. Rabbit antilysozyme antiserum (1:250; Dako), anti–cryptdin 2 (1:1,000), or anti-Ki67 protein antiserum (1:2,000; provided by T. Scholzen, Research Center Borstel, Borstel, Germany) was incubated for 1 h at room temperature and detected using a Texas red–conjugated anti–rabbit secondary antibody (1:50; Jackson ImmunoResearch Laboratories). Cryosections were acetone fixed and incubated with rabbit anti-CRAMP antiserum at a final dilution of 1:500 for 90 min at room temperature. Primary antibody detection was performed with a goat anti–rabbit Cy3-conjugated secondary antibody (Jackson ImmunoResearch Laboratories). Counterstaining was performed with MFP488 phalloidin (MoBiTec) or fluorescein-conjugated wheat germ agglutinin at a dilution of 1:50 (Vector Laboratories), as indicated in the figures. Slides were mounted in DAPI containing Vectashield (Vector Laboratories) and visualized using an ApoTome-equipped microscope (Axioplan 2) connected to a digital camera (AxioCam Mr; all purchased from Carl Zeiss, Inc.). CRAMP expression was visualized by flow cytometry after fixation (Cytofix; BD Biosciences), with permeabilization in Ca2+- and Mg2+-free PBS containing 0.5% saponin and 2% FCS using a rabbit anti-CRAMP antiserum (1:500) or control serum in combination with a TR-conjugated anti–rabbit secondary antibody. Cells were analyzed on a FACSCalibur apparatus (BD Biosciences). Immunoblotting was performed as recently described (10). Membranes were incubated overnight at 4°C, with the primary antibody provided by B. Agarberth (Karolinska Institute, Stockholm, Sweden) at a 1:1,000 dilution. Detection was performed using peroxidase-conjugated goat anti–rabbit secondary antibody (Jackson ImmunoResearch Laboratories) in combination with the ECL kit (GE Healthcare). To confirm equal loading, actin staining was used with a rabbit polyclonal actin antiserum (Sigma-Aldrich).
Determination of CRAMP antibacterial activity and bacterial challenge.
Mature CRAMP peptide ISRLAGLLRKGGEKIGEKLKKIGQKIKNFFQKLVPQPE was synthesized with a purity of >95% (Innovagen) and tested by HPLC and mass spectrometry. L. murinus, L. reuteri, and S. gallinaceus were mouse small intestinal isolates identified biochemically and confirmed by 16S ribosomal RNA gene sequencing. S. Typhimurium 14028 was received from the American Type Culture Collection, and the nonpathogenic E. coli strain D21 and the clinical isolate L. monocytogenes type 1 were received from the laboratory strain collection. Encapsulated E. coli K1 and S. agalactiae (GBS) were human clinical isolates provided by R. Berner (University of Freiburg, Freiburg, Germany). The antibacterial activity of peptides was evaluated with a broth microdilution assay in 10 mM sodium phosphate buffer supplemented with 1% tryptic soy broth supplemented with 0.5% yeast extract (TSB-Y; Difco) for L. monocytogenes, E. coli K1, S. gallinaceus, and S. agalactiae. Sodium phosphate buffer was supplemented with 1% MRS broth for lactobacilli species and with 1% Luria-Bertani broth for E. coli D21 and S. Typhimurium, as previously described (37). 104 bacteria grown to mid-logarithmic phase were coincubated in 100 μl with or without the addition of peptide at the concentrations indicated in the figures (0.5, 1, 5, 10, 20, and 50 μg/ml). The number of viable bacteria (CFU) was analyzed after 2 h by serial dilution. Cramp-deficient (Cramp−/−) as well as Cramp+/+ 129/SvJ mice were bred at the Animal Facility of the Microbiology and Tumor Biology Center (Karolinska Institutet). For in vivo analysis, 5-d-old Cramp+/+ and Cramp−/− mice were orally infected with 106 CFU L. monocytogenes in 5 μl of bicarbonate buffer. 24 h after infection, mice were killed and the small intestine was removed and homogenized. The number of viable bacteria per gram of tissue was determined by serial dilution. All in vivo experiments were conducted in agreement with European Communities Council Directive 86/609/EEC and Swedish animal protection legislation.
Statistical analysis.
Results are given as the mean ± SD or median ± interquartile range, as indicated. Statistical analyses were performed using the Student's t test. P < 0.05 was considered significant.
Online supplemental material.
Fig. S1 A shows no detectable Cramp expression in primary IECs isolated from small intestinal tissue of 8- and 15-wk-old mice using quantitative mRNA analysis. The absence of an effect of CRAMP on epithelial cell proliferation and cell viability is demonstrated in Fig. S1 (B and C). Fig. S1 (D and E) illustrate the strong endotoxin-blocking activity of CRAMP on naive cells but the absence of a detectable effect of CRAMP on LPS-tolerized IECs. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20071022/DC1.
Supplemental Material
Acknowledgments
We thank Reinhard Berner, Thomas Scholzen, and Birgitta Agarberth for providing bacterial strains, antisera, and technical help, as well as Anja Schöpflin and Anette Widmer for excellent technical assistance and Christian Bogdan for helpful discussions.
This work has been supported by grants from the German Research Foundation (HO 2236/5), the Swedish Research Council (K2003-31P-14792 to M.W. Hornef, K2005-06X-15405 to M. Aandersson, and K2003-06XD-14653 to K. Putsep), the Swedish Cancer Foundation (Cancerfonden), the Thyssen Foundation (AZ 10.05.2.173), the University of Freiburg (M.W. Hornef), the Swedish Foundation for International Cooperation in Research and Higher Education (M. Andersson), the Ruth and Richard Juhlin's Foundation (K. Putsep), and the Fondation de la Recherche Médical (S. Ménard).
The authors have no conflicting financial interests.
Abbreviations used: CRAMP, cathelin-related antimicrobial peptide; CRS, cryptdin-related sequence; GBS, group B streptococci; Hprt1, hypoxanthine guanine phosphoribosyl transferase 1; IEC, intestinal epithelial cell; Mbd, mouse β-defensin; MMP, metalloproteinase; ZO-1, Zonula occludens protein 1.
S. Ménard's present adresses is Institut National de la Santé et de la Recherche Médicale, U793, Faculté Necker-Enfants-Malades, 75730 Paris, Cedex 15, France.
E. Glocker's present address is Royal Free and University College Medical School, London WC1E 6BT, England, UK.
References
- 1.Bevins, C.L. 2004. The Paneth cell and the innate immune response. Curr. Opin. Gastroenterol. 20:572–580. [DOI] [PubMed] [Google Scholar]
- 2.Wilson, C.L., A.J. Ouellette, D.P. Satchell, T. Ayabe, Y.S. Lopez-Boado, J.L. Stratman, S.J. Hultgren, L.M. Matrisian, and W.C. Parks. 1999. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 286:113–117. [DOI] [PubMed] [Google Scholar]
- 3.Salzman, N.H., D. Ghosh, K.M. Huttner, Y. Paterson, and C.L. Bevins. 2003. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 422:522–526. [DOI] [PubMed] [Google Scholar]
- 4.Wehkamp, J., N.H. Salzman, E. Porter, S. Nuding, M. Weichenthal, R.E. Petras, B. Shen, E. Schaeffeler, M. Schwab, R. Linzmeier, et al. 2005. Reduced Paneth cell alpha-defensins in ileal Crohn's disease. Proc. Natl. Acad. Sci. USA. 102:18129–18134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards, C.A., and A.M. Parrett. 2002. Intestinal flora during the first months of life: new perspectives. Br. J. Nutr. 88(Suppl. 1):S11–S18. [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg, R.L., and C. Thompson. 2003. The infectious origins of stillbirth. Am. J. Obstet. Gynecol. 189:861–873. [DOI] [PubMed] [Google Scholar]
- 7.van Es, J.H., P. Jay, A. Gregorieff, M.E. van Gijn, S. Jonkheer, P. Hatzis, A. Thiele, M. van den Born, H. Begthel, T. Brabletz, et al. 2005. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat. Cell Biol. 7:381–386. [DOI] [PubMed] [Google Scholar]
- 8.Bry, L., P. Falk, K. Huttner, A. Ouellette, T. Midtvedt, and J.I. Gordon. 1994. Paneth cell differentiation in the developing intestine of normal and transgenic mice. Proc. Natl. Acad. Sci. USA. 91:10335–10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darmoul, D., D. Brown, M.E. Selsted, and A.J. Ouellette. 1997. Cryptdin gene expression in developing mouse small intestine. Am. J. Physiol. 272:G197–G206. [DOI] [PubMed] [Google Scholar]
- 10.Lotz, M., D. Guetle, S. Walther, S. Menard, C. Bogdan, and M.W. Hornef. 2006. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J. Exp. Med. 203:973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Putsep, K., L.G. Axelsson, A. Boman, T. Midtvedt, S. Normark, H.G. Boman, and M. Andersson. 2000. Germ-free and colonized mice generate the same products from enteric prodefensins. J. Biol. Chem. 275:40478–40482. [DOI] [PubMed] [Google Scholar]
- 12.Hornef, M.W., K. Putsep, J. Karlsson, E. Refai, and M. Andersson. 2004. Increased diversity of intestinal antimicrobial peptides by covalent dimer formation. Nat. Immunol. 5:836–843. [DOI] [PubMed] [Google Scholar]
- 13.Koshikawa, N., S. Hasegawa, Y. Nagashima, K. Mitsuhashi, Y. Tsubota, S. Miyata, Y. Miyagi, H. Yasumitsu, and K. Miyazaki. 1998. Expression of trypsin by epithelial cells of various tissues, leukocytes, and neurons in human and mouse. Am. J. Pathol. 153:937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bens, M., A. Bogdanova, F. Cluzeaud, L. Miquerol, S. Kerneis, J.P. Kraehenbuhl, A. Kahn, E. Pringault, and A. Vandewalle. 1996. Transimmortalized mouse intestinal cells (m-ICc12) that maintain a crypt phenotype. Am. J. Physiol. 270:C1666–C1674. [DOI] [PubMed] [Google Scholar]
- 15.Heilborn, J.D., M.F. Nilsson, G. Kratz, G. Weber, O. Sørensen, N. Borregaard, and M. Ståhle-Bäckdahl. 2003. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J. Invest. Dermatol. 120:379–389. [DOI] [PubMed] [Google Scholar]
- 16.Tokumaru, S., K. Sayama, Y. Shirakata, H. Komatsuzawa, K. Ouhara, Y. Hanakawa, Y. Yahata, X. Dai, M. Tohyama, H. Nagai, et al. 2005. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J. Immunol. 175:4662–4668. [DOI] [PubMed] [Google Scholar]
- 17.Mookherjee, N., K.L. Brown, D.M. Bowdish, S. Doria, R. Falsafi, K. Hokamp, F.M. Roche, R. Mu, G.H. Doho, J. Pistolic, et al. 2006. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J. Immunol. 176:2455–2464. [DOI] [PubMed] [Google Scholar]
- 18.Kim, B.M., J. Mao, M.M. Taketo, and R.A. Shivdasani. 2007. Phases of canonical Wnt signaling during the development of mouse intestinal epithelium. Gastroenterology. 133:529–538. [DOI] [PubMed] [Google Scholar]
- 19.Lecuit, M., S. Dramsi, C. Gottardi, M. Fedor-Chaiken, B. Gumbiner, and P. Cossart. 1999. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 18:3956–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lecuit, M., S. Vandormael-Pournin, J. Lefort, M. Huerre, P. Gounon, C. Dupuy, C. Babinet, and P. Cossart. 2001. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science. 292:1722–1725. [DOI] [PubMed] [Google Scholar]
- 21.Mallow, E.B., A. Harris, N. Salzman, J.P. Russell, R.J. DeBerardinis, E. Ruchelli, and C.L. Bevins. 1996. Human enteric defensins. Gene structure and developmental expression. J. Biol. Chem. 271:4038–4045. [DOI] [PubMed] [Google Scholar]
- 22.Yoshio, H., H. Lagercrantz, G.H. Gudmundsson, and B. Agerberth. 2004. First line of defense in early human life. Semin. Perinatol. 28:304–311. [DOI] [PubMed] [Google Scholar]
- 23.Starner, T.D., B. Agerberth, G.H. Gudmundsson, and P.B. McCray Jr. 2005. Expression and activity of beta-defensins and LL-37 in the developing human lung. J. Immunol. 174:1608–1615. [DOI] [PubMed] [Google Scholar]
- 24.Dorschner, R.A., K.H. Lin, M. Murakami, and R.L. Gallo. 2003. Neonatal skin in mice and humans expresses increased levels of antimicrobial peptides: innate immunity during development of the adaptive response. Pediatr. Res. 53:566–572. [DOI] [PubMed] [Google Scholar]
- 25.Nizet, V., T. Ohtake, X. Lauth, J. Trowbridge, J. Rudisill, R.A. Dorschner, V. Pestonjamasp, J. Piraino, K. Huttner, and R.L. Gallo. 2001. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 414:454–457. [DOI] [PubMed] [Google Scholar]
- 26.Putsep, K., G. Carlsson, H.G. Boman, and M. Andersson. 2002. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet. 360:1144–1149. [DOI] [PubMed] [Google Scholar]
- 27.Hase, K., M. Muratami, M. Rimura, S.P. Cole, Y. Horibe, T. Ohtake, M. Obonyo, R.L. Gallo, L. Eckmann, and M.F. Kagnoff. 2003. Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology. 125:1613–1625. [DOI] [PubMed] [Google Scholar]
- 28.Schauber, J., C. Svanholm, S. Termen, K. Iffland, T. Menzel, W. Scheppach, R. Melcher, B. Agerberth, H. Luhrs, and G.H. Gudmundsson. 2003. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut. 52:735–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schauber, J., D. Rieger, F. Weiler, J. Wehkamp, M. Eck, K. Fellermann, W. Scheppach, R.L. Gallo, and E.F. Stange. 2006. Heterogeneous expression of human cathelicidin hCAP18/LL-37 in inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 18:615–621. [DOI] [PubMed] [Google Scholar]
- 30.Gallo, R.L., K.J. Kim, M. Bernfield, C.A. Kozak, M. Zanetti, L. Merluzzi, and R. Gennaro. 1997. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J. Biol. Chem. 272:13088–13093. [DOI] [PubMed] [Google Scholar]
- 31.Chromek, M., Z. Slamova, P. Bergman, L. Kovacs, L. Podracka, I. Ehren, T. Hokfelt, G.H. Gudmundsson, R.L. Gallo, B. Agerberth, and A. Brauner. 2006. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 12:636–641. [DOI] [PubMed] [Google Scholar]
- 32.Iimura, M., R.L. Gallo, K. Hase, Y. Miyamoto, L. Eckmann, and M.F. Kagnoff. 2005. Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J. Immunol. 174:4901–4907. [DOI] [PubMed] [Google Scholar]
- 33.Brodsky, I.E., N. Ghori, S. Falkow, and D. Monack. 2005. Mig-14 is an inner membrane-associated protein that promotes Salmonella typhimurium resistance to CRAMP, survival within activated macrophages and persistent infection. Mol. Microbiol. 55:954–972. [DOI] [PubMed] [Google Scholar]
- 34.Schmidtchen, A., I.M. Frick, E. Andersson, H. Tapper, and L. Bjorck. 2002. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol. Microbiol. 46:157–168. [DOI] [PubMed] [Google Scholar]
- 35.Liu, P.T., S. Stenger, H. Li, L. Wenzel, B.H. Tan, S.R. Krutzik, M.T. Ochoa, J. Schauber, K. Wu, C. Meinken, et al. 2006. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 311:1770–1773. [DOI] [PubMed] [Google Scholar]
- 36.Raqib, R., P. Sarker, P. Bergman, G. Ara, M. Lindh, D.A. Sack, K.M. Nasirul Islam, G.H. Gudmundsson, J. Andersson, and B. Agerberth. 2006. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc. Natl. Acad. Sci. USA. 103:9178–9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hultmark, D., A. Engstrom, K. Andersson, H. Steiner, H. Bennich, and H.G. Boman. 1983. Insect immunity. Attacins, a family of antibacterial proteins from Hyalophora cecropia. EMBO J. 2:571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.