Abstract
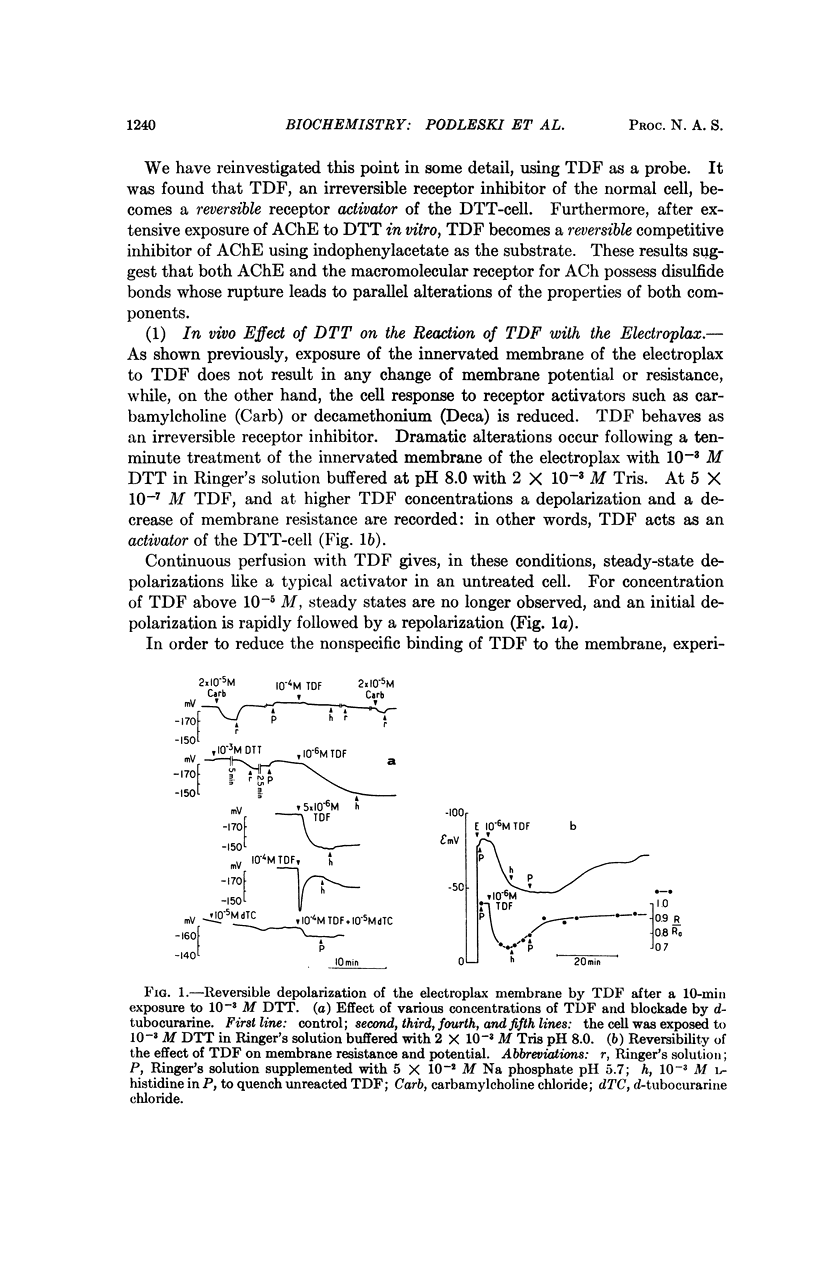
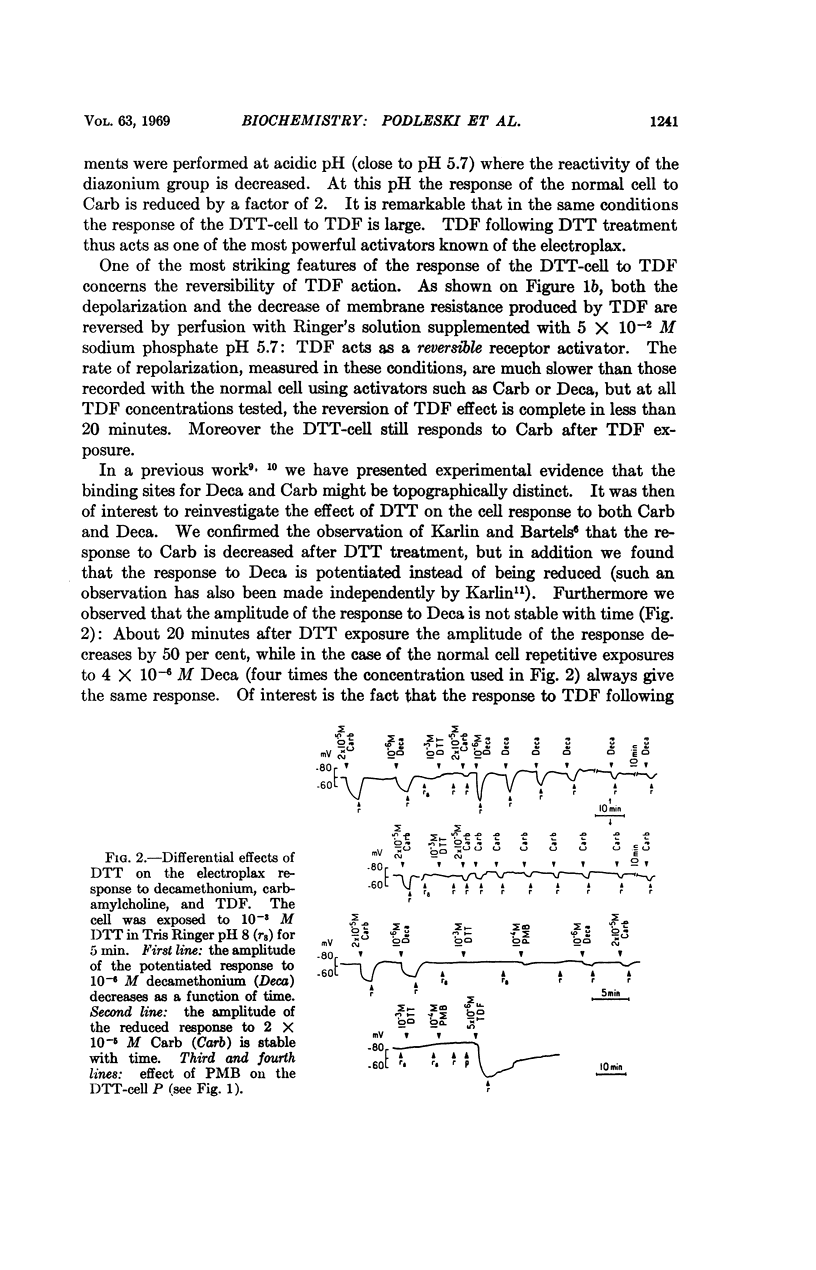
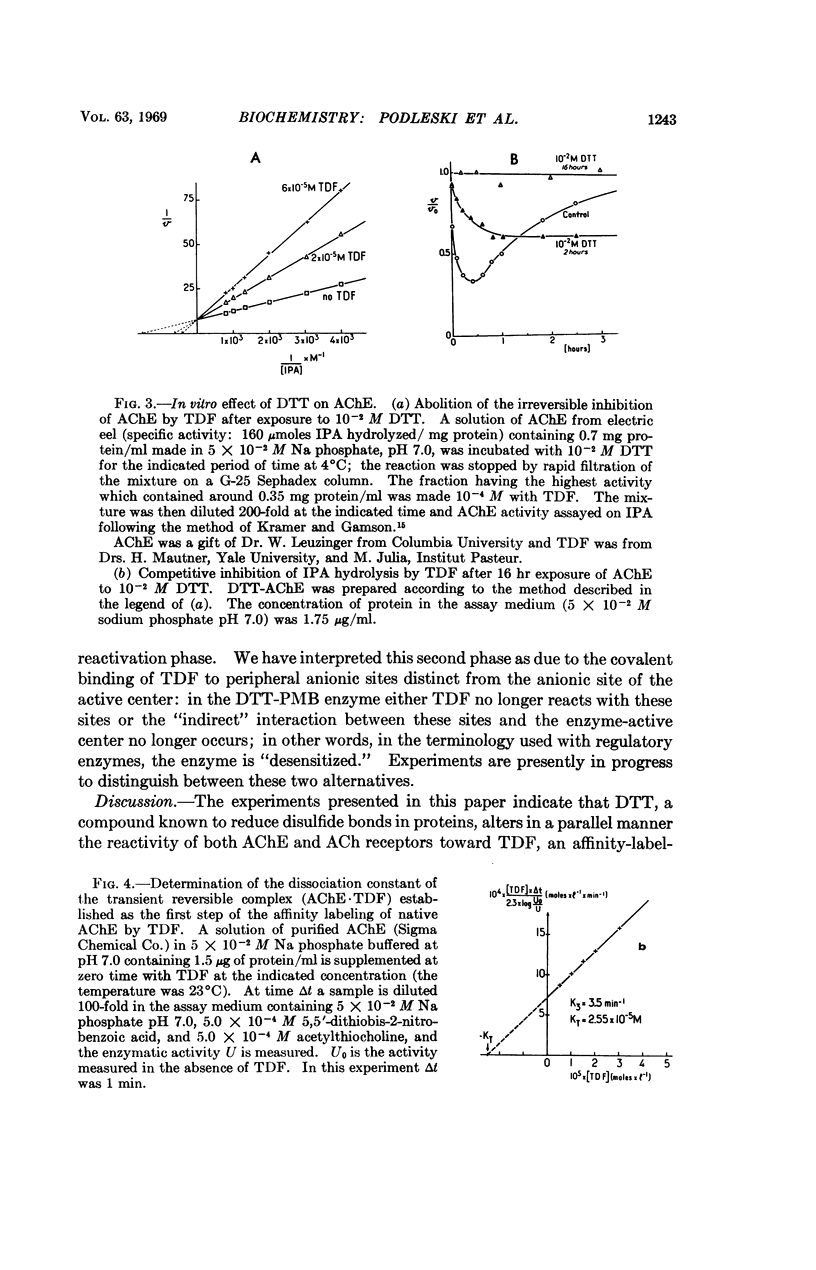
p-(trimethyl ammonium) benzene diazonium difluoroborate (TDF), an affinity-labeling reagent of the acetylcholine receptor site(s), which in the normal cell acts as an irreversible inhibitor becomes a reversible activator after in vivo exposure of the electroplax to dithiothreitol (DTT), a disulfide bond reducing agent. After in vitro exposure of acetylcholinesterase to DTT, TDF becomes a reversible competitive inhibitor of the enzyme, using indophenyl acetate as the substrate. Both acetylcholinesterase and the macromolecular receptor of acetylcholine thus contain disulfide bonds. Additional experiments with DTT suggest that there might exist several different classes of receptor sites for cholinergic agents in the excitable membrane of the electroplax.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Changeux J. P., Podleski T. R. On the excitability and cooperativity of the electroplax membrane. Proc Natl Acad Sci U S A. 1968 Mar;59(3):944–950. doi: 10.1073/pnas.59.3.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Podleski T. R., Wofsy L. Affinity labeling of the acetylcholine-receptor. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2063–2070. doi: 10.1073/pnas.58.5.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P. Responses of acetylcholinesterase from Torpedo marmorata to salts and curarizing drugs. Mol Pharmacol. 1966 Sep;2(5):369–392. [PubMed] [Google Scholar]
- Changeux J. P., Thiéry J., Tung Y., Kittel C. On the cooperativity of biological membranes. Proc Natl Acad Sci U S A. 1967 Feb;57(2):335–341. doi: 10.1073/pnas.57.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREMZNER L. T., WILSON I. B. A chromatographic procedure for the purification of acetylcholinesterase. J Biol Chem. 1963 May;238:1714–1717. [PubMed] [Google Scholar]
- Karlin A., Bartels E. Effects of blocking sulfhydryl groups and of reducing disulfide bonds on the acetylcholine-activated permeability system of the electroplax. Biochim Biophys Acta. 1966 Nov 8;126(3):525–535. doi: 10.1016/0926-6585(66)90010-0. [DOI] [PubMed] [Google Scholar]
- Karlin A. Chemical distinctions between acetylcholinesterase and the acetylcholine receptor. Biochim Biophys Acta. 1967 Jul 11;139(2):358–362. doi: 10.1016/0005-2744(67)90039-3. [DOI] [PubMed] [Google Scholar]
- Karlin A. On the application of "a plausible model" of allosteric proteins to the receptor for acetylcholine. J Theor Biol. 1967 Aug;16(2):306–320. doi: 10.1016/0022-5193(67)90011-2. [DOI] [PubMed] [Google Scholar]
- Karlin A., Winnik M. Reduction and specific alkylation of the receptor for acetylcholine. Proc Natl Acad Sci U S A. 1968 Jun;60(2):668–674. doi: 10.1073/pnas.60.2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier J. -C., Changeux J. -P. On the irreversible binding of p-(trimethylammonium) benzenediazonium fluoroborate (TDF) to acetylcholinesterase from electrogenic tissue. FEBS Lett. 1969 Feb;2(4):224–226. doi: 10.1016/0014-5793(69)80025-6. [DOI] [PubMed] [Google Scholar]
- Rubin M. M., Changeux J. P. On the nature of allosteric transitions: implications of non-exclusive ligand binding. J Mol Biol. 1966 Nov 14;21(2):265–274. doi: 10.1016/0022-2836(66)90097-0. [DOI] [PubMed] [Google Scholar]
- Sanchez C., Changeux J. P. Sur les propriétés de la L-thréonine désaminase de biosynthèse d'un mutatn d'E. coli K 12. Bull Soc Chim Biol (Paris) 1966;48(5):705–713. [PubMed] [Google Scholar]


