Abstract
The Ca2+-binding protein recoverin may regulate visual transduction in retinal rods and cones, but its functional role and mechanism of action remain controversial. We compared the photoresponses of rods from control mice and from mice in which the recoverin gene was knocked out. Our analysis indicates that Ca2+-recoverin prolongs the dark-adapted flash response and increases the rod's sensitivity to dim steady light. Knockout rods had faster Ca2+ dynamics, indicating that recoverin is a significant Ca2+ buffer in the outer segment, but incorporation of exogenous buffer did not restore wild-type behavior. We infer that Ca2+-recoverin potentiates light-triggered phosphodiesterase activity, probably by effectively prolonging the catalytic activity of photoexcited rhodopsin.
Keywords: calcium-binding proteins, knockout mice, light adaptation, photoreceptors, phototransduction
INTRODUCTION
In retinal rods, calcium orchestrates several mechanisms that counteract the effect of light on the phototransduction cascade, thus shortening the light response and preventing signal saturation (for reviews see Pugh et al., 1999; Pugh and Lamb, 2000; Fain et al., 2001). In darkness, Ca2+ enters the outer segment through cGMP-gated channels and is extruded by an electrogenic exchanger which couples the movement of 4 Na+ and 1 K+ down their electrochemical gradients to the removal of 1 Ca2+ (Cervetto et al., 1989). In the presence of light, fewer cGMP-gated channels are open, reducing Ca2+ influx in the face of continued Ca2+ extrusion; this results in a fall in the intracellular Ca2+ concentration. The lowered [Ca2+]i is thought to affect three aspects of the transduction machinery: (a) it decreases light-dependent cGMP phosphodiesterase (PDE) activity, (b) it increases the channel's sensitivity for cGMP, and (c) it increases the activity of guanylate cyclases. Since the cell's responses to light reflect the contributions of all of these control mechanisms, a full understanding of Ca2+'s actions requires experimental designs that allow quantitative assessment of the individual contributions. The purpose of this work is to investigate the contribution of recoverin, which has been proposed to mediate calcium control of PDE activity (Kawamura, 1993; Chen et al., 1995a; Klenchin et al., 1995).
Recoverin is a 23-kD Ca2+-binding protein present in certain retinal neurons: rods, cones, a subset of bipolar cells, and a minor subpopulation of cells in the ganglion cell layer (Dizhoor et al., 1991; Milam et al., 1993; Wiechmann and Hammarback, 1993). Recoverin is also expressed by pinealocytes (Korf et al., 1992), ocular ciliary epithelial cells (Bertazolli-Filho et al., 2001), olfactory receptor neurons (Bastianelli et al., 1995), and some types of carcinomas (Polans et al., 1995; Maeda et al., 2000). Related proteins are found at numerous loci in the CNS. Biochemical experiments on rod outer segment preparations in vitro demonstrated that Ca2+-recoverin inhibits phosphorylation of rhodopsin (Kawamura, 1993) by binding to rhodopsin kinase (Chen et al., 1995a; Klenchin et al., 1995). Consistent with such an effect, dialysis of exogenous Ca2+-recoverin into isolated, intact rod outer segments delayed the recovery of the flash response (Gray-Keller et al., 1993). In truncated rods, addition of exogenous recoverin was shown to lengthen the response duration by prolonging the lifetime of catalytically active, photoexcited rhodopsin (Erickson et al., 1998). These results argue that recoverin imparts Ca2+ dependence to the shutoff of rhodopsin by rhodopsin kinase. Yet, experiments on outer segments permeabilized with staphylococcal α-toxin produced no evidence that the level of Ca2+ controlled the light-dependent phosphorylation of rhodopsin (Otto-Bruc et al., 1998). Recoverin's specific role in phototransduction in vivo, as well as its mechanism of action, therefore remain unclear. We approached these questions by observing the effects of recoverin deletion on visual transduction in intact mouse rods.
MATERIALS AND METHODS
Gene Targeting
A targeting vector was constructed by inserting the gene conferring neomycin resistance, MC1Neo (Stratagene), into the Not I restriction site of the first exon of the recoverin gene (Fig. 1 A). The vector was transfected into CJ7 embryonic stem (ES) cells by electroporation and 960 individual neomycin-resistant clones were isolated and expanded in 96-well plates. Proper targeting of the vector was confirmed by Southern analysis in seven of these isolated clones using the strategy illustrated in Fig. 1 A. Two of the recombinant ES clones were selected, expanded, and injected into 3.5 d blastocysts of C57/B6 mice. Chimeric mice were then bred to obtain mice in which one or both copies of the recoverin gene were disrupted. For some experiments, recoverin knockout (rec−/−) mice were crossed with mice in which guanylate cyclase activating proteins 1 and 2 were knocked out (GCAPs−/−; Mendez et al., 2001) to obtain rods in which both sets of proteins were absent (GCAPs−/−, rec−/−).
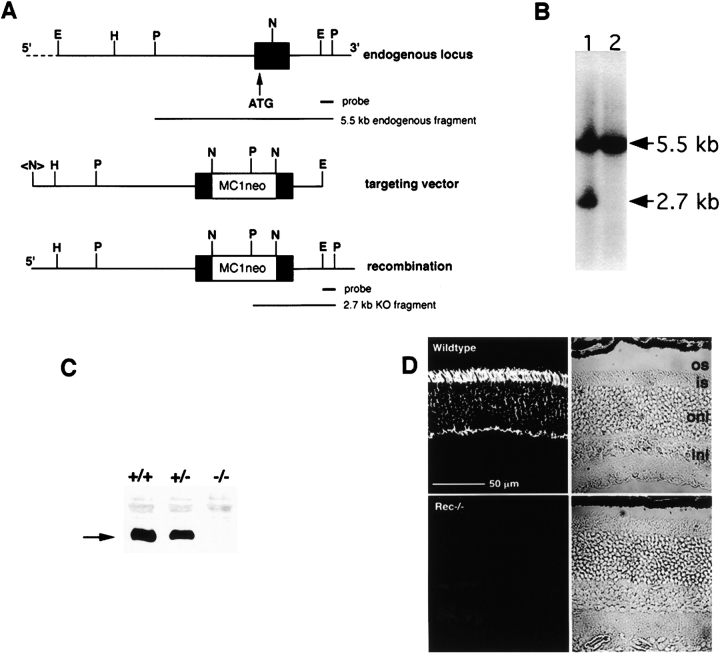
Figure 1.
Targeted disruption of recoverin. (A) Partial restriction map of the endogenous locus, targeting vector and predicted genomic structure after homologous recombination. The targeting vector contained 7 kb of 5′ and 1.5 kb of 3′ homologous sequence. An MC1neo polyA cassette was inserted into the NotI site of exon 1, disrupting the gene. The probes used for Southern blots, representing wild-type and disrupted alleles are illustrated below their corresponding genomic structures. E, EcoRI; H, HindIII; N, NotI; P, PstI; <N>, NotI site from the polylinker of the lambda phage genomic clone. (B) Representative Southern blot of ES clones. DNA prepared from the G418-resistant ES clones was digested with PstI, size fractionated, blotted, and probed for the EcoRI-Pst fragment, located on the 3′ side of the targeting vector. The 5.5-kb fragment derived from the wild-type allele, whereas the 2.7 kb band in lane 1 confirmed that the expected recombination event had occurred. (C) Recoverin immunoblot of retinal extracts prepared from wild-type (+/+), hemizygous (+/−), and homozygous knockout (−/−) mice probed with a polyclonal antibody raised against purified recoverin (Dizhoor et al., 1991). Recoverin (arrow) was reduced in the +/− lane as compared with the wild-type lane and undetectable in the −/− lane. (D) Localization of recoverin in the outer retina of normal mice and its disappearance in knockout mice. Sections from wild-type and recoverin knockout mice were immunostained for recoverin. os, outer segments; is, inner segments; onl, outer nuclear layer; inl, inner nuclear layer.
Immunocytochemistry
Eyecups from 2-mo-old mice were fixed in 4% paraformaldehyde, 0.5% glutaraldehyde in 0.1 M phosphate buffer solution for 1 h on ice, rinsed in cold buffer, and embedded in acrylamide (Johnson and Blanks, 1984). Frozen sections, 10 μm in thickness, were collected and incubated with antirecoverin antibody (dilution, 1:200; Dizhoor et al., 1991). Bound antibody was visualized using a FITC-conjugated secondary antibody (dilution, 1:100; Vector Laboratories).
Analysis of Gene Transcription
RNA samples were prepared as recommended by Affymetrix, Inc. Briefly, a sample of 20–30 μg total RNA was isolated from four retinas using Trizol (Life Technologies). Duplicate samples were prepared from four wild-type and four recoverin knockout mice. All mice were killed at the same time of day, after being dark-adapted for 2–3 d. 10 μg of total RNA from each sample was reverse transcribed to generate cDNA, which was then used to synthesize biotin-labeled cRNA by in vitro transcription (Enzo Diagnostics, Inc.). Labeled cRNA was cleaned up using Rneasy Mini kits (QIAGEN) and fragmented to sizes from 35 to 200 bases by incubating at 94°C for 35 min.
The biotinylated, fragmented cRNA (15 μg) was hybridized to Affymetrix Murine genome U74Av2 arrays for 16 h at 40°C in the GeneChip Fluidics Station 400 (Affymetrix, Inc.). These arrays represented all 6,000 genes in the Mouse UniGene database that have been functionally characterized as well as ∼6,000 expressed sequence tag clusters. After hybridization, the arrays were washed, stained with streptavidin-conjugated phycoerythrin, and scanned with a Hewlett-Packard Scanner. To compare results obtained with different arrays, GeneChip algorithms (Affymetrix, Inc.) were used to normalize the hybridization intensity for each probe by the average hybridization intensity of all the chips. Then pairwise comparisons in the gene expression levels were made between retinas from wild-type and recoverin knockout mice to search for genes that were consistently up- or down-regulated by more than twofold.
Western Analysis of Retinal Proteins
Retinas from C57/B6 × 129svev control and rec−/− mice, 6-wk- to 3-mo-old, were homogenized in buffer (80 mM Tris-HCl, pH 7.4; 10 mM EDTA; 4 mM MgCl2; 2 mM CaCl2; 0.5 mg/ml complete mini protease inhibitors; Boehringer). Equal amounts of retinal homogenates from control and rec−/− mice were fractionated on 12% SDS-PAGE and transferred to nitrocellulose membranes (Protran, Schleicher and Schuell). The membranes were blocked with 5% nonfat dry milk in TBST (20 mM Tris-HCl, pH 7.6, 137 mM NaCl, 0.05% Tween 20), incubated with primary antibodies diluted in TBST and 1% bovine serum albumin, followed by secondary antibodies conjugated to horseradish peroxidase (Sigma-Aldrich), and visualized using ECL (Amersham Biosciences). The primary antibodies consisted of antirhodopsin R2-12N (Hargrave et al., 1986), antirhodopsin kinase 8585 (Inglese et al., 1992) and G8 (Zhao et al., 1998), antirecoverin (Dizhoor et al., 1991), antiarrestin C10C10 (Knospe et al., 1988), antirod transducin α-subunit Tα1A (compare Calvert et al., 2000), antirod transducin β-subunit βN1 (Amatruda et al., 1988) and Gβ1 (Santa Cruz Biotechnology), anti-PDE α-, β- (CytoSignal), and γ-subunits (Arshavsky et al., 1992), anti-RGS-9 (Chen et al., 2000), and antiguanylate cyclase-E (Yang and Garbers, 1997). Each protein was analyzed one to three times.
Single Cell Physiology
Photoresponses were recorded from single rods of rec−/− and control mice as described in Sung et al. (1994). Control mice were obtained from Jackson ImmunoResearch Laboratories, as well as from the California Institute of Technology. Briefly, mice were dark adapted overnight, anesthetized with CO2, and then killed by cervical dislocation. The retinas were isolated into Leibovitz's L-15 medium under infrared light and stored on ice. Small samples were removed, chopped finely in L-15 containing ∼1.5 μg/ml DNase1 (Type IV, Sigma-Aldrich), and transferred to an experimental chamber perfused continuously with an enriched Locke's solution heated to 35–38°C. Locke's contained (mM): Na+ 144, K+ 3.6, Ca2+ 1.2, Mg2+ 2.4, Cl− 123.3, HEPES 10, HCO3 − 20, EDTA 0.02, glucose 10, glutamate 0.5, succinate 3, BME vitamins, BME amino acid supplement, pH 7.4. A rod outer segment was drawn into a silanized glass pipette filled with a similar solution except that vitamins and amino acids were omitted and HCO3 − was replaced with an equimolar amount of Cl−. Cells were stimulated with an electronically shuttered tungsten halogen lamp. Monochromatic light (10-nm bandwidth) at 500 nm (flashes or steps of light) or at 520 nm (steps) was obtained with interference filters. Photoresponses were recorded with a current-to-voltage converter (Axopatch 200; Axon Instruments, Inc.) and digitized online with a Macintosh computer. Records were low-pass filtered at 30 Hz (−3 dB, 8-pole Bessel, AI2040; Axon Instruments, Inc.), except where noted otherwise. No corrections were made for the delay introduced by filtering. In addition, the traces in Figs. 2, 3, 4 and 7 were digitally filtered at 7 Hz (Igor version 3.15; Wavemetrics).
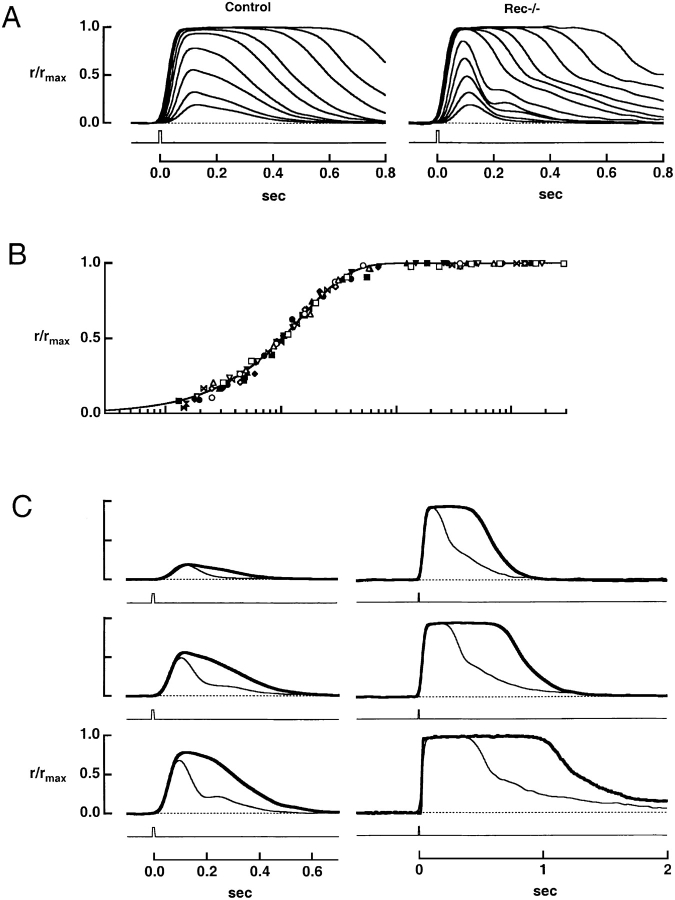
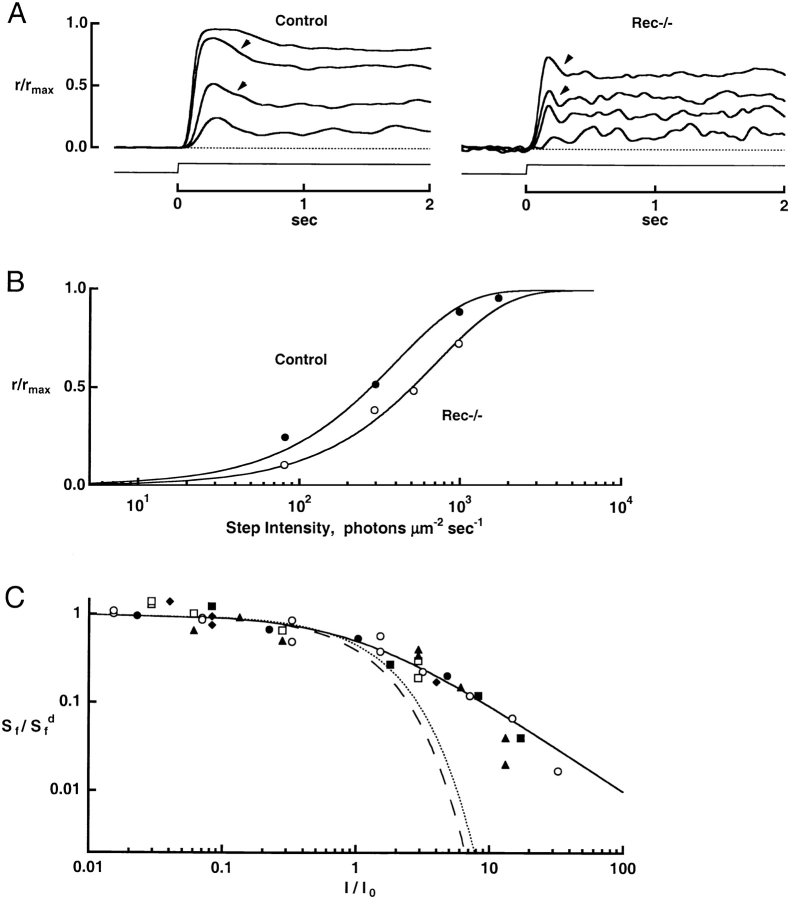
Figure 2.
The effect of recoverin deletion on flash responses. (A) Averaged responses of a control rod and a rec−/− rod to flashes of increasing strength. The maximal response amplitudes were 16.4 and 12.9 pA for control and rec−/− rods, respectively. Flash strengths for the control rod were: 11.8, 20.6, 42.8, 74.9, 139, 243, 504, 882, and 1,690 photons μm−2, while for the rec−/− rod, they were 12.8, 22.4, 46.5, 81.4, 151, 548, 960, 1,840, 3,230, 6,700, and 11,700 photons μm−2. (B) Stimulus-response relation for seven control (filled symbols) and seven rec−/− rods (open symbols). The continuous line is a saturating exponential, r/rmax= 1 − exp(−ki/i1/2), where rmax is the saturating response amplitude, k = ln(2), i is the flash strength, and i1/2 is the flash strength producing a half-maximal response for each cell. The cells in A are shown by the (▾, control), and by the (□, knockout). (C) Faster flash response recovery in a rec−/− rod. Each pair of traces shows the averaged responses elicited by flashes of similar strength from the control (thick trace) and rec−/− (thin trace) rods in A. The flash strengths were (top to bottom, left to right): 11.8, 42.8, 74.9, 504, 1,690, and 6,160 photons μm−2 for the control rod and 12.8, 46.5, 81.4, 548, 1,840, and 6,700 photons μm−2 for the rec−/− rod.
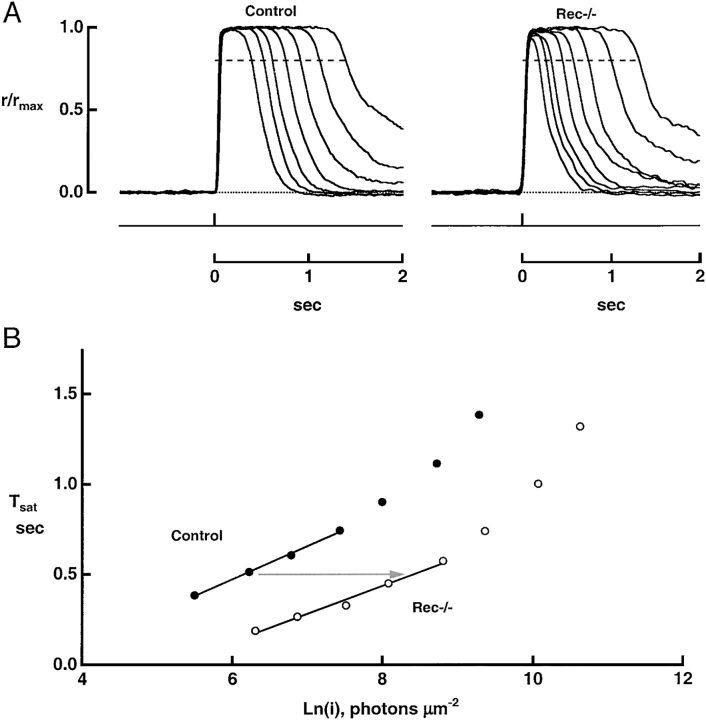
Figure 3.
Faster recovery of rec−/− rods from bright flashes. (A) Averaged responses of a control rod and a rec−/− rod. Tsat was measured from midflash to 20% recovery of the circulating current, demarcated by the dashed line. Maximal response amplitudes were 16.4 and 10.4 pA for control and rec−/− rods, respectively. (B) Pepperberg plots from the responses in A. Tsat was lower for the rec−/− rod than for the wild-type rod at every flash strength. Linear regression over the indicated regions gave slopes of 184 ms for the control rod and 154 ms for the rec−/− rod. The rec−/− rod required 9.7-fold more light to produce a Tsat of 0.5 s (arrow).
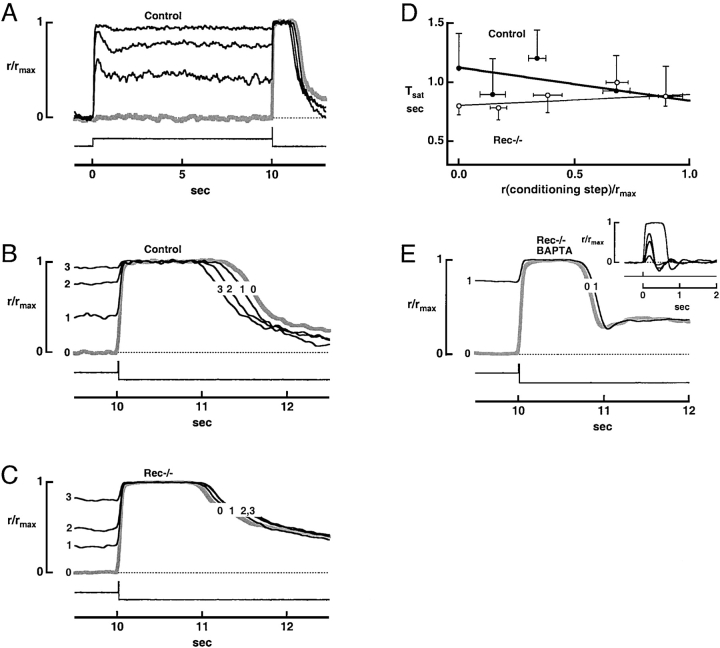
Figure 4.
Absence of phosphodiesterase adaptation in rec−/− rods. (A) Step-flash paradigm. A saturating flash of 57,800 photons μm−2 at 500 nm, presented to a control rod at time = 10 s, was preceded by darkness (response shown with thick gray line) or by a conditioning step of light of intensity: 2,080, 4,540, or 9,470 photons μm−2 s−1 at 520 nm, which reduced [Ca2+]i. The maximal response amplitude was 7.3 pA. (B) Decrease in response saturation time for flashes given after a step of light. Responses are the same as those shown in A. (C) Lack of decrease in flash response saturation time after conditioning steps in a rec−/− rod. The flash delivered 55,200 photons μm−2 at 500 nm while the step intensities were: 920, 1,920 and 4,190 photons μm−2 s−1 at 520 nm. The maximal response amplitude was 11.4 pA. (D) Convergence of control and rec−/− rod flash response saturation times after a conditioning step of light to diminish Ca2+. Averaged responses to the conditioning step after 10 s of exposure relative to the maximal response were plotted on the abscissa. Error bars show SEM. Test flash strength ranged from 4,310 and 4,830 photons μm−2 at 500 nm. Continuous lines plot the mean slopes and intercepts for rods of each type, after linear regressions of the results from individual rods. For controls, the slope was −0.283 s while for rec−/−, it was 0.092 s. Rec−/− rods exhibited differences in both slope (P < 0.0006) and intercept (P < 0.02). (E) Failure of calcium buffer to restore PDE adaptation in a rec−/− rod. Rods were incubated in 100 μM BAPTA-AM for a minimum of 10 min at room temperature, before being recorded. The flash strength was 36,300 photons μm−2 at 500 nm, dimmer than in A–C. The step intensity was 5,020 photons μm−2 s−1 at 520 nm. The maximal response amplitude was 14.3 pA. (Inset) Averaged flash responses of a rec−/− rod treated with BAPTA. The time to peak of the dim flash response was delayed to 170 ms but the integration time, measured to the first baseline crossing, was only 140 ms. The flash densities were: 7.9, 26.4, 46.2, and 3,800 photons μm−2 at 500 nm.
Figure 7.
Responses to steps of light and to flashes superimposed on steps. (A) Averaged responses of a control rod and a rec−/− rod to steps of light. Arrowheads mark the partial recovery in current resulting from light adaptation. Maximal response amplitudes were 14.5 and 8.0 pA for control and rec−/− rods, respectively. (B) Diminished sensitivity of a rec−/− rod to steps of light. Results from A were fitted with a saturating exponential function (continuous lines). Half-maximal responses were elicited with intensities of 273 and 495 photons μm−2 s−1 at 500 nm in the control and rec−/− rods, respectively. (C) Normal incremental flash sensitivity in rec−/− rods. Rods were adapted to a background light for a minimum of 40 s and then probed with dim test flashes. Dim flashes were also given in darkness before and after presentation of the background to monitor the condition of the cells. The continuous line plots the Weber-Fechner relation (Eq. 1). For comparison, the dashed and dotted lines plot the behaviors predicted by saturation alone (Eq. 2) for control and rec−/− rods, respectively. Collected results from four control (filled symbols) and two rec−/− rods (open symbols).
For the purposes of estimating the intracellular concentrations of ions and proteins, we calculated a rod outer segment aqueous volume of 9.2 to 15.4 fl. The calculation was based on a rod diameter of 1.4 μm (Carter-Dawson and LaVail, 1979) and a recorded outer segment length of 12 to 20 μm. Half of the space was assumed to be occupied by the disks and was excluded.
RESULTS
Rec−/− Retinal Morphology and Protein Content
The targeting vector disrupted the first exon of the recoverin gene and prevented its expression (Fig. 1). The effect of recoverin knockout on gene expression in the retina was analyzed using Affymetrix Murine genome U74Av2 arrays, which contain all known phototransduction genes in addition to ∼12,000 functionally characterized genes and expressed sequence tags. Comparisons of expression profiles between dark-adapted rec−/− and control retinas revealed only two significant changes. There was a 10-fold decrease in the signal for recoverin. The persistence of a low signal for recoverin in knockouts could result from the presence of a partial mRNA sequence for recoverin or from weak crosshybridization with transcript for another protein. In addition, two probe sets for transducin β-subunit mRNA revealed 6- and 16-fold decreases, respectively. This large difference in expression levels for transducin β was verified using semiquantitative, real time RT-PCR. The lack of immunohistochemical staining of photoreceptor and bipolar cells in retinal sections and the absence of recoverin in Western blots of retinal protein confirmed that recoverin was indeed absent from knockout mice. Western analyses, however, did not show large changes in the amount of transducin β-subunit. Levels of rhodopsin, rhodopsin kinase, arrestin, transducin α-subunit, the α-, β-, and γ-subunits of PDE, RGS9-1, and guanylate cyclase E were also similar in knockout and control retinas. Disruption of recoverin expression was therefore considered to be functionally selective in that it did not give rise to compensatory changes in the levels of phototransduction proteins or to changes in the levels of mRNA for proteins not directly involved in phototransduction.
Knockout of recoverin did not appear to adversely affect the development or long-term viability of the retina. Rods elaborated outer segments of normal size and the outer nuclear layer, consisting of photoreceptor nuclei, attained normal thickness. The thickness of the inner nuclear layer, which contains the somas of bipolar cells, was also normal. There was no sign of a retinal degeneration in rec−/− mice up to 1 yr of age, the oldest examined.
Faster Flash Response Recovery in Dark-adapted rec−/− Rods
Fig. 2 A shows flash response families from a representative rec−/− and control rod. Although the rising phases of the responses were similar, rec−/− responses recovered more rapidly—a characteristic difference that is analyzed more fully below. After flashes of intermediate strength, the recovery phase of the knockout rod response often displayed a prominent “kink” usually not present in responses of control rods. The kink in the flash response of rec−/− rods may explain the anomalous electroretinograms of rec−/− mice, which exhibit two corneal positive b-waves rather than one in response to a single midrange scotopic flash (Ron Bush, personal communication; Hurley and Chen, 2001).
Collected results on the relation between normalized peak response amplitude and normalized flash strength are shown in Fig. 2 B. The smooth curve, a saturating exponential (Lamb et al., 1981), provided a good fit to the results from both populations. Although the half-saturating flash strength was lower for rec−/− rods, the difference was small, as shown in Table I. The table also summarizes other parameters of the flash responses from control and rec−/− rods. It can be noted that the dark current and the time-to-peak of the dim flash response were very similar for the two populations, as was the estimated amplitude of the single photon response.
TABLE I.
Response Parameters of Control and Rec−/− Rods
| Control | Rec−/− | p | |
|---|---|---|---|
| Dark current (pA) | 8.9 ± 0.3, 96 | 9.6 ± 0.5, 47 | ns |
| Na+/Ca2+-K+ exchange | |||
| Time constant (ms) | 113 ± 7, 73 | 86 ± 9, 51 | 2e−3 |
| Initial amplitude (pA) | −0.53 ± 0.02, 73 | −0.59 ± 0.05, 51 | ns |
| Fractional amplitude | 0.062 ± 0.003, 73 | 0.060 ± 0.04, 73 | ns |
| Half-saturating flash strength, i1/2 (photons μm−2 at 500 nm) | 47 ± 3, 59 | 37 ± 2, 39 | 2e−2 |
| Single photon response characteristics | |||
| Integration time, Ti (ms) | 250 ± 11, 76 | 157 ± 9, 47 | 6e−8 |
| Time to peak, tp (ms) | 138 ± 3, 76 | 135 ± 3, 47 | ns |
| Amplitude (pA) | 0.39 ± 0.04, 39 | 0.43 ± 0.04, 17 | ns |
| Step sensitivity, I1/2 (photons μm−2 s−1 at 500 nm) | 250 ± 31, 12 | 410 ± 71, 11 | 4e−2 |
| Light adaptation, I0 (photons μm−2 s−1 at 500 nm) | 178 ± 33, 4 | 192 ± 123, 2 | ns |
Values given as mean ± SEM, n. The kinetic parameters for the single photon response were taken from the responses to dim flashes, since their shapes are identical. Flashes eliciting a mean response less than a fifth of the maximum were considered dim. The amplitude of the single photon response was estimated by taking the ratio of the ensemble variance to the mean amplitude of the response to a dim flash. Sensitivity to steps of light was evaluated with either 500-nm or with 520-nm light. The intensity giving rise to a half maximal response, I1/2, was given for 500-nm light, taking rod sensitivity to 520 nm relative to that at 500 nm as 0.833 (Calvert et al., 2000). I0, found from light adaptation experiments of the type shown in Fig. 7 C, is the intensity of background light that halved flash sensitivity from the value observed in darkness. The value given for I0 includes a conversion from 520- to 500-nm photons. Comparisons were made using a t test.
Fig. 2 C compares the responses of the rods in Fig. 2 A to flashes whose strength varied over a wide range. For subsaturating flashes, the responses of the rec−/− rod recovered more rapidly than those of the control rod. The integration time of the dim flash response, defined as the area under the response divided by its peak height, was on average 37% lower in rec−/− rods than in control rods (Table I). The difference in effective response duration became more pronounced at higher flash strengths that saturated the response (Fig. 3, see also Hurley and Chen, 2001).
The diminished time in saturation (Tsat) of rec−/− responses to bright flashes corresponds to an effective decrease in phototransduction gain. The magnitude of the decrease was assessed from the ratio of the flash strengths required to hold control (n = 20) and rec−/− (n = 11) rods in saturation for a given time (e.g., Fig. 3). Although the slope of the relation for the two types of rod differed slightly, a flash approximately ninefold brighter was required to hold knockout rods in saturation, indicating a ninefold lower gain (Fig. 3 B). The general conclusion from these experiments is that wild-type rods had a higher transduction gain during the recovery phase of the dark-adapted flash response.
Effect of Background Light on PDE Activity Evoked by a Bright Flash
Early biochemical studies suggested that recoverin exerts its effect in the Ca2+-bound form (Kawamura, 1993). If Ca2+-bound recoverin prolongs the response to a bright flash delivered in darkness, then at low Ca2+, control and rec−/− rods should behave identically. This idea was tested using the step-flash protocol devised by Fain et al. (1989), as shown in Fig. 4 A. Steady background lights of varying intensities were used to lower the rod's [Ca2+]i, before the presentation of a saturating test flash. As expected (Fain et al., 1989), background light shortened the saturation time of the response to the flash in control rods (Fig. 4, A and B). Because the test flash response itself always lowered [Ca2+]i to a minimal level and thus activated guanylate cyclase maximally, the reduction in Tsat should be caused by a reduction in the flash-evoked PDE activity (Matthews, 1996). The shortening of Tsat was a robust phenomenon, being present in each of 34 control cells.
Background light failed to shorten the Tsat of rec−/− rods (19 of 20 rods) and in most cases increased it. Records from one such experiment are shown in Fig. 4 C. Tsat was extended slightly with conditioning step intensity in rec−/− rods because recovery from the step alone was generally more prolonged at higher intensities. There was some evidence that the same effect partially masked the decline in Tsat with conditioning step intensity for control rods. In 7 out of 24 control rods where a wide range of conditioning step intensities were tested, Tsat change little or even increased after dim to moderate conditioning steps, but eventually decreased at higher step intensities. Background light did shorten Tsat slightly in one rec−/− rod. But the recovery phases of the responses to steps and steps plus flashes in this rod had undershoots that were not present in the responses to the flashes alone. Similar behavior was sometimes observed in control rods and hence appears to be unrelated to the deletion of recoverin. The fact that background light caused the saturation time of the response to a bright test flash in control rods and rec−/− rods to become more similar (Fig. 4, B–D) is consistent with the notion that Ca2+-recoverin boosts the latter portion of the bright flash response.
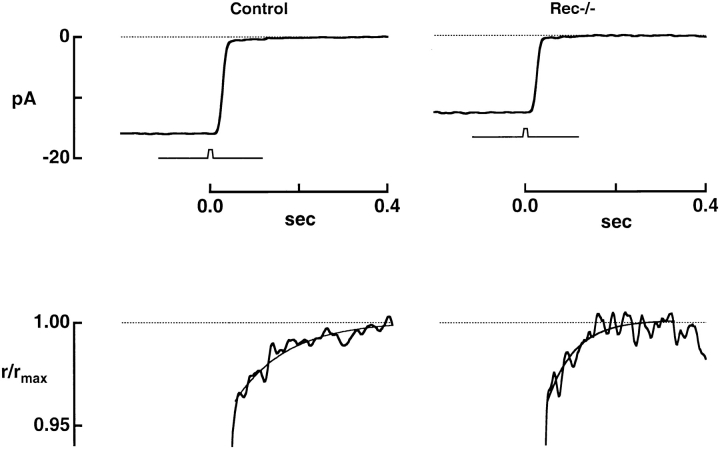
Altered Kinetics of Na+/Ca2+-K+ Exchange in rec−/− Rods
The light response is generated by changes in the concentration of cGMP, which in turn reflect not only activation of PDE by light but also Ca2+ mediated activation of guanylate cyclase by GCAPs (guanylate cyclase activating proteins). Recoverin's abundance in photoreceptors (17–40 μM; Kawamura and Murakami, 1991; Klenchin et al., 1995), suggests that it could contribute significantly to a rod's Ca2+ buffering capacity. Thus, the deletion of recoverin could speed the light-induced decline in calcium and the resulting activation of guanylate cyclase. To assess the effect of recoverin deletion on Ca2+ dynamics, we recorded the Na+/Ca2+-K+ exchange currents of control and knockout rods. A bright flash, which rapidly closed the light-sensitive channels, revealed the exchange current as a small, slowly decaying inward current (Fig. 5). The exchange transients are shown on an expanded, normalized ordinate scale in the lower panels (jagged traces) where they are fitted with single exponentials (smooth curves). In control rods the exponential time constant was 113 ± 7 ms (mean ± SEM), while in rec−/− rods, it was 86 ± 9 ms (Table I). In the same rods the initial amplitude of the exchange current, extrapolated to the time at which the bright flash response reached half its peak amplitude (Yau and Nakatani, 1985), was −0.53 ± 0.02 and −0.59 ± 0.05 pA in control and rec−/− rods, respectively. Because the stoichiometry of ionic exchange is fixed (Cervetto et al., 1989) the time integral of the exchange current is directly proportional to the amount of Ca2+ extruded. The initial amplitudes were the same for the two types of rods, consistent with their maintaining a similar free [Ca2+]i in darkness. However, the longer time constant for control rods made the integrated area of the exchange current larger, indicating that more Ca2+ was extruded from control rods. The 9.1 fC of additional charge entering control rods during the exchange current corresponds to 6 to 10 μM Ca2+ in the outer segment, assuming a volume range of 9.2 and 15.4 fl (see materials and methods). Since most of a rod's internal Ca2+ is bound (Lagnado et al., 1992), the extra Ca2+ content of controls presumably represents a fraction bound to recoverin. This fraction, which is apparently released and extruded rapidly after a bright flash, comprises ∼15% of the total Ca2+ present in the outer segment.
Figure 5.
Accelerated Ca2+ dynamics in rec−/− rods. The top panel shows the averaged responses of a control and a knockout rod to bright, saturating flashes of 1,690 and 5,520 photons μm−2, respectively. The response amplitude was 15.9 pA for the control rod and 12.6 pA for the knockout rod. The low amplitude, slowly declining inward current reflects the extrusion of Ca2+ by the Na+/Ca2+-K+ exchanger. The exchange currents are expanded, normalized, and fitted with a single exponential in the bottom panel. For the control rod, the exponential time constant was 112 ms, while for the knockout, it was 61 ms. The initial amplitudes of the exchange currents were −0.7 and −0.6 pA for control and rec−/−, respectively.
Does the rec−/− phenotype result simply from a faster fall in intracellular Ca2+ concentration and earlier activation of guanylate cyclase? We examined this in two ways.
First, we used BAPTA-AM incorporation to bolster the Ca2+ buffering capacity and slow the changes in intracellular free Ca2+ concentration in four rec−/− rods from one retina. Ca2+ buffering was significantly increased as demonstrated by the fact that the exchange current time constant was twofold longer than that in four untreated rods from the other retina of the same mouse (82 ± 13 ms, rec−/−; 157 ± 29 ms, rec−/− with BAPTA). BAPTA did not restore a normal phenotype to rec−/− rods; instead it delayed the time to peak of the flash response and introduced an oscillation in the recovery phase (Fig. 4 E, inset). Furthermore, background light still failed to reduce the saturation time of a bright flash response (Fig. 4 E). These experiments suggest that recoverin does not act only as a Ca2+ buffer in the outer segment.
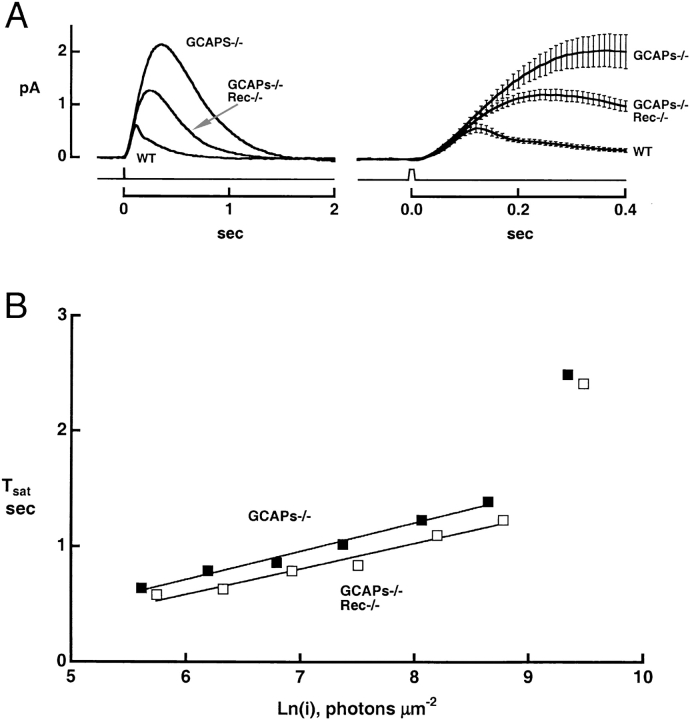
Second, we determined the effect of deleting recoverin in rods lacking calcium-regulated GC activity. We did so by comparing transduction in rods lacking guanylate cyclase–activating proteins (GCAPs−/−; Mendez et al., 2001) with that in rods lacking both GCAPs and recoverin (GCAPs−/− rec−/− double knockouts). If recoverin solely acts by controlling the time course of cyclase activation, deleting recoverin should have no effect in the GCAPS−/− background. Fig. 6 illustrates single photon responses averaged from a number of GCAPs−/−, GCAPs−/− rec−/− double knockouts, and wild-type rods; Table II summarizes several parameters of the flash responses of the cell populations. As reported previously, GCAPs deletion increased the amplitude, time-to-peak, and integration time of the single photon response (Mendez et al., 2001). Deletion of recoverin in the GCAPs−/− background gave a sizeable reduction in the single photon response amplitude (Fig. 6, Table II) and a corresponding increase in the half-saturating flash strength (Table II). Recoverin deletion also shortened the time-to-peak and integration time of the dim flash response in the GCAPs−/− background. This indicates that in the absence of recoverin, light-stimulated PDE activity was effectively shorter. In addition, the “kinks” observed in subsaturating rec−/− responses were absent in the GCAPs−/− rec−/− responses. Apparently, the kinks in the responses of the rec−/− rods can be attributed to abnormally rapid cyclase activation. The characteristic differences in the flash responses of normal, rec−/−, GCAPs−/−, and GCAPs−/− rec−/− were satisfactorily predicted by a simple quantitative model of the response dynamics (Fig. 8 and ).
Figure 6.
Changes in the flash responses of GCAPs−/− rods after deletion of recoverin. (A) Single photon responses of GCAPs−/− and GCAPs−/− rec−/− rods. Traces show the averaged responses of 15 rods of each type on two time scales. Error bars demarcate the SEM. Low pass filtered at 20 Hz. The GCAPs−/− and wild type responses are reproduced from Mendez et al. (2001). (B) Saturation functions. The relation for the GCAPs−/− rec−/− rod was shifted to flash strengths ∼1.7-fold greater than for the GCAPs−/− rod. Linear regression yielded slopes of 240 and 222 ms for GCAPs−/− and GCAPs−/− rec−/− rods, respectively.
TABLE II.
Flash Response Parameters of Rods after Deletion of GCAPs and Recoverin
| GCAPs−/− | GCAPs−/− Rec−/− | p | |
|---|---|---|---|
| Dark current (pA) | 13.3 ± 0.8, 42 | 12.3 ± 0.9, 19 | ns |
| Half-saturating flash strength, i1/2 (photons μm−2 at 500 nm) | 11.3 ± 0.8, 33 | 19.0 ± 1.2, 18 | 1e−6 |
| Single photon response characteristics | |||
| Integration time, Ti (ms) | 589 ± 19, 42 | 504 ± 27, 15 | 2e−2 |
| Time to peak, tp (ms) | 315 ± 13, 42 | 255 ± 20, 17 | 2e−2 |
| Amplitude (pA) | 2.31 ± 0.20, 41 | 1.34 ± 0.10, 15 | 6e−3 |
Values are given as mean ± SEM, n. Results from GCAPs−/− rods were reported previously by Mendez et al. (2001).
Saturation times of bright flash responses in GCAPs−/− rods were shifted to twofold higher flash strengths upon recoverin deletion (e.g., Fig. 6 B; n = 21 GCAPs−/− and 11 GCAPs−/− rec−/− rods). In additional experiments (not depicted), exposure to a conditioning step shortened the saturation time of the response to a bright flash in each of 11 GCAPs−/− rods, as expected from the presence of recoverin's action, but this effect was absent in each of 7 GCAPs−/− rec−/− rods.
In summary, these results suggest that recoverin has a dual action on the flash response: (a) Ca2+-recoverin extends the effective duration of rhodopsin's catalytic lifetime, and (b) by acting as a Ca2+ buffer, recoverin slightly delays the activation of guanylate cyclase.
Decreased Sensitivity of rec−/− Rods to Steps of Light
The onset of a step of light elicited a response that rose to a peak and then partially recovered, as the rod adapted (e.g., Fig. 4 A). The initial restoration of the current was faster in rec−/− rods than in controls (Fig. 7 A, arrowheads), but was not faster in rods lacking both GCAPs and recoverin (GCAPs−/− rec−/−; unpublished data). This suggests that the initial, faster restoration of the current in rec−/− rods arises from accelerated Ca2+ activation of guanylate cyclase. The absence of recoverin decreased step sensitivity, measured at the initial peak of the step response (Fig. 7 B and Table I). For control rods, an intensity of 250 photons μm−2 s−1 elicited a half-maximal response while for rec−/− rods, 410 photons μm−2 s−1 was required (Table I). This 1.6-fold difference is attributable to the 1.6-fold lower integration time of the dim flash response in rec−/− rods.
Incremental Flash Responses in Background Light
Deleting recoverin had little effect on light adaptation, as assessed by measuring the dependence of flash sensitivity on background light intensity (Fig. 7 C). The plot shows the relative sensitivity to a dim test flash as a function of normalized background light intensity, with both scales logarithmic. The closed symbols show results from control rods, and open symbols show results from rec−/− rods. The normalizing factor for the background light intensity is I0, the intensity that reduced the cell's flash sensitivity to half the value in darkness. Values of I0 were similar in control and rec−/− rods: 178 and 192 photons μm−2 s−1, respectively (Table I ). The smooth curve is drawn according to the Weber-Fechner relation:
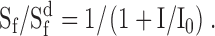 |
(1) |
This expression provides an empirical description of the behavior observed in several types of mammalian rods (Tamura et al., 1989, 1991; Nakatani et al., 1991). For comparison, the dashed and dotted curves plot the relation expected for rods whose adaptation results strictly from response saturation:
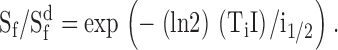 |
(2) |
The behavior of both types of rod was very similar, and both populations truly adapted rather than simply saturating. We conclude that deletion of recoverin had little effect on the mechanisms that fix the dependence of incremental flash sensitivity on background light intensity.
DISCUSSION
Functional Role of Recoverin in Phototransduction
A photon absorbed in bright background light normally produces a smaller contribution to the steady-state PDE activity than does a photon absorbed in darkness (Koutalos et al., 1995). This effect, referred to as “PDE adaptation”, depends at least in part on the light-induced fall in steady-state free [Ca2+] within the outer segment and can amount to a seven-fold reduction in the incremental PDE activity (Matthews, 1996). By reducing the steady level of PDE activity that the background generates, PDE adaptation reduces the background's ability to elicit a saturating response, during which incremental stimuli cannot be effectively transduced. Our results identify recoverin as the Ca2+ sensor that triggers calcium-dependent PDE adaptation. Thus, the dark-adapted response to bright flashes was shorter in rec−/− rods than in wild-type rods, and this difference disappeared in background light, which lowered the free [Ca2+]i. There was no evidence that any other component of PDE adaptation persisted after the recoverin gene was knocked out. Evidence that the shorter incremental response of rec−/− rods arose from reduced PDE activity rather than an effect on the activity of guanylate cyclase is provided by the fact that recoverin's action was still present in GCAPs−/− rods, whose cyclase activity does not vary with background light level (Mendez et al., 2001; Burns et al., 2002). It should be emphasized that recoverin potentiates the dark-adapted response to light rather than having an effect on the response in background light. Thus, Ca2+ recoverin increases the duration of the light-triggered PDE activity. As a result, the integration time of the dark-adapted flash response is longer and the amplitude of the response to a dim step is larger.
Deletion of recoverin had little effect on the dependence of relative flash sensitivity on background light intensity (Fig. 7 C). This result, perhaps surprising at first, may be explained in the following way. At high background intensities, recoverin is in the Ca2+-free state, where it exerts no effect, and thus knockout rods and control rods should behave identically. At low background intensities, Ca2+-recoverin potentiates the late phase of the incremental flash response in control rods. However, flash sensitivity, measured at the peak response amplitude, is almost insensitive to recoverin's action, so that again little difference is expected between knockout and control rods. Had step sensitivity been plotted as a function of background intensity, rods containing recoverin would have exhibited a modest extension of response range, being nearly twofold more sensitive in darkness and converging with the rec−/− rods' behavior at high background intensity. We cannot explain the electroretinographic recordings that suggested a slightly elevated step sensitivity in rec−/− mice (Hurley and Chen, 2001).
Matthews (1997) showed that in salamander rod outer segments at room temperature there was very little PDE adaptation during the response to a bright flash, yet a conditioning step of light preceding the bright flash gave a substantial effect. Negative feedback during the flash response itself was negligible because the target controlling PDE adaptation had mostly disappeared by the time the free [Ca2+]i fell. Probably there is also little PDE adaptation (calcium-dependent modulation of rhodopsin activity) during the flash response of mouse rods because slowing Ca2+ dynamics in GCAPs−/− rods has no effect on the kinetics of the flash response (Burns et al., 2002). The rapid disappearance of Ca2+-recoverin's target suggests that recoverin mediates an effect in which the response to an incident photon can desensitize only the response to a subsequent photon. This is in contrast to the effect mediated by guanylate cyclase activation, which provides negative feedback during the single photon response.
Molecular Mechanism of Recoverin's Action
Our experiments show that Ca2+-recoverin effectively prolongs light-triggered PDE activity, but they do not identify the target of its action. Because the initial rate of rise of the dark-adapted flash response was the same in rec−/− and control rods it is unlikely that recoverin affects the initial efficiency of transducin activation by photoexcited rhodopsin, the efficiency of PDE activation by activated transducin, or the catalytic activity of PDE itself. Therefore, Ca2+-recoverin apparently acts on the effective catalytic lifetime of one of the species in the excitation chain. In truncated outer segments of salamander rods, Ca2+-recoverin failed to affect the rate of deactivation of transducin (Erickson et al., 1998). Furthermore, Kawamura (1993) presented evidence that Ca2+-recoverin inhibits the phosphorylation of rhodopsin by rhodopsin kinase, and subsequent experiments demonstrated that Ca2+-recoverin binds rhodopsin kinase (Gorodovikova and Philippov, 1993; Chen et al., 1995a; Klenchin et al., 1995). Ca2+-recoverin may impede the shutoff of rhodopsin simply by decreasing the effective concentration of free rhodopsin kinase. The feasibility of such a mechanism was demonstrated by models of rod photoresponses (compare Pugh et al., 1999; Hamer, 2000; Nikonov et al., 2000). Consistent with this notion, addition of exogenous recoverin into a rod outer segment prolonged the flash response (Gray-Keller et al., 1993; Erickson et al., 1998) and extended the catalytic lifetime of photoexcited rhodopsin (Erickson et al., 1998). Also consistent, reduction of the rhodopsin kinase content of mouse rods by knockout of one allele slowed the recovery phase of the flash response (Chen et al., 1999).
If recoverin is to effectively control the concentration of free rhodopsin kinase, its concentration must exceed that of rhodopsin kinase. Is this condition met? Recoverin has a relatively low affinity for Ca2+ (Ames et al., 1995) so that if the dark concentration of free Ca2+ is 250 nM (Woodruff et al., 2002), most of the recoverin will be in the Ca2+-free form. Assuming that recoverin binds two Ca2+ with a Hill coefficient of 1.4, that recoverin exists in a simple equilibrium between Ca2+-free and Ca2+-bound forms and that Ca2+-bound recoverin partitions into the membrane, then
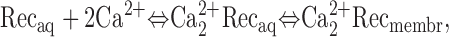 |
where k1 = [Rec]aq[Ca2+]free 1.4/[Ca2+ 2Rec]aq = (17 μM)1.4 and k2 = [Ca2+ 2Rec]aq/[Ca2+ 2Rec]membr = 0.1 (Ames et al., 1995; Baldwin and Ames, 1998; Erickson et al., 1998). For [Ca2+]free = 0.25 μM and [Ca2+ 2Rec]aq + [Ca2+ 2Rec]membr = 3–5 μM (from the analysis of the exchanger current, above), the total recoverin concentration in a dark-adapted outer segment would be 106–176 μM. This range of values is larger than the biochemical determinations of 30–40 μM for recoverin (Kawamura and Murakami, 1991; Klenchin et al., 1995; but see Kawamura et al., 1993, 1996). At >100 μM, recoverin exceeds the estimated rhodopsin kinase concentration of ∼10 μM by tenfold (Shichi and Somers, 1978), making the model for kinase inhibition feasible. Therefore, the simplest interpretation of our data is that calcium-recoverin prolongs the lifetime of photoexcited rhodopsin, probably via its interaction with rhodopsin kinase. During light adaptation, intracellular Ca2+ falls and the effect of recoverin is removed.
Acknowledgments
We thank Drs. V. Arshavsky, L. Donoso, P. Hargrave, J. Hurley, R. Lefkowitz, and K. Palczewski for generously providing us with antibodies, Mr. S. Nagpal for the microchip analyses, Ms. C. Mfalila for assistance with the statistical analysis, and Mr. R. Schneeveis for skillful, technical support.
This research was funded by a Career Development Award from Research to Prevent Blindness (C.L. Makino), the Arnold and Mabel Beckman Foundation (J. Chen), the National Institute of General Medical Sciences (R.L. Dodd), the National Institute on Aging AG12288 (M.I. Simon), and the National Eye Institute EY12944 (C.L. Makino), EY12703 (J. Chen), EY14047 (M.E. Burns), EY05750 (D.A. Baylor).
Olaf S. Andersen served as editor.
APPENDIX
The effect of deletion of recoverin on the photocurrents generated by flashes was modeled using Rieke and Baylor's (1998) treatment. The model makes several simplifying assumptions: catalytically active rhodopsin in dark-adapted control rods decays exponentially, the ROS volume is well-stirred, and regulation of the cGMP-gated channel by Ca2+ is negligible. The model successfully described the responses of rec−/− rods if the absence of recoverin or low intracellular Ca2+ was assumed to cause an abrupt decline of the rhodopsin activity, the remaining activity then decaying with an exponential time course (Fig. 8 A).
Figure 8.
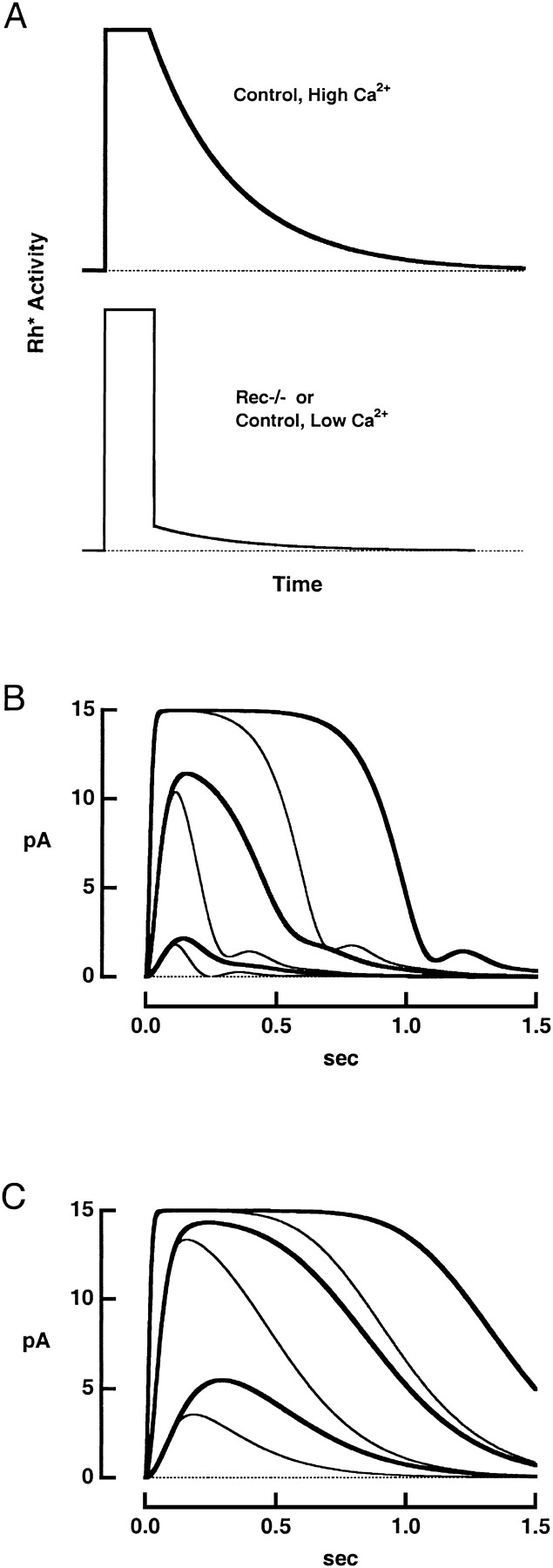
Calculated flash responses. (A) Assumed time courses of rhodopsin's catalytic activity. In the control rod (top), activity shut off exponentially after a delay of 100 ms (Chen et al., 1995b, 1999). The time constant of 205 ms was found from the mean slope of the relation between Tsat and the natural logarithm of the flash strength (compare Pepperberg et al., 1992). In the rec−/− rod (bottom), rhodopsin activity suddenly fell ninefold after 100 ms and then decayed exponentially with a time constant of 205 ms. (B) Photocurrents generated by the flash response model () for control (thick traces) and rec−/− (thin traces) rods. Rhodopsin's catalytic activity was increased ten-fold for successive pairs of responses. (C) Responses from the model in the absence of Ca2+ feedback onto guanylate cyclase for rods with (thick traces) and without (thin traces) recoverin. The catalytic activity levels of rhodopsin are the same as those in B.
The change in PDE activity induced by photoexcited rhodopsin is given by
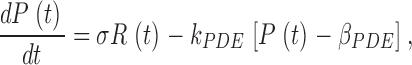 |
(A1) |
where R is rhodopsin activity, P is the total PDE activity, σ is the rate of PDE activation for unit R, βPDE is the basal PDE activity, and k PDE is the rate of decay of light-triggered PDE activity. The cGMP concentration, G(t), depends on its rate of synthesis, γ, and its rate of hydrolysis:
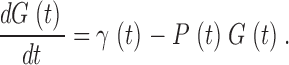 |
(A2) |
The synthesis of cGMP is Ca2+ dependent:
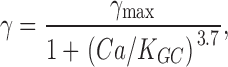 |
(A3) |
where Ca is the free [Ca2+]i and K GC is the Ca at which cGMP synthesis is half maximal. Changes in Ca follow
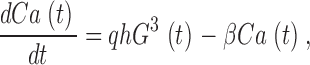 |
(A4) |
where q is a constant relating membrane current to changes in Ca, β is the rate constant for Ca2+ extrusion, and h (=0.551 pA μM−3) is a constant relating the membrane current to the cube of the cGMP concentration.
In our simulations (Fig. 8 B), the maximal light-suppressible current was 15 pA, and Ca was set to 250 nM in darkness and assumed to fall to 20 nM in saturating light (Woodruff et al., 2002) along a single exponential time course. For control responses, the time constant β was set to 113 ms, while for rec−/−, it was set to 86 ms. PDE decay was 7 s−1, basal PDE activity set to 10 s−1, and K GC was 0.25 μM with a Hill coefficient of 3.7 (Burns et al., 2002). Feedback onto guanylate cyclase was removed in order to simulate the behavior of the rod after knockout of both recoverin and GCAPs by setting K GC to 10,000 (Fig. 8 C).
References
- Amatruda, T.T., III, N. Gautam, H.K.W. Fong, J.K. Northup, and M.I. Simon. 1988. The 35- and 36-kDa β subunits of GTP-binding regulatory proteins are products of separate genes. J. Biol. Chem. 263:5008–5011. [PubMed] [Google Scholar]
- Ames, J.B., T. Porumb, T. Tanaka, M. Ikura, and L. Stryer. 1995. Amino-terminal myristoylation induces cooperative calcium binding to recoverin. J. Biol. Chem. 270:4526–4533. [DOI] [PubMed] [Google Scholar]
- Arshavsky, V.Y., C.L. Dumke, and M.D. Bownds. 1992. Noncatalytic cGMP-binding sites of amphibian rod cGMP phosphodiesterase control interaction with its inhibitory γ-subunits. A putative regulatory mechanism of the rod photoresponse. J. Biol. Chem. 267:24501–24507. [PubMed] [Google Scholar]
- Baldwin, A.N., and J.B. Ames. 1998. Core mutations that promote the calcium-induced allosteric transition of bovine recoverin. Biochemistry. 37:17408–17419. [DOI] [PubMed] [Google Scholar]
- Bastianelli, E., A.S. Polans, H. Hidaka, and R. Pochet. 1995. Differential distribution of six calcium-binding proteins in the rat olfactory epithelium during postnatal development and adulthood. J. Comp. Neurol. 354:395–409. [DOI] [PubMed] [Google Scholar]
- Bertazolli-Filho, R., S. Ghosh, W. Huang, G. Wollmann, and M. Coca-Prados. 2001. Molecular evidence that human ocular ciliary epithelium expresses components involved in phototransduction. Biochem. Biophys. Res. Commun. 284:317–325. [DOI] [PubMed] [Google Scholar]
- Burns, M.E., A. Mendez, J. Chen, and D.A. Baylor. 2002. Dynamics of cyclic GMP synthesis in retinal rods. Neuron. 36:81–91. [DOI] [PubMed] [Google Scholar]
- Calvert, P.D., N.V. Krasnoperova, A.L. Lyubarsky, T. Isayama, M. Nicolo, B. Kosaras, G. Wong, K.S. Gannon, R.F. Margolskee, R.L. Sidman, et al. 2000. Phototransduction in transgenic mice after targeted deletion of the rod transducin α-subunit. Proc. Natl. Acad. Sci. USA. 97:13913–13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Dawson, L.D., and M.M. LaVail. 1979. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J. Comp. Neurol. 188:245–262. [DOI] [PubMed] [Google Scholar]
- Cervetto, L., L. Lagnado, R.J. Perry, D.W. Robinson, and P.A. McNaughton. 1989. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature. 337:740–743. [DOI] [PubMed] [Google Scholar]
- Chen, C.-K., J. Inglese, R.J. Lefkowitz, and J.B. Hurley. 1995. a. Ca2+-dependent interaction of recoverin with rhodopsin kinase. J. Biol. Chem. 270:18060–18066. [DOI] [PubMed] [Google Scholar]
- Chen, J., C.L. Makino, N.S. Peachey, D.A. Baylor, and M.I. Simon. 1995. b. Mechanisms of rhodopsin inactivation in vivo as revealed by a COOH-terminal truncation mutant. Science. 267:374–377. [DOI] [PubMed] [Google Scholar]
- Chen, C.-K., M.E. Burns, M. Spencer, G.A. Niemi, J. Chen, J.B. Hurley, D.A. Baylor, and M.I. Simon. 1999. Abnormal photoresponses and light-induced apoptosis in rods lacking rhodopsin kinase. Proc. Natl. Acad. Sci. USA. 96:3718–3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C.-K., M.E. Burns, W. He, T.G. Wensel, D.A. Baylor, and M.I. Simon. 2000. Slowed recovery of rod photoresponse in mice lacking the GTPase accelerating protein RGS9-1. Nature. 403:557–560. [DOI] [PubMed] [Google Scholar]
- Dizhoor, A.M., S. Ray, S. Kumar, G. Niemi, M. Spencer, D. Brolley, K.A. Walsh, P.P. Philipov, J.B. Hurley, and L. Stryer. 1991. Recoverin: a calcium sensitive activator of retinal rod guanylate cyclase. Science. 251:915–918. [DOI] [PubMed] [Google Scholar]
- Erickson, M.A., L. Lagnado, S. Zozulya, T.A. Neubert, L. Stryer, and D.A. Baylor. 1998. The effect of recombinant recoverin on the photoresponse of truncated rod photoreceptors. Proc. Natl. Acad. Sci. USA. 95:6474–6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain, G.L., T.D. Lamb, H.R. Matthews, and R.L.W. Murphy. 1989. Cytoplasmic calcium as the messenger for light adaptation in salamander rods. J. Physiol. 416:215–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain, G.L., H.R. Matthews, M.C. Cornwall, and Y. Koutalos. 2001. Adaptation in vertebrate photoreceptors. Physiol. Rev. 81:117–151. [DOI] [PubMed] [Google Scholar]
- Gorodovikova, E.N., and P.P. Philippov. 1993. The presence of a calcium-sensitive P26-containing complex in bovine retina rod cells. FEBS Lett. 335:277–279. [DOI] [PubMed] [Google Scholar]
- Gray-Keller, M.P., A.S. Polans, K. Palczewski, and P.B. Detwiler. 1993. The effect of recoverin-like calcium-binding proteins on the photoresponse of retinal rods. Neuron. 10:523–531. [DOI] [PubMed] [Google Scholar]
- Hamer, R.D. 2000. Analysis of Ca++-dependent gain changes in PDE activation in vertebrate rod phototransduction. Mol. Vis. 6:265–286. [PMC free article] [PubMed] [Google Scholar]
- Hargrave, P.A., G. Adamus, A. Arendt, J.H. McDowell, J. Wang, A. Szary, D. Curtis, and R.W. Jackson. 1986. Rhodopsin's amino terminus is a principal antigenic site. Exp. Eye Res. 42:363–373. [DOI] [PubMed] [Google Scholar]
- Hurley, J.B., and J. Chen. 2001. Evaluation of the contributions of recoverin and GCAPs to rod photoreceptor light adaptation and recovery to the dark state. Prog. Brain Res. 131:395–405. [DOI] [PubMed] [Google Scholar]
- Inglese, J., J.F. Glickman, W. Lorenz, M.G. Caron, and R.J. Lefkowitz. 1992. Isoprenylation of a protein kinase. Requirement of farnesylation/α-carboxyl methylation for full enzymatic activity of rhodopsin kinase. J. Biol. Chem. 267:1422–1425. [PubMed] [Google Scholar]
- Johnson, L.V., and J.C. Blanks. 1984. Application of acrylamide as an embedding medium in studies of lectin and antibody binding in the vertebrate retina. Curr. Eye Res. 3:969–974. [DOI] [PubMed] [Google Scholar]
- Kawamura, S. 1993. Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature. 362:855–857. [DOI] [PubMed] [Google Scholar]
- Kawamura, S., and M. Murakami. 1991. Calcium-dependent regulation of cyclic GMP phosphodiesterase by a protein from frog retinal rods. Nature. 349:420–423. [DOI] [PubMed] [Google Scholar]
- Kawamura, S., O. Hisatomi, S. Kayada, F. Tokunaga, and C.-H. Kuo. 1993. Recoverin has S-modulin activity in frog rods. J. Biol. Chem. 268:14579–14582. [PubMed] [Google Scholar]
- Kawamura, S., O. Kuwata, M. Yamada, S. Matsuda, O. Hisatomi, and F. Tokunaga. 1996. Photoreceptor protein s26, a cone homologue of S-modulin in frog retina. J. Biol. Chem. 271:21359–21364. [DOI] [PubMed] [Google Scholar]
- Klenchin, V.A., P.D. Calvert, and M.D. Bownds. 1995. Inhibition of rhodopsin kinase by recoverin. Further evidence for a negative feedback system in phototransduction. J. Biol. Chem. 270:16147–16152. [DOI] [PubMed] [Google Scholar]
- Knospe, V., L.A. Donoso, J.P. Banga, S. Yue, E. Kasp, and D.S. Gregerson. 1988. Epitope mapping of bovine retinal S-antigen with monoclonal antibodies. Curr. Eye Res. 7:1137–1147. [DOI] [PubMed] [Google Scholar]
- Korf, H.-W., B.H. White, N.C. Schaad, and D.C. Klein. 1992. Recoverin in pineal organs and retinae of various vertebrate species including man. Brain Res. 595:57–66. [DOI] [PubMed] [Google Scholar]
- Koutalos, Y., K. Nakatani, and K.-W. Yau. 1995. The cGMP-phosphodiesterase and its contribution to sensitivity regulation in retinal rods. J. Gen. Physiol. 106:891–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado, L., L. Cervetto, and P.A. McNaughton. 1992. Calcium homeostasis in the outer segments of retinal rods from the tiger salamander. J. Physiol. 455:111–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb, T.D., P.A. McNaughton, and K.-Y. Yau. 1981. Spatial spread of activation and background desensitization in toad rod outer segments. J. Physiol. 319:463–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda, A., H. Ohguro, T. Maeda, I. Wada, N. Sato, Y. Kuroki, and T. Nakagawa. 2000. Aberrant expression of photoreceptor-specific calcium-binding protein (recoverin) in cancer cell lines. Cancer Res. 60:1914–1920. [PubMed] [Google Scholar]
- Matthews, H.R. 1996. Static and dynamic actions of cytoplasmic Ca2+ in the adaptation of responses to saturating flashes in salamander rods. J. Physiol. 490:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews, H.R. 1997. Actions of Ca2+ on an early stage in phototransduction revealed by the dynamic fall in Ca2+ concentration during the bright flash response. J. Gen. Physiol. 109:141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez, A., M.E. Burns, I. Sokal, A.M. Dizhoor, W. Baehr, K. Palczewski, D.A. Baylor, and J. Chen. 2001. Role of guanylate cyclase-activating proteins (GCAPs) in setting the flash sensitivity of rod photoreceptors. Proc. Natl. Acad. Sci. USA. 98:9948–9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milam, A.H., D.M. Dacey, and A.M. Dizhoor. 1993. Recoverin immunoreactivity in mammalian cone bipolar cells. Vis. Neurosci. 10:1–12. [DOI] [PubMed] [Google Scholar]
- Nakatani, K., T. Tamura, and K.-W. Yau. 1991. Light adaptation in retinal rods of the rabbit and two other nonprimate mammals. J. Gen. Physiol. 97:413–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov, S., T.D. Lamb, and E.N. Pugh, Jr. 2000. The role of steady phosphodiesterase activity in the kinetics and sensitivity of the light-adapted salamander rod photoresponse. J. Gen. Physiol. 116:795–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto-Bruc, A.E., R.N. Fariss, J.P. van Hooser, and K. Palczewski. 1998. Phosphorylation of photolyzed rhodopsin is calcium-insensitive in retina permeabilized by α-toxin. Proc. Natl. Acad. Sci. USA. 95:15014–15019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperberg, D.R., M.C. Cornwall, M. Kahlert, K.P. Hofmann, J. Jin, G.J. Jones, and H. Ripps. 1992. Light-dependent delay in the falling phase of the retinal rod photoresponse. Vis. Neurosci. 8:9–18. [DOI] [PubMed] [Google Scholar]
- Polans, A.S., D. Witkowska, T.L. Haley, D. Amundson, L. Baizer, and G. Adamus. 1995. Recoverin, a photoreceptor-specific calcium-binding protein, is expressed by the tumor of a patient with cancer-associated retinopathy. Proc. Natl. Acad. Sci. USA. 92:9176–9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh, E.N., Jr., and T.D. Lamb. 2000. Phototransduction in vertebrate rods and cones: molecular mechanisms of amplification, recovery and light adaptation. In Handbook of Biological Physics, Vol. 3, Molecular Mechanisms of Visual Transduction. D.G. Stavenga, W.J. de Grip, and E.N. Pugh, Jr., editors. Elsevier, Amsterdam. 183–255.
- Pugh, E.N., Jr., S. Nikonov, and T.D. Lamb. 1999. Molecular mechanisms of vertebrate photoreceptor light adaptation. Curr. Opin. Neurobiol. 9:410–418. [DOI] [PubMed] [Google Scholar]
- Rieke, F., and D.A. Baylor. 1998. Origin of reproducibility in the responses of retinal rods to single photons. Biophys. J. 75:1836–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichi, H., and R.L. Somers. 1978. Light-dependent phosphorylation of rhodopsin. Purification and properties of rhodopsin kinase. J. Biol. Chem. 253:7040–7046. [PubMed] [Google Scholar]
- Sung, C.-H., C. Makino, D. Baylor, and J. Nathans. 1994. A rhodopsin gene mutation responsible for autosomal dominant retinitis pigmentosa results in a protein that is defective in localization to the photoreceptor outer segment. J. Neurosci. 14:5818–5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, T., K. Nakatani, and K.-W. Yau. 1989. Light adaptation in cat retinal rods. Science. 245:755–758. [DOI] [PubMed] [Google Scholar]
- Tamura, T., K. Nakatani, and K.-W. Yau. 1991. Calcium feedback and sensitivity regulation in primate rods. J. Gen. Physiol. 98:95–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiechmann, A.F., and J.A. Hammarback. 1993. Expression of recoverin mRNA in the human retina: localization by in situ hybridization. Exp. Eye Res. 57:763–769. [DOI] [PubMed] [Google Scholar]
- Woodruff, M.L., A.P. Sampath, H.R. Matthews, N.V. Krasnoperova, J. Lem, and G.L. Fain. 2002. Measurement of cytoplasmic calcium concentration in the rods of wild-type and transducin knock-out mice. J. Physiol. 542:843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, R.-B., and D.L. Garbers. 1997. Two eye guanylyl cyclases are expressed in the same photoreceptor cells and form homomers in preference to heteromers. J. Biol. Chem. 272:13738–13742. [DOI] [PubMed] [Google Scholar]
- Yau, K.-W., and K. Nakatani. 1985. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 313:579–582. [DOI] [PubMed] [Google Scholar]
- Zhao, X., J. Huang, S.C. Khani, and K. Palczewski. 1998. Molecular forms of human rhodopsin kinase (GRK1). J. Biol. Chem. 273:5124–5131. [DOI] [PubMed] [Google Scholar]