Abstract
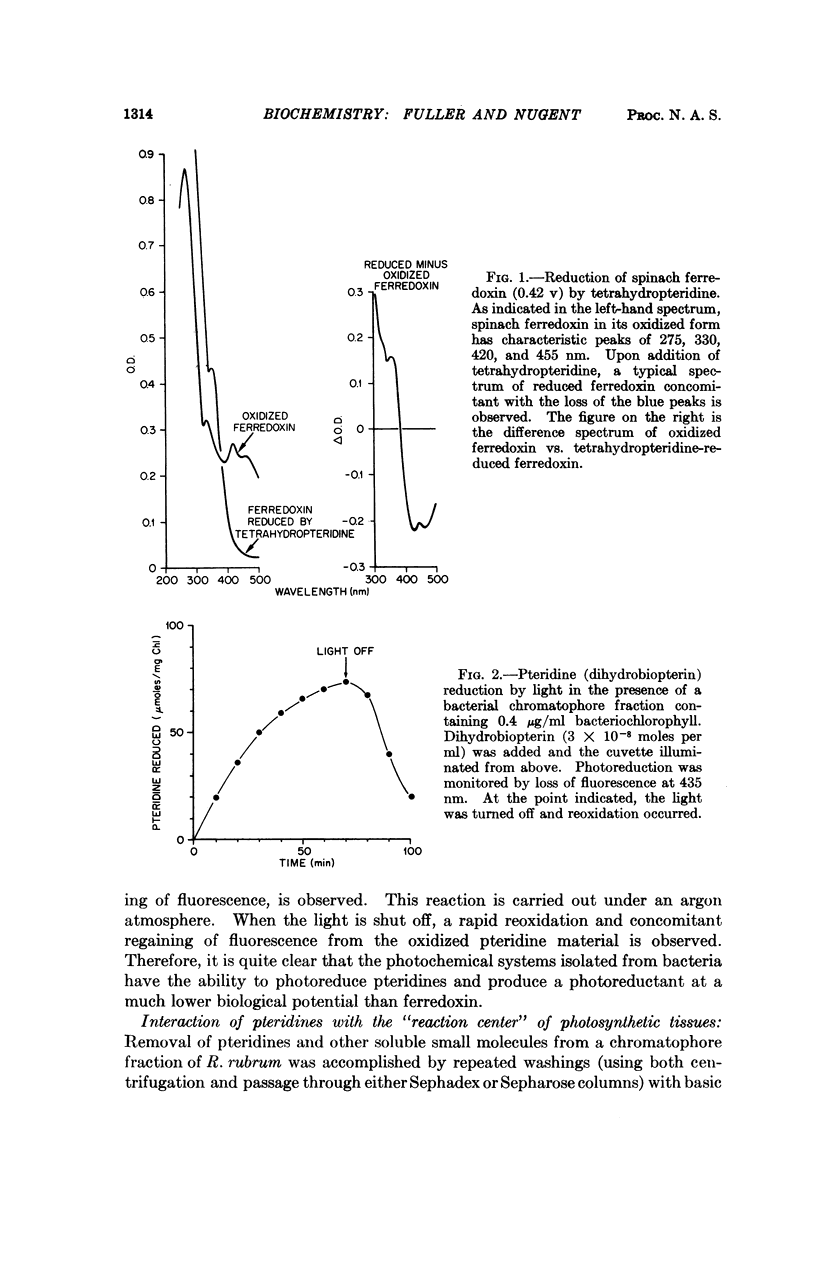
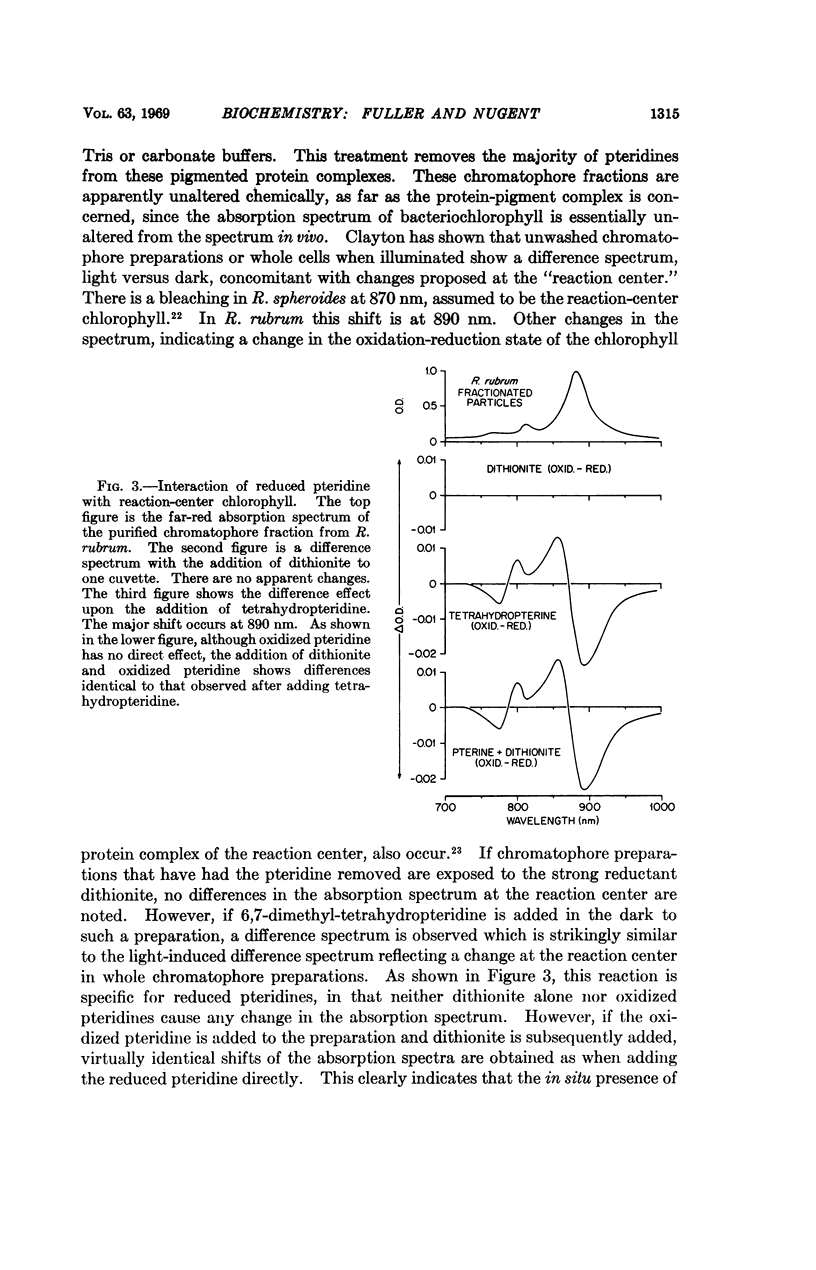
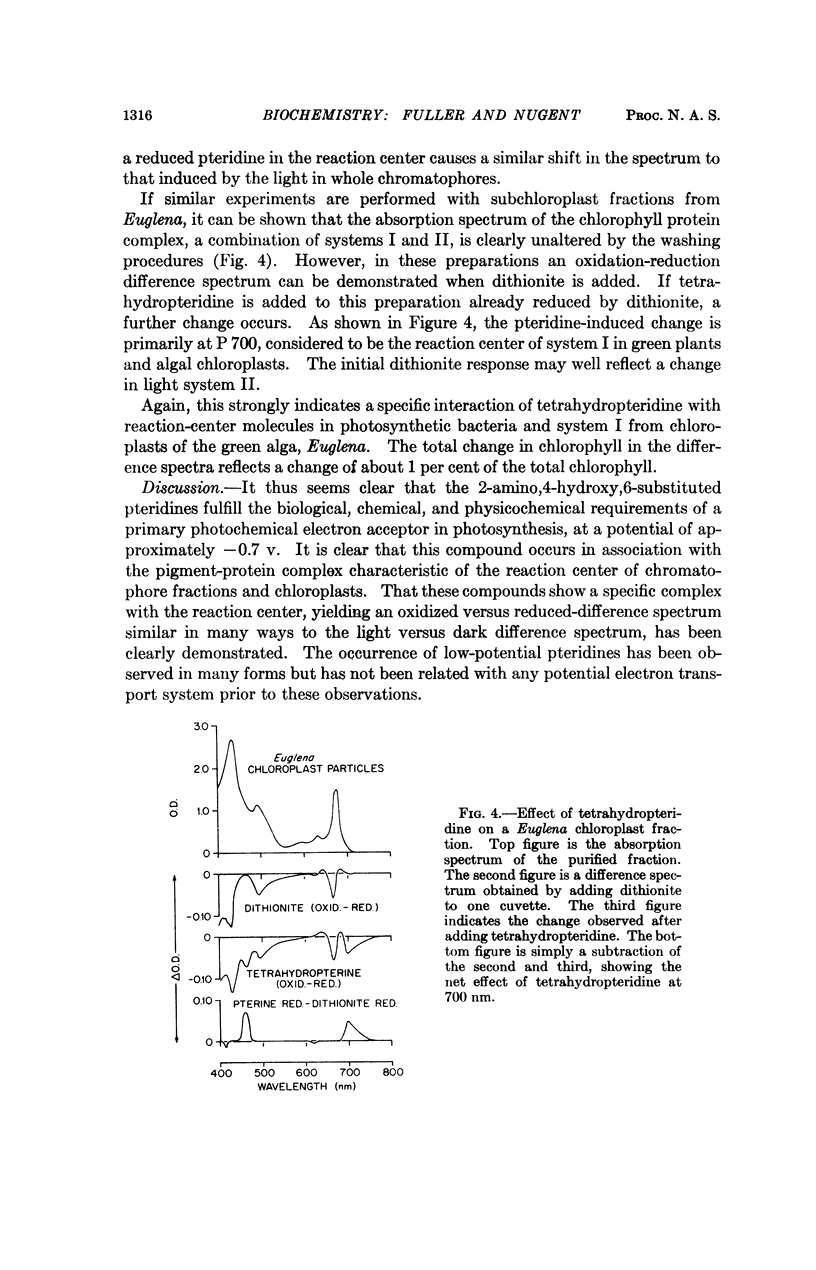
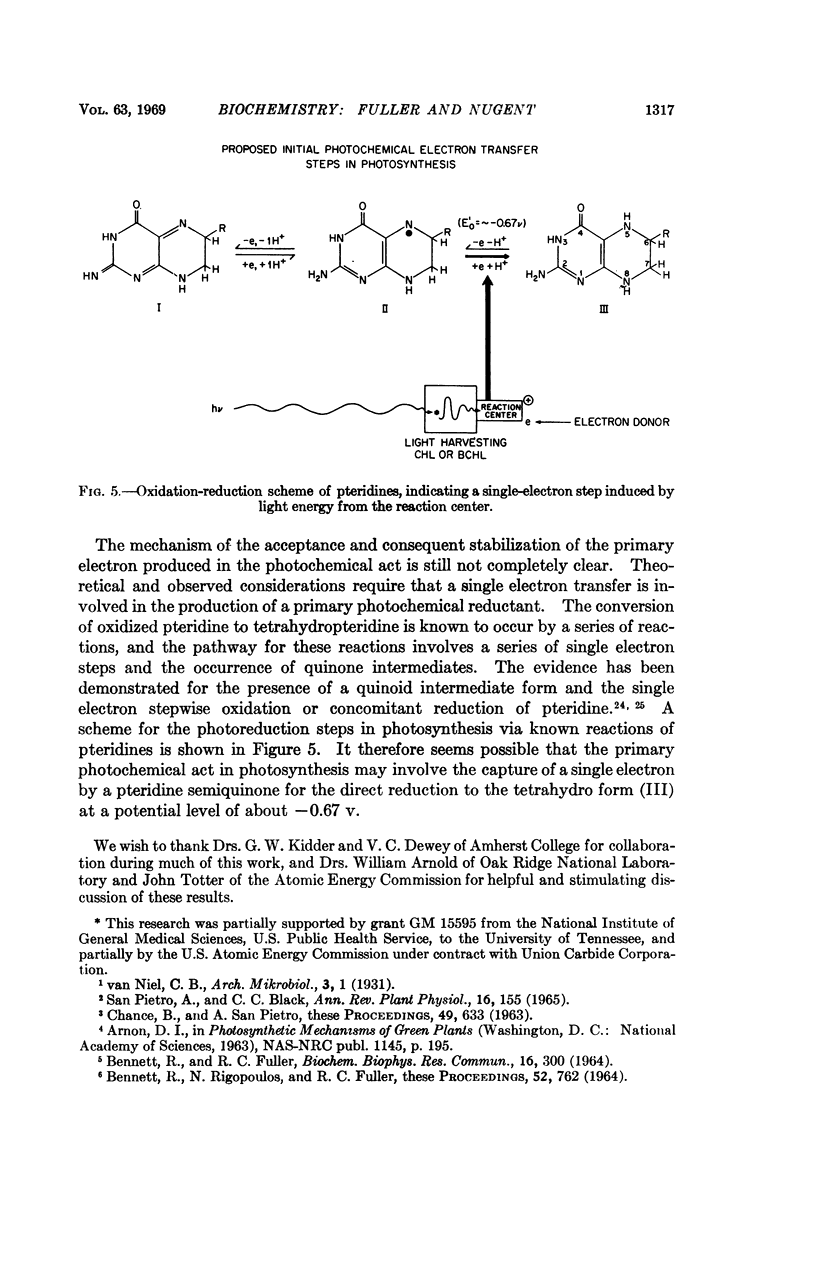
The photoreduction and interaction with the photosynthetic “reaction center” of 2-amino,4-hydroxy-6-substituted pteridine indicates that these low-potential (∼ -0.7 v), naturally occurring compounds play a primary role in photosynthetic electron transport. These unconjugated pteridines, which occur in association with the photosynthetic apparatus of green plants and photosynthetic bacteria, can be reduced by light in the presence of a bacterial chromatophore fraction from the dihydro form to the tetrahydro form. 6,7-Dimethyl-tetrahydropteridine readily reduces spinach ferredoxin. This compound also specifically interacts with reaction-center chlorophyll and bacteriochlorophyll and produces spectral shifts similar to those produced by light. It is proposed that the electron produced by excited-state chlorophyll is captured and separated by a pteridine at -0.67 v at the photosynthetic reaction center.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENNETT R., RIGOPOULOS N., FULLER R. C. THE PYRUVATE PHOSPHOROCLASTIC REACTION AND LIGHT-DEPENDENT NITROGEN FIXATION IN BACTERIAL PHOTOSYNTHESIS. Proc Natl Acad Sci U S A. 1964 Sep;52:762–768. doi: 10.1073/pnas.52.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R., Fuller R. C. The pyruvate phosphoroclastic reaction in Chromatium. A probable role for ferredoxin in a photosynthetic bacterium. Biochem Biophys Res Commun. 1964 Jul 1;16(4):300–307. doi: 10.1016/0006-291x(64)90030-0. [DOI] [PubMed] [Google Scholar]
- Black C. C., Jr Chloroplast reactions with dipyridyl salts. Biochim Biophys Acta. 1966 Jul 13;120(3):332–340. doi: 10.1016/0926-6585(66)90300-1. [DOI] [PubMed] [Google Scholar]
- Bobst A., Viscontini M. Hydroxylation non-enzymatique de la phénylalanine en tyrosine à l'aide de ptérines tétrahydrogénées. Helv Chim Acta. 1966 Mar 10;49(2):884–888. doi: 10.1002/hlca.19660490209. [DOI] [PubMed] [Google Scholar]
- Chance B., Pietro A. S. ON THE LIGHT-INDUCED BLEACHING OF PHOTOSYNTHETIC PYRIDINE NUCLEOTIDE REDUCTASE IN THE PRESENCE OF CHLOROPLASTS. Proc Natl Acad Sci U S A. 1963 May;49(5):633–638. doi: 10.1073/pnas.49.5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. C., Gibbs M. Intracellular and Phylogenetic Distribution of Ribulose 1,5-Diphosphate Carboxylase and D-Glyceraldehyde-3-Phosphate Dehydrogenases. Plant Physiol. 1959 May;34(3):324–329. doi: 10.1104/pp.34.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok B., Rurainski H. J., Owens O. V. The reducing power generated in photoact I of photosynthesis. Biochim Biophys Acta. 1965 Nov 29;109(2):347–356. doi: 10.1016/0926-6585(65)90162-7. [DOI] [PubMed] [Google Scholar]
- MACLEAN F. I., FUJITA Y., FORREST H. S., MYERS J. PHOTOSYNTHETIC PHOSPHORYLATION: STIMULATION BY PTERIDINES AND A COMPARISON WITH PHOSPHODOXIN. Science. 1965 Aug 6;149(3684):636–638. doi: 10.1126/science.149.3684.636. [DOI] [PubMed] [Google Scholar]
- Sybesma C. Light-induced reactions of P890 and P800 in the purple photosynthetic bacterium Rhodospirillum rubrum. Biochim Biophys Acta. 1969 Jan 14;172(1):177–179. doi: 10.1016/0005-2728(69)90104-2. [DOI] [PubMed] [Google Scholar]
- TAGAWA K., ARNON D. I. Ferredoxins as electron carriers in photosynthesis and in the biological production and consumption of hydrogen gas. Nature. 1962 Aug 11;195:537–543. doi: 10.1038/195537a0. [DOI] [PubMed] [Google Scholar]
- ZWEIG G., AVRON M. ON THE OXIDATION-REDUCTION POTENTIAL OF THE PHOTOPRODUCED REDUCTANT OF ISOLATED CHLOROPLASTS. Biochem Biophys Res Commun. 1965 May 3;19:397–400. doi: 10.1016/0006-291x(65)90135-x. [DOI] [PubMed] [Google Scholar]


