Abstract
Waldenstrom macroglobulinemia (WM) is an incurable low-grade lymphoplasmacytic lymphoma. We demonstrate up-regulated Akt activity in WM, and that Akt down-regulation by Akt knockdown and the inhibitor perifosine leads to significant inhibition of proliferation and induction of apoptosis in WM cells in vitro, but not in normal donor peripheral blood and hematopoietic progenitors. Importantly, down-regulation of Akt induced cytotoxicity of WM cells in the bone marrow microenvironment (BMM) context. Perifosine induced significant reduction in WM tumor growth in vivo in a subcutaneous xenograft model through inhibition of Akt phosphorylation and downstream targets. We also demonstrated that Akt pathway down-regulation inhibited migration and adhesion in vitro and homing of WM tumor cells to the BMM in vivo. Proteomic analysis identified other signaling pathways modulated by perifosine, such as activation of ERK MAPK pathway, which induces survival of tumor cells. Interestingly, MEK inhibitor significantly enhanced perifosine-induced cytotoxicity in WM cells. Using Akt knockdown experiments and specific Akt and PI3K inhibitors, we demonstrated that ERK activation is through a direct effect, rather than feedback activation, of perifosine upstream ERK pathway. These results provide understanding of biological effects of Akt pathway in WM and provide the framework for clinical evaluation of perifosine in WM patients.
Introduction
Waldenstrom macroglobulinemia (WM) is a low-grade lymphoplasmacytic lymphoma characterized by serum monoclonal immunoglobulin M.1–3 Although indolent, WM disease remains incurable, with a median overall survival of 5 to 6 years, and most patients succumb to disease progression. Current therapies give overall response rates (ORR) of 30% to 70% in upfront or relapsed settings. However, complete response rates are less than 10% and median duration of response averages 2 to 3 years.4,5 Novel therapeutic agents with demonstrated efficacy in multiple myeloma (MM) and chronic lymphocytic leukemia, such as thalidomide, bortezomib, and alemtuzumab, improve the ORR only slightly.6,7 Therefore, there is a need for the rational development of novel agents that target aberrant molecular pathways in WM, as well as to understand the molecular mechanisms of sensitivity/resistance to these agents
WM is characterized by widespread involvement of the bone marrow (BM) and lymphadenopathy in 20% of the patients, implying continuous trafficking of WM cells into and out the BM and lymph nodes.8 Migration of B cell through the blood to the BM niches requires active navigation, a process termed homing.9 Homing is a coordinated, multistep process, which is regulated by several cytokines and chemokines.10,11 It also implies that ultimately tumor cells adhere to their microenvironment.12 This process is not well understood in WM.
The PI3K/Akt pathway acts as a critical regulator of cell survival by stimulating cell proliferation and inhibiting apoptosis.13–15 In addition, the PI3K/Akt pathway regulates migration and trafficking in lymphocytes, indicating that it may regulate homing in WM.16–18 PI3K catalyzes the synthesis of a second messenger, thereby activating phosphatidylinositol-dependent kinase 1 (PDK1), which in turn activates the serine-threonine kinase Akt.19 Akt regulates the activity of multiple proteins involved in proliferation, mitosis, and survival, as well as in apoptosis.19 The PI3K/Akt pathway has been implicated in pathogenesis of various cancers, including lymphoproliferative disorders.20,21
In this study, we demonstrate that down-regulation of Akt leads to significant inhibition of proliferation and induction of apoptosis of WM cells in vitro and in vivo and inhibition of migration and adhesion in vitro and homing of WM tumor cells to the BM microenvironment in vivo.
Materials and methods
Cells
The WM cell lines (BCWM.1 and WSU-WM) and IgM secreting low-grade lymphoma cell lines, MEC1 (DMSZ, Braunschweig, Germany) and RL (American Type Culture Collection [ATCC], Manassas, VA) were used in this study. The BCWM.1 is a recently developed WM cell line at Dana- Farber Cancer Institute. WM-WSU was obtained from Dr Al Khatib (Wayne State University, Detroit, MI) and MEC1 from Dr Kay (Mayo Clinic, Rochester, MN). RL was purchased from the ATCC (Manassas, VA). Multiple myeloma cell line, MM.1S was a kind gift from Dr Steven Rosen (Northwestern University, Chicago, IL). All cell lines were cultured in RPMI-1640 containing 10% fetal bovine serum (FBS; Sigma Chemical, St Louis, MO), 2 μM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (GIBCO, Grand Island, NY).
Patient samples were obtained after approval from the Dana Farber Cancer Institute Institutional Review Board. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki. Primary WM cells were obtained from BM samples using CD19 + microbead selection (Milteny Biotec, Auburn, CA) with more than 90% purity, as confirmed by flow cytometric analysis with monoclonal antibody reactive to human CD20-PE (BD Biosciences, San Jose, CA). Peripheral blood mononuclear cells (PBMCs) and bone marrow CD19 + selected B cells were obtained from healthy volunteers after Ficoll-Hipaque density sedimentation.
Lentivirus shRNA vector construction and Akt gene transduction
To further determine the role of AKT in the regulation of ERK/MEK, we established an Akt knockout BCWM.1 cell line using a lentivirus transfection system.22–24 The sense and antisense oligonucleotide sequence for construction of Akt shRNA were as follows: clone no. 10162: target sequence GGACAAGGACGGGCACATTAA; no. 10163: target sequence CGAGTTTGAGTACCTGAAGCT.
Reagents
Perifosine (Keryx Biopharmaceuticals, New York, NY) is a novel Akt inhibitor that belongs to a class of lipid-related compounds called alkylphospholipids. Rituximab was provided by Genentech (San Francisco, CA). Triciribine, a specific Akt inhibitor, was purchased from Biomol (Plymouth, PA). JNK inhibitor SP600215, MEK1/2 inhibitor U0126, and PI3k inhibitor LY-294002 were purchased from Calbiochem (San Diego, CA). IL-6 and IGF-1 were purchased from R&D Systems (Minneapolis, MN).
Growth inhibition assay
The inhibitory effect of perifosine on WM cell growth, alone or in combination with other agents, was assessed by measuring 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Chemicon International, Temecula, CA) dye absorbance, as previously described.24
DNA synthesis
Proliferation was measured by DNA synthesis. WM cells were incubated in the presence of perifosine alone or in combination with other agents, or recombinant cytokines. DNA synthesis was measured by the [3H]-thymidine uptake (Perkin Elmer, Boston, MA) as previously published.24
Flow cytometric analysis
Cell-cycle analysis was profiled by flow cytometry using propidium iodide staining, as described.24 Apoptosis was quantitated using Apo2.7 flow cytometric analysis (Beckman Coulter, Fullerton, CA), as described.24
Colony-forming cell assay
Following informed consent, bone marrow was obtained from healthy volunteers and processed to obtain a nonadherent mononuclear fraction, as previously described.24,25 Colony-forming cell (CFC) assays were performed in the presence or absence of perifosine added to the cultures at plating. Colonies were counted at days 14 to 16.
Effect of perifosine on paracrine WM cell growth in the BM
Growth stimulation and signaling in WM cells adherent to bone marrow stromal cells (BMSCs) was measured in the presence or absence of perifosine, as described.24
Immunoblotting
Whole-cell lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride (PVDF) membrane (Bio-Rad Laboratories, Hercules, CA). The antibodies used for immunoblotting included: anti-phospho (p)-Akt (Ser473), p-Akt (Thr308), -Akt, –p-ERK (Thr202/Tyr204), -ERK1/2, –p-PDK1 (Ser241), –p-GSK3α/b (Ser21/9), –p-S6 Ribosomal (Ser240/244), –p-P38 (Thr180/Tyr182), –p-SAPK/JNK (Thr183/Tyr185), –p-c-Raf (Ser259), –pan-p-PKC, –caspase-8, –caspase-9, –caspase3, and -PARP (Cell Signaling Technology, Beverly, MA); as well as –b-actin and –α-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA).
In vitro Akt kinase assay
In vitro Akt kinase assay was performed following the manufacturer recommendations (Cell Signaling Technology). Following Akt immunoprecipitation, the cell lysis was then suspended with ATP and GSK-3 fusion protein. Kinase activity was detected by immunoblotting with anti–phospho-GSK-3α/β (Ser21/9) antibody.
Protein microarray procedure
Protein array was used to investigate expression of members of the PI3k/Akt pathway and to study perifosine-induced deregulation of proteins involved in a broad range of biological functions, such as adhesion and migration, signal transduction, cell-cycle regulation, gene transcription, and survival-apoptosis. The complete list of the 512 highly specific and sensitive monoclonal antibodies of the nanoscale Antibody Microarray (Clontech, Mountain View, CA) is available at www.bdbiosciences.com. The technique was performed as previously described.24,26 Labeling the proteins from both normal and malignant B cells with Cy3 and Cy5 allowed for microarray detection of differences in specific protein abundance between the WM and control samples. The slides were scanned using Axon GenePix 4000B scanner, as previously described. The mean of the ratios of Cy5/Cy3 of both slides were analyzed using Clontech Excel software. The Genespring software (Silicon Genetics, Redwood City, CA) was used for analysis of all 2 experiments and normalized to controls. An unsupervised clustering analysis was performed to identify changes that were 2-fold or 1.3-fold in the perifosine-treated group compared with the control group.
Transwell migration assay
Migration was determined using the transwell migration assay (Costar, Corning, NY), as previously described.27 BCWM.1 cells were treated with perifosine for 90 minutes and then placed in the migration chambers in the presence of SDF-1 (R&D). After 4 hours of incubation, cells that migrated to the lower chambers were counted using a Beckman Coulter cell counter (Beckman Coulter).
Adhesion assay
We used an in vitro adhesion assay coated with fibronectin, a ligand of VLA-4, following the manufacturer recommendations (EMD Biosciences, San Diego, CA). BCWM.1 cells were treated with perifosine for 1 hour. Calcein AM was used to measure adherent cells, and the degree of fluorescence was measured using a spectrophotometer 485-520. A bovine serum albumin (BSA)–coated well served as negative control.
In vivo flow cytometry
The effects of perifosine on homing in vivo were tested using Balb/c mice with in vivo flow cytometry as previously described.11,28 In brief, BCWM.1 cells were incubated with perifosine for 2 hours (or control phosphate- buffered saline) then injected into the mice. The cells were fluorescently labeled by incubation with the dialkylcarbocyanine membrane dye “DID” (Molecular Probes, Carlsbad, CA) 0.5 μM for 30 minutes. Fluorescence signal was detected on an appropriate artery in the ear and digitized for analysis with Matlab software developed in house.
In vivo rate confocal microscopy and 2-photon microscopy
Cell homing to BM vasculature of the skull was then analyzed using fluorescence confocal microscopy, as described.11,28 High-resolution images of excited DID cells were obtained through the intact mouse skull at depths of up to 250 μm from the surface of the skull using a 30×/0.9 NA water immersion objective lens. Images were captured after injection of the cells for 30 minutes to 1 hour per session. Quantitative evaluation is made by dividing the BM into predetermined quadrants (areas 1 to 4) and counting numbers of fluorescent cells per field.
Xenograft murine model
SCID homozygous female mice (5 weeks old) were obtained from Charles River Laboratories (Wilmington, MA) and irradiated upon arrival. All animal studies were conducted according to protocols approved by the Animal Ethics Committee of the Dana Farber Cancer Institute. The mice were inoculated subcutaneously with 2.5 × 106 BCWM.1 cells. Once tumors were measurable, mice were assigned to receive oral perifosine daily (35 mg/kg; N = 11) or oral vehicle (water; N = 8). Caliper measurements of the tumor volume were performed every alternate day using the formula: 0.52 × (width/2)2 × (length/2).29 Tumor growth was evaluated from the first day of treatment until a significant difference in mean tumors' volume was observed between the 2 groups. Mice were killed when tumors reached 2 cm or if the mice appeared moribund, in order to prevent unnecessary morbidity. Tumor tissues from mice treated with control vehicle or daily perifosine were harvested, processed, and subjected to Western blotting using anti–p-Akt, -Akt, -GSK3α/β and -α-tubulin antibodies.
Statistical analysis
Statistical significance of differences observed in drug-treated versus control cultures was determined using the Wilcoxon signed rank test. The minimal level of significance was a P value less than .05. The interaction between perifosine and other inhibitors was analyzed by isobologram analysis using the CalcuSyn software program (Biosoft, Ferguson, MO), as described.24 The Chou-Talalay method calculates a combination index (CI) to indicate synergistic effect (CI < 0.8).
Results
Baseline expression of phospho-Akt is elevated in patients with WM
We have previously demonstrated that the level of expression of members of the PI3K pathway proteins is elevated in samples of patients with WM compared with those of healthy controls.30 We therefore sought to confirm the expression level of phophorylated Akt (phospho-Akt) in WM. As shown in Figure 1A, expression of phospho-Akt was higher in CD19 + WM cells from the BM of 4 patients with WM compared with BM CD19 + cells from 2 healthy donors. In addition, we examined the baseline phosphorylation of Akt (at serine 473 and at threonine 308 sites) in WM cell lines (BCWM.1 and WSU-WM), IgM secreting low-grade lymphoma cell lines (MEC1, RL), as well as one MM cell line (MM.1S). As shown in Figure 1B, all cell lines demonstrated constitutive activation of Akt at baseline.
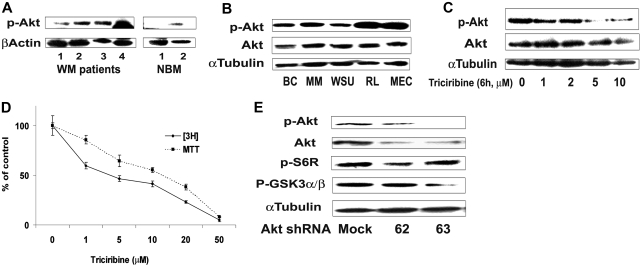
Figure 1.
Akt expression in WM (Waldenstrom macroglobulinemia) cells. (A) Baseline phosphorylation expression of Akt serine 473 in freshly selected CD19 + bone marrow cells from patients with WM disease (N = 4) compared with bone marrow CD19 + cells from healthy donors (NBM) (N = 2). β-actin was used as a control. (B) Baseline phosphorylation expression of Akt serine 473 was assessed in BCWM.1 cell line (BC), MM.1S cell line (MM), and several IgM-secreting cell lines: WM-WSU (WSU), MEC-1 (MEC), RL, by Western blotting. α-tubulin antibody is always used as a control. Specific inhibition of Akt pathway affects survival of WM cells. (C) BCWM.1 cells were cultured with triciribine for 6 hours with doses that range from 1 to 10 μM. Whole cell lysates were subjecting to Western blotting using anti–p-Akt ser473, -Akt, –p-ERK, and α-tubulin antibodies. (D) Triciribine (1 to 50 μM) induces growth inhibition and cytotoxicity in BCWM.1 cells at 48 hours by the [3H]-thymidine uptake assay and the MTT assay. (E) BCWM.1 cells were transduced with 2 Akt shRNA for 48 hours. Mock: control plasmid; 62 and 63 represent shRNA that target 2 different sequences of Akt gene. Whole cell lysates were subjected to Western blotting using anti–p-Akt, -Akt, -p-S6R, –p-GSK3α/β, and α-tubulin antibodies.
Inhibition of Akt pathway induces cytotoxicity in WM cells
We then examined whether inhibition of the Akt pathway induces cytotoxicity of WM cells. We first examine the effect of triciribine, a specific Akt inhibitor, on phosphorylation of Akt and on survival and proliferation of the WM cell line. Triciribine inhibited phosphorylation of Akt at 6 hours (Figure 1C), followed by significant growth inhibition in BCWM.1 cells using the thymidine uptake assay at 48 hours (Figure 1D). We then confirmed those results using Akt knockdown BCWM.1 cell line using lentivirus infection. Total Akt expression was significantly inhibited in BCWM.1 cells using both clones 62 and 63 of Akt shRNA (Figure 1E). Akt knockdown inhibited survival of BCWM.1 by 40% compared with mock-infected control and induced apoptosis up to 30% of control at 48 hours, indicating that Akt is an important survival pathway in WM (data not shown).
Perifosine blocks proliferation and induces cytotoxicity in WM cells
WM cell lines, IgM-secreting cell lines, and MM.1S cells were cultured for 24 hours and 48 hours in the presence of perifosine (2-50 μM). Perifosine inhibited proliferation at 48 hours, as measured by the thymidine uptake assay in BCWM.1 and MM.1S cells, with an IC50 of 5 μM (Figure 2A). We next examined the cytotoxic effect of perifosine in cell lines using the MTT assay. As shown in Figure 2B, perifosine significantly inhibited growth of these cell lines in a dose-dependent fashion at 24 and 48 hours, with IC50 of 7 to 20 μM at 48 hours. Interestingly, the effect of perifosine was most significant on the transformed WM cell line WM-WSU, with IC50 of 10 μM at 48 hours (Figure 2C). The cytotoxic effect of perifosine was confirmed in tumor cells from patients with WM (N = 3; Figure 2D). In contrast, perifosine did not trigger cytotoxicity in PBMCs from 3 healthy volunteers (Figure 2E), as well as in hematopoietic progenitor cells (Figure 2F). These results demonstrate that perifosine triggers significant cytotoxicity in WM, MM, and IgM-secreting cell lines, as well as patient WM cells, without toxicity in normal PBMCs or progenitor cells.
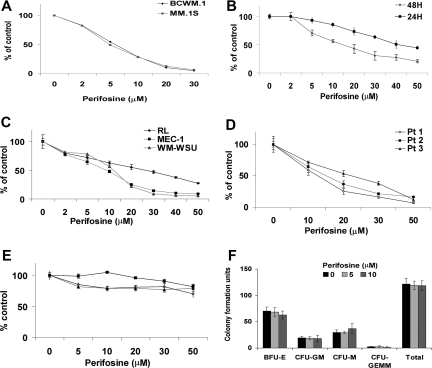
Figure 2.
Perifosine induces decrease in proliferation and cytotoxicity. (A) Thymidine uptake assay. MM.1S and BCWM.1 cells were cultured with perifosine (2 to 30 μM) for 48 hours. BCWM.1 (■), MM.1S (♦). (B) BCWM.1 cells were cultured with perifosine (2 to 50 μM) for 24 hours (♦) and 48 hours (■). (C) Several IgM-secreting cell lines, RL (♦), MEC-1 (■), WM-WSU (Δ) were cultured with perifosine (2 to 50 μM). (D,E) Freshly isolated BM CD19 + tumor cells from 3 patients with WM (D) and PBMCs from 3 healthy donors (E) were cultured with perifosine (5 to 50 μM). Cytotoxicity was assessed by the MTT assay (C-E). All results represent mean (± SD) of triplicate experiments. (F) Colony forming-cell assay. Nonadherent mononuclear cells were cultured using methylcellulose semisolid technique in absence and presence of perifosine (5 μM and 10 μM) for 14 days. Burst forming units-erythroid (BFU-E), colony-forming units-granulocyte/macrophage (CFU-GM), colony-forming units-macrophage (CFU-M), and colony-forming units-granulocyte/erythroid/macrophage/megakaryocyte (CFU-GEMM).
Perifosine-induced cytotoxicity signals through inhibition of Akt
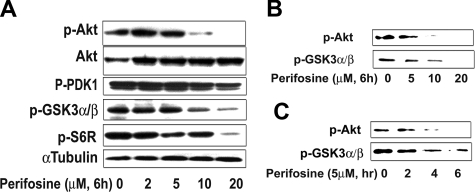
We next determined whether perifosine inhibited Akt activity in WM cells. We demonstrated using proteomic technique that perifosine inhibited Akt phosphorylation and downstream targets (Table 1). First we found down-regulated expression for several proteins downstream of the Akt pathway such as ribosomal protein S6 kinase alpha-1 and inhibitor of nuclear factor kappa-B kinase alpha subunit. We then confirmed those results by immunobloting in both a dose- (Figure 3A) and time-dependent fashion (data not shown), while total Akt expression was not modified. Perifosine (10 μM) significantly inhibited phosphorylation of Akt at 6 hours. In addition, perifosine inhibited phospho-S6 ribosomal protein, a downstream target protein of Akt. In contrast, perifosine (10 μM and higher doses) only slightly inhibited phosphorylation of PDK1, a molecule upstream of Akt (Figure 3A). We then confirmed the effect of perifosine on Akt activity using an in vitro Akt kinase assay in a dose- and time-dependent fashion (Figure 3B,C). Perifosine inhibited Akt kinase activity at early time points, with 5 μM inhibiting Akt function at 4 hours, as shown in Figure 3C. These results demonstrate that perifosine directly inhibited baseline phosphorylation of Akt and its downstream target proteins.
Table 1.
Proteins dysregulated by perifosine (10 μM for 12 hours) using nanoscale antibody microarray
| Classes, up-regulated proteins | No.* | Down-regulated proteins | No. |
|---|---|---|---|
| Cell-signaling pathways/kinases | |||
| Mitogen-activated protein kinase kinase 4 (JNK-activating kinase 1) | P45985 | Ribosomal protein S6 kinase alpha-1 | Q15418 |
| Mitogen-activated protein kinase 1/ERK2 | P28482 | Inhibitor of nuclear factor kappa-B kinase alpha subunit | O15111 |
| Protein phosphatase 1, regulatory (inhibitor) subunit 2 | P41236 | — | — |
| Phospholipase C, gamma 1 (formerly subtype 148) | P19174 | — | — |
| Protein kinase, cAMP-dependent, regulatory, type I alpha/PRKAR1A | P10644 | — | — |
| Ras-GTPase-activating protein SH3-domain-binding protein | Q13283 | — | — |
| Rho GDP dissociation inhibitor (GDI) beta | P52566 | — | — |
| RAB24, member RAS oncogene family | Q969Q5 | — | — |
| Polo-like kinase (Drosophila) | P53350 | — | — |
| Insulin receptor substrate 1 | P35568 | — | — |
| Active BCR-related gene/ABR | Q12979 | — | — |
| Apoptosis | |||
| Caspase 7, apoptosis-related cysteine protease | P55210 | — | — |
| BCL2 | P10415 | — | — |
| Cell-cycle regulators and inhibition of proliferation | |||
| Transforming growth factor beta 1 induced transcript 1 | O43294 | — | — |
| Cyclin-dependent kinase 7 | P50613 | — | — |
| Leucine zipper putative tumor suppressor 1 | Q9Y250 | — | — |
| CDC37 cell division cycle 37 homolog (S cerevisiae) | Q16543 | — | — |
| Serpin B5 | P36952 | — | — |
| Chromosome/cell division | |||
| Pericentrin 2 (kendrin) | P48725 | MRE11 meiotic recombination 11 homolog A | P49959 |
| Histone deacetylase 3 | O15379 | Microtubule-associated protein 4 | P27816 |
| Thymopoietin | P42166 | DEAH (Asp-Glu-Ala-His) box polypeptide 16 | O60231 |
| Nuclear mitotic apparatus protein 1 | Q14980 | — | — |
| Immunomodulator | |||
| Interleukin 12B | P29459 | Linker for activation of T cells/LAT | O43561 |
| Interleukin 1, beta | P01584 | — | — |
| Semaphorin-4D | Q92854 | — | — |
| Others | |||
| Endoglin (Osler-Rendu-Weber syndrome 1) | P17813 | Junction plakoglobin/Desmoplakin-3 | P14923 |
| Stromal interaction molecule 1 | Q13586 | Adaptor-related protein complex 2, alpha 1 subunit/AP-2A1 | O95782 |
| Potassium voltage-gated channel, subfamily H, member 6 | Q9H252 | Protein kinase, cAMP-dependent, regulatory, type II, beta | P31323 |
| Sorting nexin 1 | Q13596 | — | — |
| Syntaxin binding protein 1 | Q64320 | — | — |
| Neuronal Shc | Q92529 | — | — |
| 5-hydroxytryptamine (serotonin) receptor 2C | P28335 | — | — |
— indicates no entry.
Swiss protein accession numbers.
Figure 3.
Perifosine inhibits Akt phosphorylation. (A) BCWM.1 cells were cultured with perifosine for 6 hours with doses that ranged from 2 to 20 μM. Whole cell lysates were subjecting to Western blotting using anti–p-Akt ser473, -Akt, –p-PDK1, –p-GSK3α/β, –p-S6R, and α-tubulin antibodies. (B,C) BCWM.1 cells were cultured with perifosine. Whole cell lysates were immunoprecipitated with anti-Akt antibody. Then the immunoprecipitated were washed and subjected to in vitro kinase assay according to manufacturer's protocol. Western blotting used -p-Akt, and fusion protein -p-GSK3α/β antibodies. (B) Dose effect of perifosine at 6 hours at doses that range from 5 to 20 μM. (C) Time-effect of perifosine at 10 μM for 2 to 6 hours.
Neither growth factors nor adherence to BMSCs protect against perifosine-induced WM cell cytotoxicity
Since the BM microenvironment confers WM cell growth and drug resistance, we next studied whether perifosine can overcome resistance induced by the BM microenvironment. BCWM.1 cells, along with MM.1S cells as a control, were cultured with perifosine (5-20 μM) in the presence or absence of BMSCs. Adherence of MM.1S cells and BCWM.1 cells to BMSCs triggered 2.2-fold (P < .01) and 2-fold (P < .01) increase in the [3H]-thymidine uptake, respectively. Importantly, perifosine inhibited WM cell growth in the context of the BM microenvironment in a dose-dependent fashion (P < .001; Figure 4A), confirming that perifosine retains significant antitumor activity even in the presence of BM stromal cells. The adhesion of WM cells in coculture with stromal cells led to increased activation of Akt, and perifosine 10 μM was able to overcome Akt activity, even in the presence of stromal cells, as shown in Figure 4B.
Figure 4.
Growth factors and coculture with BMSCs do not protect against perifosine-induced WM cell cytotoxicity. (A) BCWM.1 cells were cultured with control media and with perifosine (5 to 20 μM) for 48 hours in the presence or absence of BMSCs. Cell proliferation was assessed using the [3H]-thymidine uptake assay. (B) BCWM.1 cells were cultured with either perifosine (10 μM) alone and in presence of BMSCs for 6 hours. Whole cell lysates were subjected to Western blotting using anti–p-Akt, -Akt and α-tubulin antibodies. (C) BCWM.1 cells were cultured with perifosine (5 to 20 μM) in the absence and presence of IL-6 (25 ng/mL) or IGF-1 (50 ng/mL) for 48 hours. Cytotoxicity was assessed by the MTT assay. All data represent mean (± SD) of triplicate experiments (A,C). (D,E) BCWM.1 cells were cultured with perifosine (10 μM) for 6 hours in the absence and presence of (D) IGF-1 (50 ng/mL) or (E) IL-6 (25 ng/mL) for the last 10 minutes. Whole cell lysates were subjected to Western blotting using anti–p-Akt ser473, -Akt and α-tubulin antibodies.
Previous studies using gene expression analysis in WM have demonstrated that interleukin 6 (IL-6) signaling is up-regulated in WM.31 IL-6 also has been reported to be an important factor in promoting plasmacytoid lymphocyte growth in WM, as well as a marker reflecting tumor burden and disease severity.24 Both IL-6 and IGF-1 induce activation of Akt in MM.18,32 We therefore tested whether perifosine can overcome the protective effect of IL-6 and IGF-1 in WM cells. As shown in Figure 4C, IL-6 (25 ng/mL) and IGF-1 (50 ng/mL) induced modest proliferation (10% to 30%) of WM cells, which was blocked by perifosine (5-20 μM). Using immunobloting, both IL-6 and IGF-1 induced rapid Akt phosphorylation at 10 minutes of cytokine stimulation, and perifosine (10 μM) inhibited Akt activation at 6 hours even in the presence of IGF-1 or IL-6, as shown in Figure 4D and E. These results suggest that perifosine overcomes Akt activation and associated growth triggered by IL-6 or IGF-1.
Perifosine induces SAPK/JNK MAPK caspase-dependent WM cell apoptosis
We first demonstrated that perifosine induced significant apoptosis evidenced by propidium iodide and Apo2.7 staining and flow cytometric analysis. Perifosine induced dose-dependent apoptosis in BCWM.1 and MM.1S cells with 50 μM, inducing 60% apoptosis at 48 hours (Figure 5A). To determine the mechanism of perifosine-induced apoptosis, we investigated the effect of perifosine (5-20 μM) in BCWM.1 cells using immunoblotting and protein array analysis. First, using protein array technique, we found up-regulation of expression of mitogen-activated protein kinase 4 and caspase 7 involved in induction of apoptosis, as well as regulators of cell cycle, cyclin-dependent kinase 7, and leucine zipper, a putative tumor suppressor (Table 1). We then confirmed the protein array data using immunoblotting. Perifosine induced caspase-8, caspase-9, caspase-3, and PARP cleavage in a dose- and time-dependent fashion (Figure 5B), with caspase activation and PARP cleavage starting after 4 hours treatment with perifosine (10 μM).
Figure 5.
Perifosine induces SAPK/JNK-dependent MM cell apoptosis. (A) MM.1S and BCWM.1 cells were cultured with perifosine (5-50 μM) for 48 hours. Then the percentage of cells undergoing apoptosis was studied using Apo2.7 staining and flow cytometry. (B) BCWM.1 cells were cultured with perifosine (10 μM) for the indicated periods. Whole cell lysates were subjected to Western blotting using anti-caspase 9, -caspase 8, -PARP, and α-tubulin antibodies. Horizontal lines have been inserted to indicate a repositioned gel lane. (C) BCWM.1 cells were cultured with perifosine (2-20 μM) for 6 hours. Whole cell lysates were subjected to Western blotting using anti–p-SAPK/JNK and α-tubulin antibodies. (D) BCWM.1 was cultured with control media, perifosine (10 μM), SP600125 (5-20 μM), or perifosine (10 μM) plus SP600125 (10 μM and 20 μM) for 10 hours. Whole cell lysates were subjected to Western blotting using anti–p-SAPK/JNK, -caspase 9, -caspase 8, -PARP, and α-tubulin antibodies. Horizontal lines have been inserted to indicate a repositioned gel lane. (E) BCWM.1 cells were cultured for 48 hours with media and with perifosine (5-20 μM) in the absence or presence of 5 to 20 μM of JNK inhibitor SP600125. Cytotoxicity was assessed by the MTT assay. All data represent mean (± SD) of triplicate experiment (A,E).
We next examined the effect of perifosine on JNK phosphorylation. As shown in Figure 5C, perifosine (2-20 μM, 6 hours) induced phosphorylation of JNK1/2 in BCWM.1 cells in a dose-dependent fashion. To determine the role of JNK activity in mediating perifosine-induced cytotoxicity, we examined whether specific inhibition of JNK1/2 expression could block perifosine-induced cytotoxicity. BCWM.1 cells were treated with perifosine (5-20 μM) in the presence or absence of the JNK inhibitor SP600125 (5-20 μM). Cytotoxicity triggered by perifosine (5-20 μM for 6 hours) was blocked by SP600125 (5-20 μM for 6 hours) (Figure 5E), confirming a role for JNK mediating perifosine-induced apoptosis. SP600125 markedly inhibited perifosine-induced phosphorylation of JNK and caspase-9 cleavage, but not caspase-8 cleavage (Figure 5D).
Perifosine inhibits human WM cell growth in vivo.
We next determined the effect of perifosine on WM cell growth using an in vivo immunodeficient mouse xenograft model. Mice were treated with perifosine 35 mg/kg per day daily by oral gavage as previously described.29 Drug treatment was started after the development of measurable tumor. The mean (± SD) tumor size was not significantly different between the 2 groups at baseline. Administration of perifosine significantly reduced WM tumor growth compared with control starting at week 6 (P = .049) (Figure 6A). By week 12, 75% of the control group was killed because of tumor growth, compared with only 18% in the perifosine treatment group (P = .04). The main toxicity observed in the perifosine-treated mice was loss of weight (average, 4 grams ± 1). Whole cell lysates from tumors harvested from the perifosine-treated and control mice were assessed by Western blotting for phosphorylation of Akt. As shown in Figure 6B, perifosine induced significant in vivo inhibition of Akt phosphorylation and downstream ribosomal phospho-S6.
Figure 6.
Perifosine inhibits human WM cell growth in vivo. (A) Immunodeficient irradiated SCID mice were inoculated subcutaneously in the flank with 3 × 106 BCWM.1 cells in 100 μL RPMI 1640 medium. Oral perifosine was administered daily (35 mg/kg per day) starting after the development of measurable tumors (N = 11). Perifosine significantly inhibited WM cell growth at week 12 (P = .04) compared with the control group (N = 8) treated with vehicle only (water). Error bars represent the variation in tumor volume for the mice per group. (B) Tumor tissues from mice treated with control vehicle (N = 2) or daily perifosine (N = 2) were harvested, processed, and subjected to Western blotting using anti-Akt, –p-Akt, –p-S6R and –β-actin antibodies. Adhesion and migration in vitro and homing in vivo. (C) Transwell migration assay showing inhibition of migration of BCWM.1 in the presence of perifosine 1 to 5 μM. SDF-1 30 nM was placed in the lower chambers and induced migration as compared with control with no SDF-1 (CTL, control). SDF-1 was placed in the lower chambers of the perifosine-treated wells. (D) Adhesion assay with BCWM.1 in the presence or absence of perifosine 2 to 10 μM. BCWM.1 demonstrated increased adhesion in fibronectin-coated wells (CTL, control) as compared with BSA-coated wells. Perifosine inhibited adhesion in fibronectin-coated wells in a dose-dependent manner. All data represent mean (± SD) of triplicate experiments (C,D). (E) In vivo flow cytometry. DID-labeled cells, either treated with perifosine 5 μM ( ) or untreated control (
) or untreated control ( ), were injected in the tail vein of 2 balb/c mice. Cells were counted every 5 minutes for 1 hour. The cell count decreased by 75% in the control and only by 40% in the treated mouse, P = .001. (F) Histogram showing that the number of cells present in the perivascular bone marrow niches of the skull was significantly higher in the control mice compared with the perifosine-treated group at 24 hours after injection (P = .039), using in vivo confocal imaging of quadrants 3 and 4 of the skull of mice.
), were injected in the tail vein of 2 balb/c mice. Cells were counted every 5 minutes for 1 hour. The cell count decreased by 75% in the control and only by 40% in the treated mouse, P = .001. (F) Histogram showing that the number of cells present in the perivascular bone marrow niches of the skull was significantly higher in the control mice compared with the perifosine-treated group at 24 hours after injection (P = .039), using in vivo confocal imaging of quadrants 3 and 4 of the skull of mice.
Perifosine inhibits migration and adhesion of WM cells in vitro.
Previous studies have demonstrated that the PI3K/Akt pathway regulates migration and adhesion in B cells.16,18 We sought to determine the effect of perifosine on migration and adhesion of WM cells. We first demonstrated that stromal derived factor-1 (SDF-1), one of the important regulators of migration in B cells,33 induced migration in BCWM.1 cells at 30 nM SDF-1 (Figure 6C). To study the effect of perifosine on the migration of WM cells, BCWM.1 cells were incubated with low doses of perifosine 1 to 5 μM for 2 hours (these doses and duration of incubation did not induce apoptosis in WM cells as confirmed by trypan blue and Apo2.7 staining by flow cytometry, data not shown). Perifosine induced significant inhibition of BCWM.1 cell migration toward SDF-1 at 5 μM (P = .001; Figure 6C). To confirm that Akt plays an important role in the regulation of migration in WM, we show that perifosine inhibits Akt functional activity at 4 hours using the Akt kinase assay, as shown in Figure 3C. In addition, we used the Akt knockdown cell line and showed that there was a significant inhibition of migration in response to SDF-1 with the Akt knockdown BCWM.1 cells compared with the mock-infected cells (data not shown). We also tested the effect of perifosine on adhesion in WM. BCWM.1 cells were treated with perifosine 2 to 10 μM for 1 hour and demonstrate significant inhibition of adhesion to fibronectin (P = .02; Figure 6D). We finally demonstrated that there was no change in the surface expression of CXCR4 or VLA-4 in response to perifosine at 6, 12, and 24 hours, indicating that perifosine regulates migration and adhesion at the intracellular signaling pathways level through inhibition of the Akt pathway and not by modulating surface expression of chemokine and adhesion molecules (data not shown).
Perifosine inhibited homing of WM cells to the bone marrow.
Adhesion of malignant cells to stromal cells confers resistance to apoptosis.24 Therefore, we sought to investigate the effect of low-dose perifosine on homing in vivo in WM. DID-labeled BWM.1S cells treated with perifosine (5 μM, 2 hours) or control (PBS) were injected in the tail veins of balb/c mice, followed by in vivo flow cytometry every 5 minutes for one hour after injection. As showed in Figure 6E, the number of circulating BCWM.1 cells decreased dramatically (75% decrease) after 1 hour in the control experiment, indicating homing, while in mice injected with BCWM.1 pretreated with perifosine, there was only a 40% reduction in the cells at 1 hour (P = .001). Similarly, we demonstrated that the number of cells present in the perivascular BM niches of the skull was significantly higher in the control mice compared with the perifosine-treated group at 24 hours after injection (Figure 6F; P = .039). The effect of perifosine on signaling pathways that regulate adhesion and cell trafficking was confirmed using proteomic analysis. BCWM.1 cells were treated 12 hours at 10 μM, and the nanoscale antibody microarray was used to determine proteins that were up- or down-regulated in response to perifosine compared with control, as previously described.26 Proteins that were dysregulated in response to perifosine are outlined in Table 1. Several of these proteins belonged to families involved in trafficking, cell-cell interaction, and adhesion.
Perifosine up-regulates the MEK/ERK pathway through activation of upstream proteins
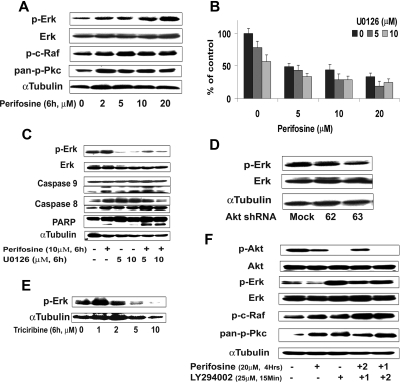
Using the protein array analysis, we found that MAPK proteins, including ERK, were activated in response to perifosine. The MEK/ERK and p38 MAPK pathways mediate proliferation and interact with the PI3K/Akt pathway in many malignancies.34–36 We therefore sought to examine the effect of perifosine on MAPK pathways using immunobloting analysis. Perifosine induced ERK1/2 phosphorylation in a dose-dependent fashion (Figure 7A), but not p38 MAPK (data not shown). We then studied whether inhibiting MEK/ERK signaling would enhance perifosine-induced cytotoxicity in WM cells. As shown in Figure 7B, cytotoxicity triggered by perifosine (5-20 μM) was enhanced in the presence of MEK1/2 inhibitor U0126 (5 μM and 10 μM). The combination was additive at low doses of perifosine with perifosine (10 μM) and U0126 (5 μM) inducing 30% inhibition of cell proliferation (CI = 0.8). The molecular mechanisms whereby perifosine-induced cytotoxicity was enhanced by U0126 were next studied. BCWM.1 cells were treated for 6 hours with either perifosine 10 μM, U0126 5 μM, and 10 μM, and the combination. Up-regulation of ERK phosphorylation triggered by perifosine was inhibited by U0126, whereas cleavage of caspase-9, -8 and PARP were up-regulated by the combination (Figure 7C).
Figure 7.
Inhibition of ERK signaling augments perifosine-induced cytotoxicity. (A) BCWM.1 cells were cultured with perifosine for 6 hours at doses that range from 2 to 20 μM. Whole cell lysates were subjected to Western blotting using anti–p-ERK, –ERK1/2, –p-c-Raf Ser259, –pan-p-PKC, and α-tubulin antibodies. (B) BCWM.1 cells were cultured for 48 hours with media and with perifosine (5-20 μM) in the absence or presence of 5 and 10 μM of MEK1/2 inhibitor U0126. Cytotoxicity was assessed by the MTT assay. Data represent mean (± SD) of triplicate experiments. (C) BCWM.1 cells were cultured with control media and either perifosine (10 μM), U0126 (5 μM and 10 μM), or the combination for 10 hours. Whole cell lysates were subjected to Western blotting using anti–p-ERK, -ERK1/2, -caspase 9, -caspase 8, -PARP and –α-tubulin antibodies. Horizontal lines have been inserted to indicate a repositioned gel lane. (D) BCWM.1 cells were transduced with 2 Akt shRNA for 48 hours. Mock: control plasmid; 62 and 63 represent shRNA that target 2 different sequences of Akt gene. Whole cell lysates were subjected to Western blotting using anti–p-ERK, -ERK1/2, and α-tubulin antibodies. (E) BCWM.1 cells were cultured with triciribine for 6 hours with doses that ranged from 1 to 10 μM. Whole cell lysates were subjected to Western blotting using anti–p-ERK and α-tubulin antibodies. (F) BCWM.1 was cultured with perifosine (20 μM, 4 hours) in the absence or presence of 25 μM of PI3K inhibitor LY294002. BCWM.1 was cultured with control media, perifosine (20 μM), LY294002 (25 μM, 15 minutes), LY294002 for 15 minutes (1) followed by perifosine for 4 hours (2), or perifosine for 4 hours (1) followed by LY294002 for 15 minutes (2) Whole cell lysates were subjected to Western blotting using anti–p-ERK, -ERK1/2, –p-Akt Ser473, -Akt, –p-c-Raf Ser259, –pan-p-PKC and α-tubulin antibodies.
To further investigate whether activation of ERK1/2 was a compensatory response to inhibition of Akt or a direct effect of perifosine on the ERK MAPK pathway, we studied ERK expression in the Akt knockdown BCWM.1 cells. As shown in Figure 7D, Akt expression was silenced after infection of BCWM.1 cells with Akt shRNA. Interestingly, ERK was not activated in response to Akt inhibition. Similarly, the specific Akt inhibitor triciribine did not induce ERK activation (Figure 7E). These data indicate that the effect of perifosine on ERK is not through a compensatory feedback mechanism. Therefore, we hypothesized that the effect of perifosine is through modulation of upstream pathways, specifi-cally PI3K, PKC, and c-Raf/MEK pathways. We first demonstrated that perifosine induced PKC and c-Raf activation (Figure 7F). In addition, the specific PI3K inhibitor LY294002 (25 μM for 15 minutes) completely abrogated Akt phosphorylation and induced significant ERK activation, similar to the effect of perifosine. We also demonstrated that LY294002 activated c-Raf and pan-pPKC at 4 hours, as does perifosine (Figure 7F). These data suggest that perifosine activates the MEK/ERK pathway through upstream activation of PKC and c-Raf.
Discussion
WM is a distinct B-cell lymphoproliferative disease.1 Little is known about signaling pathways implicated in WM pathogenesis, and the majority of therapeutic agents currently used to treat WM have been applied due to their activity in related lymphoproliferative diseases, such as chronic lymphocytic leukemia and MM. Indeed, to date there have been no preclinical studies to identify the effect of novel targeted agents on the dysregulated signaling pathways in WM. In this study, we first demonstrated that PI3K/Akt signaling cascade is activated in primary WM cells and WM cell lines. In addition, inhibition of Akt by triciribine and Akt knockdown resulted in apoptosis of WM cells, indicating that the PI3K/Akt pathway is an important regulator of survival in WM.
We then studied the effect of the novel Akt inhibitor perifosine on WM cell growth and signaling in vitro and in vivo. Perifosine is an alkylphospholipid that interacts with several kinase pathways and thereby modulates intracellular survival and proliferation signal transduction pathways.14,21,37 It has demonstrated significant in vitro and in vivo activity in many tumors, including B-cell malignancies.29,38 We demonstrated that perifosine inhibited proliferation and induced apoptosis in WM cell lines and patient samples by activation of the JNK pathway and, conversely, that blocking of JNK by SP00125 abrogated perifosine-induced apoptosis. Perifosine also activated both the intrinsic and extrinsic pathways of apoptosis, resulting in caspases -8 and -9 cleavage. Importantly, perifosine had no effect on normal mononuclear cells or growth of bone marrow colonies, suggesting a favorable therapeutic index.
The role of the bone marrow microenvironment in regulation of growth and drug resistance of malignant cells in WM has not previously been studied. However, previous studies in other B-cell malignancies have demonstrated that cytokines such as IL-6 and IGF-1, as well as BMSCs, are critical regulators of tumor growth and drug resistance.39 In this study, we showed that BMSCs and cytokines induce proliferation in WM cells, associated with activation of Akt. In addition, we demonstrated that perifosine overcomes the proliferative effect of BMSCs and cytokines. Studies to further define the role of the BM microenvironment in WM pathogenesis are ongoing. Interestingly, our data also showed that perifosine inhibited in vitro adhesion and migration as well as delayed in vivo homing to the BM. These experiments reinforce the role of perifosine in blocking the ability of WM cells to home to their protective niches within the BM.
Migration and adhesion of malignant cells to the bone marrow milieu also regulates proliferation and resistance to therapy. In this study, we determined the effect of the chemokine SDF-1 on the migration of WM cells. We demonstrated that perifosine regulates migration to SDF-1 and also inhibits adhesion of these cells to fibronectin. These results indicate that perifosine not only induces direct apoptosis of WM cells, but also prevents their migration and adhesion to the microenvironment. We next demonstrated that perifosine inhibits homing of WM cells to the BM in vivo using in vivo flow cytometry and confocal microscopy.
To further investigate potential mechanisms of resistance to perifosine, we determined the effect of this agent on other signaling cascades, specifically the MAPK pathways. Previous studies have demonstrated variable results about the effects of perifosine on MAPK signaling.29 Delineating the signaling pathways that regulate ERK MAPK activation in response to perifosine may help define mechanisms of resistance to this agent in vivo, as well as identify proteins to be targeted to overcome resistance. In this study, we demonstrated that perifosine induced activation of ERK1/2 in a dose- and time-dependent fashion. Interestingly, we demonstrated, using both Akt knockdown and specific inhibitors to Akt, that ERK activation is not a compensatory up-regulation in response to Akt inhibition, but rather due to activation of upstream PKC and c-Raf, in a fashion similar to that triggered by PI3K inhibitor LY294002. It is therefore possible that growth receptor stimulation in the presence of perifosine leads to PLC and RTK activation, which in turn induces PIP2 stimulation, upstream of PI3K and PKC. Since PI3K is blocked by perifosine, PIP2 activation leads to activation of PKC, which then induces growth stimulation, associated with activation of downstream c-Raf and MEK/ERK. In addition, growth receptors also may activate c-Raf through the Ras/Raf/MEK pathway, independent of PKC. Importantly, inhibition of ERK MAPK by U0126 in combination with perifosine led to a synergistic inhibition of growth and induction of apoptosis in WM. These studies provide a better understanding of molecular mechanisms that regulate resistance to perifosine. Future combinations of perifosine with MEK inhibitors, such as AZD6244, may overcome this resistance and induce significant activity in WM.
In summary, we demonstrated that the PI3K/Akt pathway is constitutively activated in WM and that the Akt inhibitor perifosine induced apoptosis and growth inhibition in WM cells both in vitro and in vivo, without toxicity in normal mononuclear cells and hematopoietic progenitor. In addition, perifosine was able to overcome drug resistance and inhibit proliferation of tumor cells even in the BM microenvironment. Perifosine was able to inhibit migration and adhesion of WM cells in vitro and homing in vivo to the BM. We also showed that perifosine induced activation of the ERK MAPK pathway through activation of PKC and c-Raf-1 and, conversely, that the combination of ERK inhibitor U0126 with perifosine overcomes the growth associated by ERK activation. We also demonstrated that perifosine inhibited the growth of WM cells in vivo in a SCID subcutaneous tumor model. Importantly, perifosine inhibited phospho-Akt and downstream targets in vivo in the tumor cells from treated animals. Together these studies provide the framework for clinical studies of perifosine to improve patient outcome in WM.
Acknowledgments
This work was supported in part by National Institutes of Health grant R21 1R21CA126119-01A1, the International Waldenstrom Macroglobulinemia Foundation, the Leukemia and Lymphoma Research Foundation, an American Society of Hematology Scholar Award, the Bing Center for Waldenstrom Macroglobulinemia, and the Jerome Lipper Multiple Myeloma Center. X.L. is supported by a grant from the Franco-American Fulbright Foundation. I.M.G. is a Lymphoma Research Foundation Scholar.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: X.L. designed and performed the research, analyzed the data, and wrote the paper; R.C., S.T., T.H., K.A., C.L., and I.M.G. designed the research, analyzed the data, and wrote the paper; X.J., H.N., A.S.M., A.R., G.O'S., M.F., and E.H. performed in vitro research; and J.R., C.P., J.S., D.M.M., D.M., and T.K. performed in vivo research.
Conflict-of-interest disclosure: I.M.G. and K.C.A. declare grant support by Keryx. All other authors declare no competing financial interests.
Correspondence: Irene M. Ghobrial, Medical Oncology, Dana-Farber Cancer Institute, 44 Binney St, Mayer 548A, Boston, MA 02115; e-mail: irene_ghobrial@dfci.harvard.edu.
References
- 1.Ghobrial IM, Gertz MA, Fonseca R. Waldenstrom macroglobulinaemia. Lancet Oncol. 2003;4:679–685. doi: 10.1016/s1470-2045(03)01246-4. [DOI] [PubMed] [Google Scholar]
- 2.Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol. 2003;30:110–115. doi: 10.1053/sonc.2003.50082. [DOI] [PubMed] [Google Scholar]
- 3.Treon SP, Dimopoulos M, Kyle RA. Defining Waldenstrom's macroglobulinemia. Semin Oncol. 2003;30:107–109. doi: 10.1053/sonc.2003.50081. [DOI] [PubMed] [Google Scholar]
- 4.Ghobrial IM, Witzig TE. Waldenstrom macroglobulinemia. Curr Treat Options Oncol. 2004;5:239–247. doi: 10.1007/s11864-004-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gertz MA, Anagnostopoulos A, Anderson K, et al. Treatment recommendations in Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol. 2003;30:121–126. doi: 10.1053/sonc.2003.50039. [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulos MA, Anagnostopoulos A, Kyrtsonis MC, Castritis E, Bitsaktsis A, Pangalis GA. Treatment of relapsed or refractory Waldenstrom's macroglobulinemia with bortezomib. Haematologica. 2005;90:1655–1658. [PubMed] [Google Scholar]
- 7.Treon SP, Gertz MA, Dimopoulos M, et al. Update on treatment recommendations from the Third International Workshop on Waldenstrom's macroglobulinemia. Blood. 2006;107:3442–3446. doi: 10.1182/blood-2005-02-0833. [DOI] [PubMed] [Google Scholar]
- 8.Ghobrial IM, Fonseca R, Gertz MA, et al. Prognostic model for disease-specific and overall mortality in newly diagnosed symptomatic patients with Waldenstrom macroglobulinaemia. Br J Haematol. 2006;133:158–164. doi: 10.1111/j.1365-2141.2006.06003.x. [DOI] [PubMed] [Google Scholar]
- 9.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 10.Kucia M, Jankowski K, Reca R, et al. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 11.Alsayed Y, Ngo H, Runnels J, et al. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood. 2007;109:2708–2717. doi: 10.1182/blood-2006-07-035857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakayama T, Hieshima K, Izawa D, Tatsumi Y, Kanamaru A, Yoshie O. Cutting edge: profile of chemokine receptor expression on human plasma cells accounts for their efficient recruitment to target tissues. J Immunol. 2003;170:1136–1140. doi: 10.4049/jimmunol.170.3.1136. [DOI] [PubMed] [Google Scholar]
- 13.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 14.Dancey JE. Molecular targeting: PI3 kinase pathway. Ann Oncol. 2004;15(suppl 4):233–239. doi: 10.1093/annonc/mdh932. [DOI] [PubMed] [Google Scholar]
- 15.Pene F, Claessens YE, Muller O, et al. Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase pathways in the proliferation and apoptosis in multiple myeloma. Oncogene. 2002;21:6587–6597. doi: 10.1038/sj.onc.1205923. [DOI] [PubMed] [Google Scholar]
- 16.Brader S, Eccles SA. Phosphoinositide 3-kinase signalling pathways in tumor progression, invasion and angiogenesis. Tumori. 2004;90:2–8. doi: 10.1177/030089160409000102. [DOI] [PubMed] [Google Scholar]
- 17.Yoeli-Lerner M, Toker A. Akt/PKB signaling in cancer: a function in cell motility and invasion. Cell Cycle. 2006;5:603–605. doi: 10.4161/cc.5.6.2561. [DOI] [PubMed] [Google Scholar]
- 18.Tai YT, Podar K, Catley L, et al. Insulin-like growth factor-1 induces adhesion and migration in human multiple myeloma cells via activation of beta1-integrin and phosphatidylinositol 3′-kinase/AKT signaling. Cancer Res. 2003;63:5850–5858. [PubMed] [Google Scholar]
- 19.Cantrell DA. Phosphoinositide 3-kinase signalling pathways. J Cell Sci. 2001;114:1439–1445. doi: 10.1242/jcs.114.8.1439. [DOI] [PubMed] [Google Scholar]
- 20.Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Chang F, Lee JT, Navolanic PM, et al. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 22.Dillon CP, Sandy P, Nencioni A, Kissler S, Rubinson DA, Van Parijs L. Rnai as an experimental and therapeutic tool to study and regulate physiological and disease processes. Annu Rev Physiol. 2005;67:147–173. doi: 10.1146/annurev.physiol.67.040403.130716. [DOI] [PubMed] [Google Scholar]
- 23.Rubinson DA, Dillon CP, Kwiatkowski AV, et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- 24.Moreau AS, Jia X, Ngo HT, et al. Protein kinase C inhibitor enzastaurin induces in vitro and in vivo antitumor activity in Waldenstrom macroglobulinemia. Blood. 2007;109:4964–4972. doi: 10.1182/blood-2006-10-054577. [DOI] [PubMed] [Google Scholar]
- 25.Menaa C, Devlin RD, Reddy SV, Gazitt Y, Choi SJ, Roodman GD. Annexin II increases osteoclast formation by stimulating the proliferation of osteoclast precursors in human marrow cultures. J Clin Invest. 1999;103:1605–1613. doi: 10.1172/JCI6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghobrial IM, McCormick DJ, Kaufmann SH, et al. Proteomic analysis of mantle-cell lymphoma by protein microarray. Blood. 2005;105:3722–3730. doi: 10.1182/blood-2004-10-3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hideshima T, Chauhan D, Hayashi T, et al. The biological sequelae of stromal cell-derived factor-1alpha in multiple myeloma. Mol Cancer Ther. 2002;1:539–544. [PubMed] [Google Scholar]
- 28.Sipkins DA, Wei X, Wu JW, et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hideshima T, Catley L, Yasui H, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107:4053–4062. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatjiharissi E, Ngo H, Leontovich AA, et al. Proteomic analysis of Waldenstrom macroglobulinemia. Cancer Res. 2007;67:3777–3784. doi: 10.1158/0008-5472.CAN-06-3089. [DOI] [PubMed] [Google Scholar]
- 31.Chng WJ, Schop RF, Price-Troska T, et al. Gene-expression profiling of Waldenstrom macroglobulinemia reveals a phenotype more similar to chronic lymphocytic leukemia than multiple myeloma. Blood. 2006;108:2755–2763. doi: 10.1182/blood-2006-02-005488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20:5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- 33.Cyster JG. Homing of antibody secreting cells. Immunol Rev. 2003;194:48–60. doi: 10.1034/j.1600-065x.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 34.Moelling K, Schad K, Bosse M, Zimmermann S, Schweneker M. Regulation of Raf-Akt Cross-talk. J Biol Chem. 2002;277:31099–31106. doi: 10.1074/jbc.M111974200. [DOI] [PubMed] [Google Scholar]
- 35.Sato S, Fujita N, Tsuruo T. Involvement of 3-phosphoinositide-dependent protein kinase-1 in the MEK/MAPK signal transduction pathway. J Biol Chem. 2004;279:33759–33767. doi: 10.1074/jbc.M402055200. [DOI] [PubMed] [Google Scholar]
- 36.Koh G, Teong HF, Clement MV, Hsu D, Thiagarajan PS. A decompositional approach to parameter estimation in pathway modeling: a case study of the Akt and MAPK pathways and their crosstalk. Bioinformatics. 2006;22:e271–e280. doi: 10.1093/bioinformatics/btl264. [DOI] [PubMed] [Google Scholar]
- 37.Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339–345. [PubMed] [Google Scholar]
- 38.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 39.Hideshima T, Bergsagel PL, Kuehl WM, Anderson KC. Advances in biology of multiple myeloma: clinical applications. Blood. 2004;104:607–618. doi: 10.1182/blood-2004-01-0037. [DOI] [PubMed] [Google Scholar]