Abstract
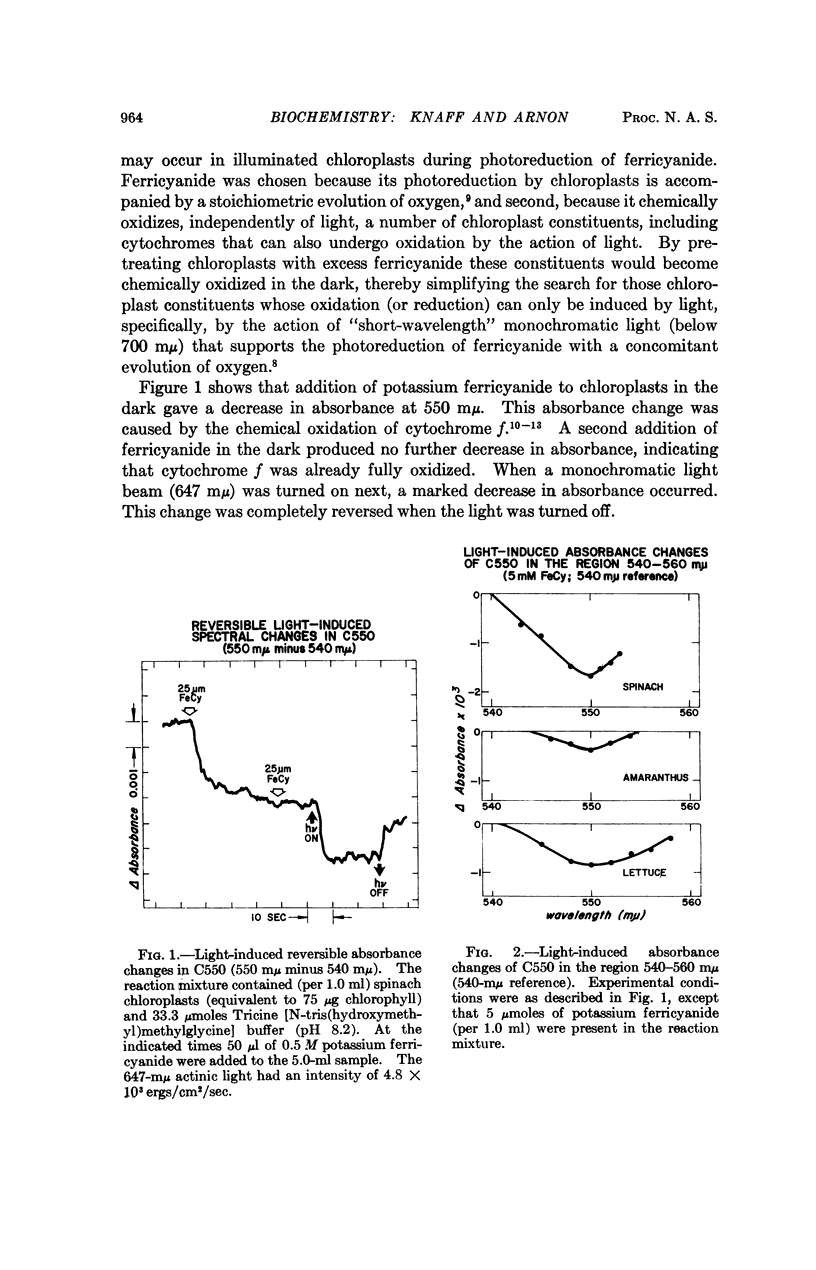
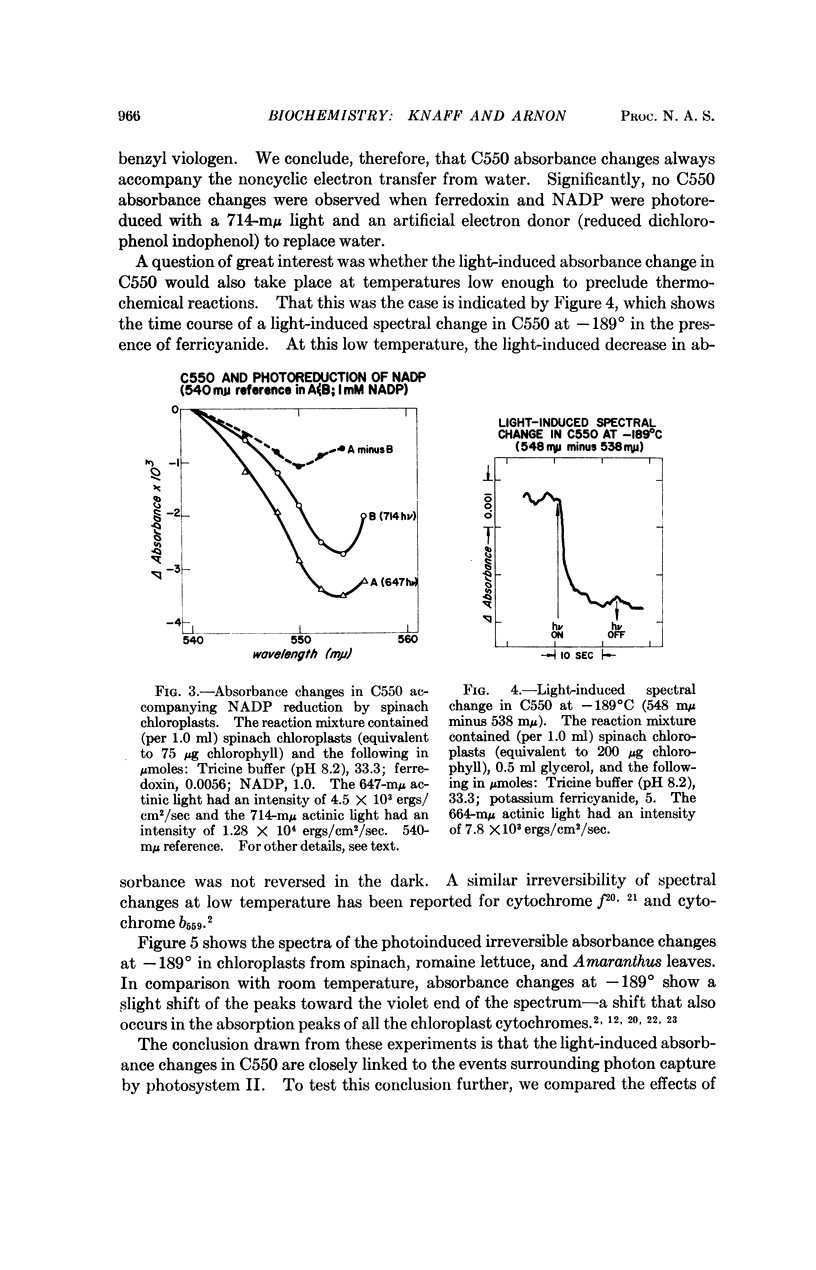
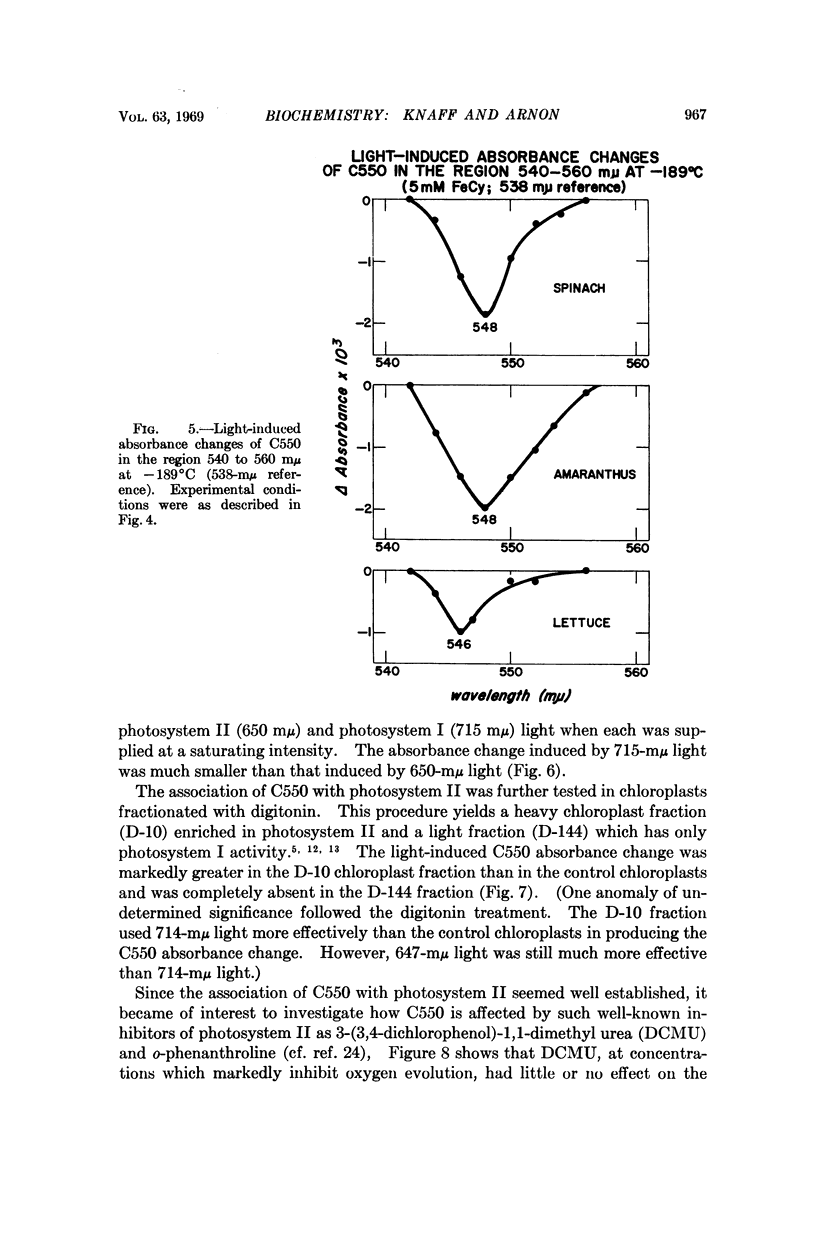
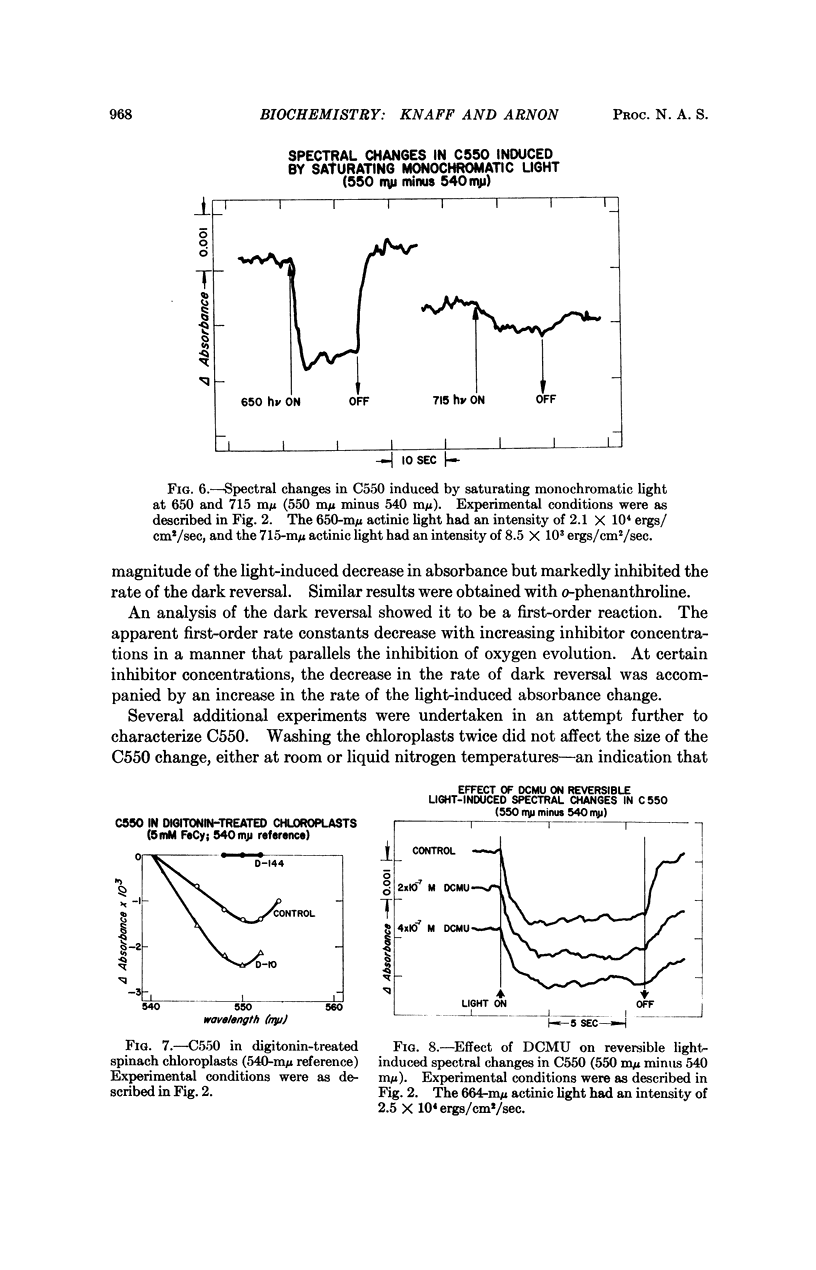
On illuminating chloroplasts with “short-wavelength” monochromatic light that supports oxygen evolution, spectral evidence was obtained for a new photoreactive chloroplast component, provisionally designated C550, which shows a reversible decrease of absorbance with a maximum at 550 mμ. The light-induced absorbance changes in C550 have been separated from those due to cytochromes in the same spectral region.
The light-induced decrease of absorbance in C550 appears to be independent of temperature, persisting even at -189° and is therefore likely to be linked to the primary light reaction associated with oxygen evolution in photosynthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNON D. I., TSUJIMOTO H. Y., MCSWAIN B. D. ROLE OF FERREDOXIN IN PHOTOSYNTHETIC PRODUCTION OF OXYGEN AND PHOSPHORYLATION BY CHLOROPLASTS. Proc Natl Acad Sci U S A. 1964 Jun;51:1274–1282. doi: 10.1073/pnas.51.6.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. M., Boardman N. K. Fractionation of the photochemical systems of photosynthesis. I. Chlorophyll contents and photochemical activities of particles isolated from spinach chloroplasts. Bibl Laeger. 1966 Mar 14;112(3):403–421. doi: 10.1016/0926-6585(66)90244-5. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I., Tsujimoto H. Y., McSwain B. D. Ferredoxin and photosynthetic phosphorylation. Nature. 1967 May 6;214(5088):562–566. doi: 10.1038/214562a0. [DOI] [PubMed] [Google Scholar]
- Bendall D. S. Oxidation-reduction potentials of cytochromes in chloroplasts from higher plants. Biochem J. 1968 Sep;109(3):46P–47P. doi: 10.1042/bj1090046pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman N. K., Anderson J. M. Fractionation of the photochemical systems of photosynthesis. II. Cytochrome and carotenoid contents of particles isolated from spinach chloroplasts. Biochim Biophys Acta. 1967 Jul 5;143(1):187–203. doi: 10.1016/0005-2728(67)90120-x. [DOI] [PubMed] [Google Scholar]
- HILL R. The cytochrome b component of chloroplasts. Nature. 1954 Sep 11;174(4428):501–503. doi: 10.1038/174501b0. [DOI] [PubMed] [Google Scholar]
- KATOH S., SHIRATORI I., TAKAMIYA A. Purification and some properties of spinach plastocyanin. J Biochem. 1962 Jan;51:32–40. doi: 10.1093/oxfordjournals.jbchem.a127497. [DOI] [PubMed] [Google Scholar]
- KOK B. Partial purification and determination of oxidation reduction potential of the photosynthetic chlorophyll complex absorbing at 700 millimicrons. Biochim Biophys Acta. 1961 Apr 15;48:527–533. doi: 10.1016/0006-3002(61)90050-6. [DOI] [PubMed] [Google Scholar]
- Kalberer P. P., Buchanan B. B., Arnon D. I. Rates of photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1542–1549. doi: 10.1073/pnas.57.6.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaff D. B., Arnon D. I. LIGHT-INDUCED OXIDATION OF A CHLOROPLAST B-TYPE CYTOCHROME AT -189 degrees C. Proc Natl Acad Sci U S A. 1969 Jul;63(3):956–962. doi: 10.1073/pnas.63.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSwain B. D., Arnon D. I. Enhancement effects and the identity of the two photochemical reactions of photosynthesis. Proc Natl Acad Sci U S A. 1968 Nov;61(3):989–996. doi: 10.1073/pnas.61.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUMBERG B., WITT H. T. ANALYSE DER PHOTOSYNTHESE MIT BLITZLICHT. I. DIE PHOTOOXYDATION VON CHLOROPHYLL-A1-430-703. Z Naturforsch B. 1964 Aug;19:693–707. [PubMed] [Google Scholar]
- SHIN M., ARNON D. I. ENZYMIC MECHANISMS OF PYRIDINE NUCLEOTIDE REDUCTION IN CHLOROPLASTS. J Biol Chem. 1965 Mar;240:1405–1411. [PubMed] [Google Scholar]
- TAGAWA K., ARNON D. I. Ferredoxins as electron carriers in photosynthesis and in the biological production and consumption of hydrogen gas. Nature. 1962 Aug 11;195:537–543. doi: 10.1038/195537a0. [DOI] [PubMed] [Google Scholar]
- TAGAWA K., TSUJIMOTO H. Y., ARNON D. I. SEPARATION BY MONOCHROMATIC LIGHT OF PHOTOSYNTHETIC PHOSPHORYLATION FROM OXYGEN EVOLUTION. Proc Natl Acad Sci U S A. 1963 Sep;50:544–549. doi: 10.1073/pnas.50.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITT H. T., MUELLER A., RUMBERG B. Oxidized cytochrome and chlorophyll C2-plus in photosynthesis. Nature. 1961 Dec 9;192:967–969. doi: 10.1038/192967a0. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Butler W. L. Photoreduction and photophosphorylation with tris-washed chloroplasts. Plant Physiol. 1968 Dec;43(12):1978–1986. doi: 10.1104/pp.43.12.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


