Abstract
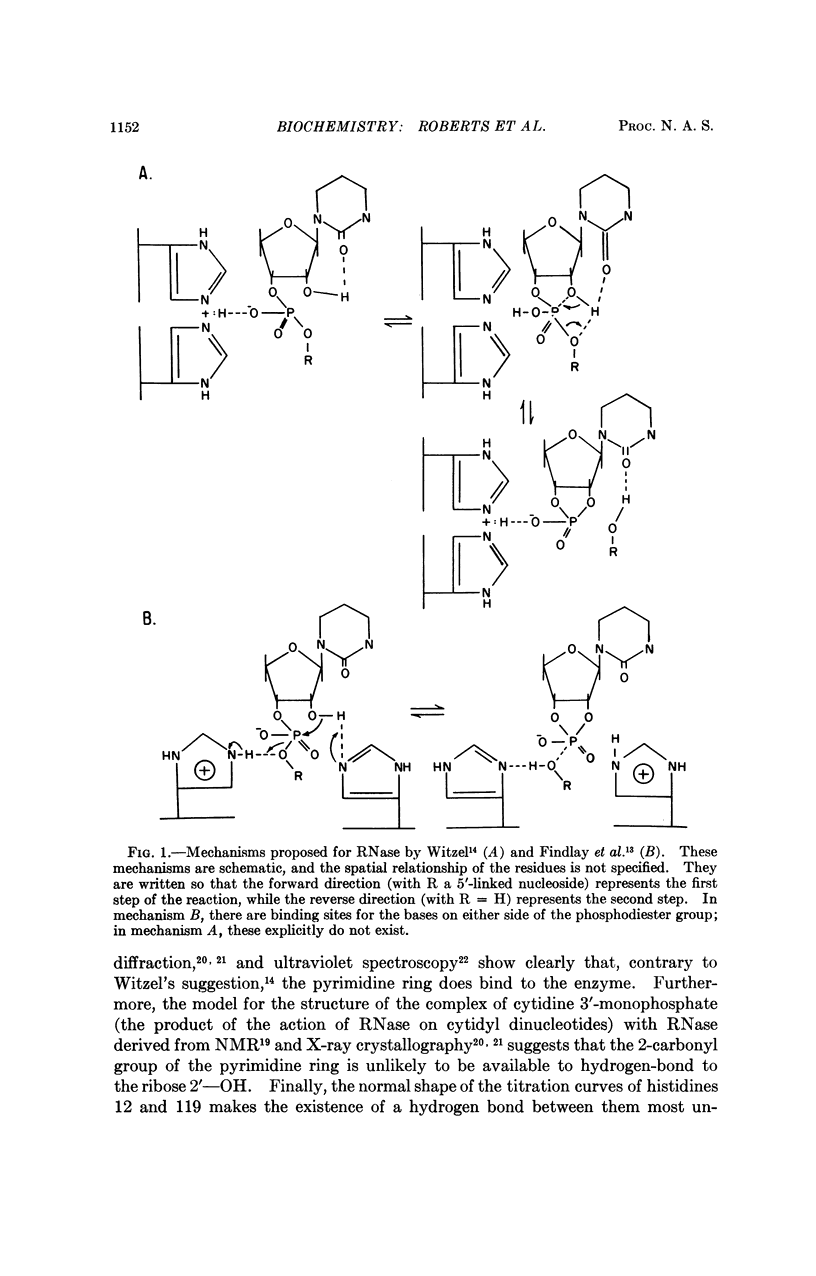
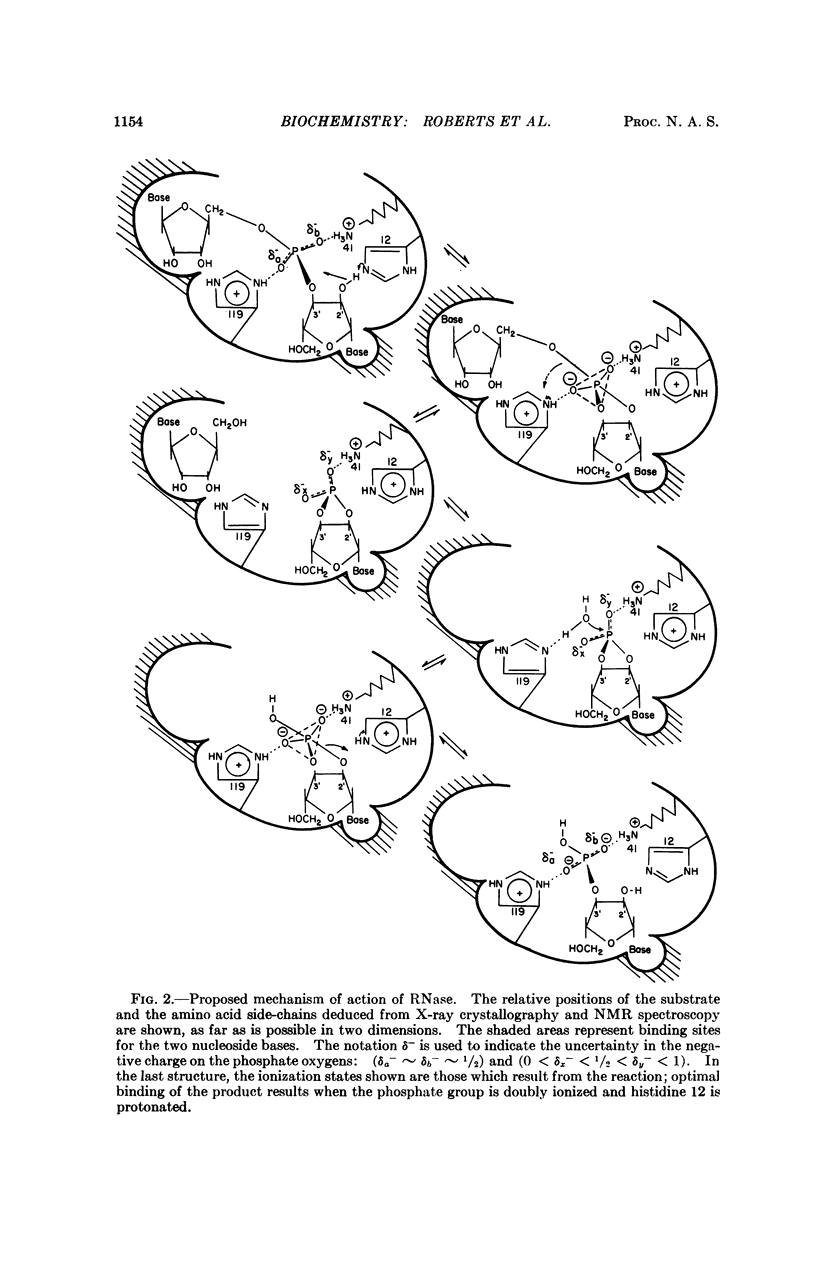
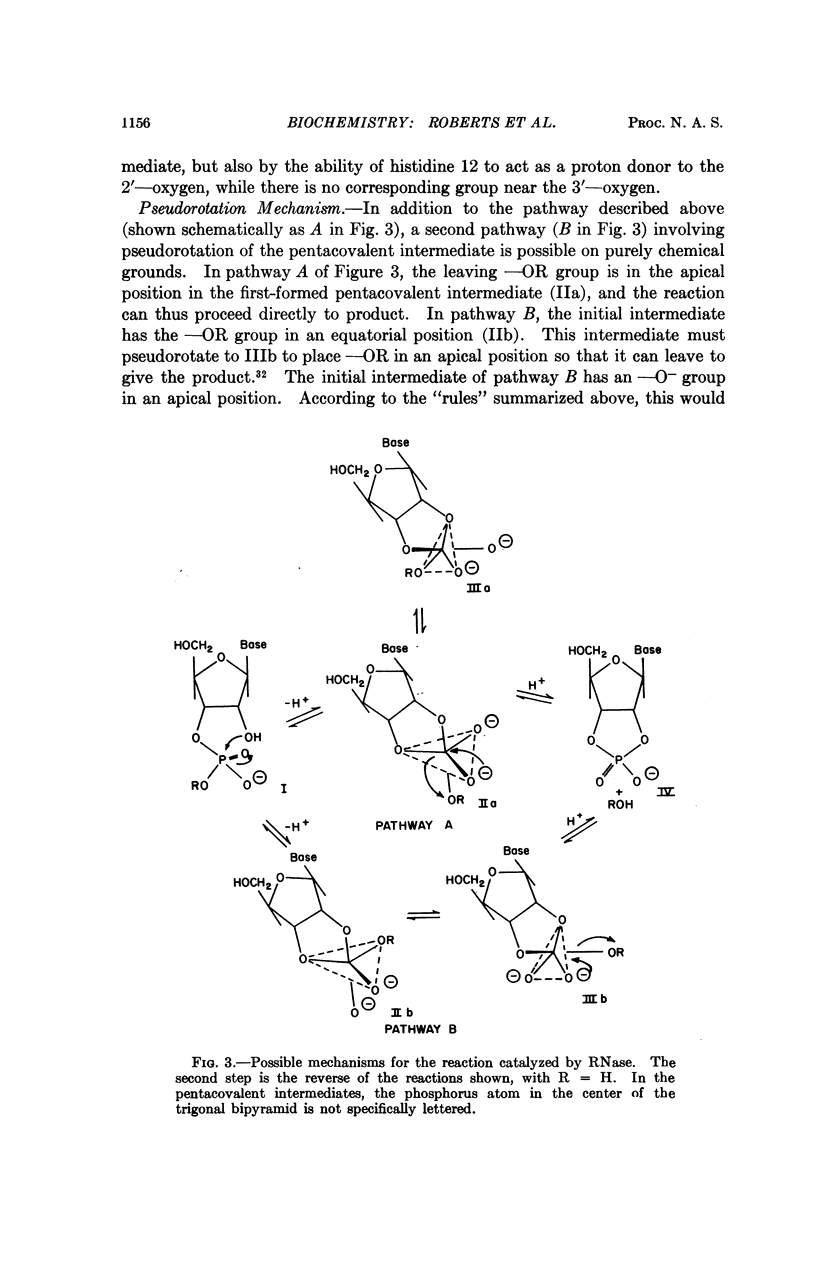
The possible mechanisms of action of bovine pancreatic ribonuclease are discussed in the light of the detailed knowledge of the geometry of the active site that has been derived from studies of inhibitor binding by X-ray diffraction and nuclear magnetic resonance. When combined with a knowledge of the mechanism of phosphate ester hydrolysis, this information imposes severe geometric constraints on possible mechanisms of action of the enzyme. Two types of mechanism can be distinguished, the linear and the pseudorotation. The linear mechanism includes a catalytic role for both histidine residues at the active site and does not involve pseudorotation of the intermediate. In contrast, in the pseudorotation mechanism one histidine residue performs all the catalytic functions, while the other serves only to bind the phosphate anion; this necessarily involves pseudorotation of the intermediate and specific protonation of the leaving group by the enzyme.
The mode of binding of the product of the reaction, cytidine-3′-monophosphate, has been elucidated by X-ray diffraction and nuclear magnetic resonance. If the substrate binds in an analogous way, only the linear mechanism is possible. This mechanism is described in detail.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRESTFIELD A. M., STEIN W. H., MOORE S. Alkylation and identification of the histidine residues at the active site of ribonuclease. J Biol Chem. 1963 Jul;238:2413–2419. [PubMed] [Google Scholar]
- Deavin A., Mathias A. P., Rabin B. R. Mechanism of action of bovine pancreatic ribonuclease. Nature. 1966 Jul 16;211(5046):252–255. doi: 10.1038/211252a0. [DOI] [PubMed] [Google Scholar]
- Dennis E. A., Westheimer F. H. The geometry of the transition state in the hydrolysis of phosphate esters. J Am Chem Soc. 1966 Jul 20;88(14):3432–3433. doi: 10.1021/ja00966a046. [DOI] [PubMed] [Google Scholar]
- Findlay D., Herries D. G., Mathias A. P., Rabin B. R., Ross C. A. The active site and mechanism of action of bovine pancreatic ribonuclease. 7. The catalytic mechanism. Biochem J. 1962 Oct;85(1):152–153. doi: 10.1042/bj0850152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMMEL J. P., KALNITSKY G. MECHANISMS OF CERTAIN PHOSPHOTRANSFERASE REACTIONS: CORRELATION OF STRUCTURE AND CATALYSIS IN SOME SELECTED ENZYMES. Annu Rev Biochem. 1964;33:15–50. doi: 10.1146/annurev.bi.33.070164.000311. [DOI] [PubMed] [Google Scholar]
- Hirs C. H., Halmann M., Kycia J. H. Dinitrophenylation and inactivation of bovine pancreatic ribonuclease A. Arch Biochem Biophys. 1965 Jul;111(1):209–222. doi: 10.1016/0003-9861(65)90343-7. [DOI] [PubMed] [Google Scholar]
- Irie M., Sawada F. Do the exposed tyrosine residues of ribonuclease A interact with nucleotides? J Biochem. 1967 Aug;62(2):282–284. doi: 10.1093/oxfordjournals.jbchem.a128662. [DOI] [PubMed] [Google Scholar]
- Kartha G., Bello J., Harker D. Tertiary structure of ribonuclease. Nature. 1967 Mar 4;213(5079):862–865. doi: 10.1038/213862a0. [DOI] [PubMed] [Google Scholar]
- MARKHAM R., SMITH J. D. The structure of ribonucleic acids. II. The smaller products of ribonuclease digestion. Biochem J. 1952 Dec;52(4):558–565. doi: 10.1042/bj0520558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows D. H., Jardetzky O., Epand R. M., Ruterjans H. H., Scheraga H. A. Assignment of the histidine peaks in the nuclear magnetic resonance spectrum of ribonuclease. Proc Natl Acad Sci U S A. 1968 Jul;60(3):766–772. doi: 10.1073/pnas.60.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows D. H., Jardetzky O. Nuclear magnetic resonance studies of the structure and binding sites of enzymes. IV. Cytidine 3'-monophosphate binding to ribonuclease. Proc Natl Acad Sci U S A. 1968 Oct;61(2):406–413. doi: 10.1073/pnas.61.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERAGA H. A., RUPLEY J. A. Structure and function of ribonuclease. Adv Enzymol Relat Subj Biochem. 1962;24:161–261. doi: 10.1002/9780470124888.ch4. [DOI] [PubMed] [Google Scholar]
- Usher D. A. On the mechanism of ribonuclease action. Proc Natl Acad Sci U S A. 1969 Mar;62(3):661–667. doi: 10.1073/pnas.62.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITZEL H., BARNARD E. A. Mechanism and binding sites in the ribonuclease reaction. II. Kinetic studies on the first step of the reaction. Biochem Biophys Res Commun. 1962 May 4;7:295–299. doi: 10.1016/0006-291x(62)90194-8. [DOI] [PubMed] [Google Scholar]
- Wang J. H. Facilitated proton transfer in enzyme catalysis. It may have a crucial role in determining the efficiency and specificity of enzymes. Science. 1968 Jul 26;161(3839):328–334. doi: 10.1126/science.161.3839.328. [DOI] [PubMed] [Google Scholar]
- Wyckoff H. W., Hardman K. D., Allewell N. M., Inagami T., Johnson L. N., Richards F. M. The structure of ribonuclease-S at 3.5 A resolution. J Biol Chem. 1967 Sep 10;242(17):3984–3988. [PubMed] [Google Scholar]


