Abstract
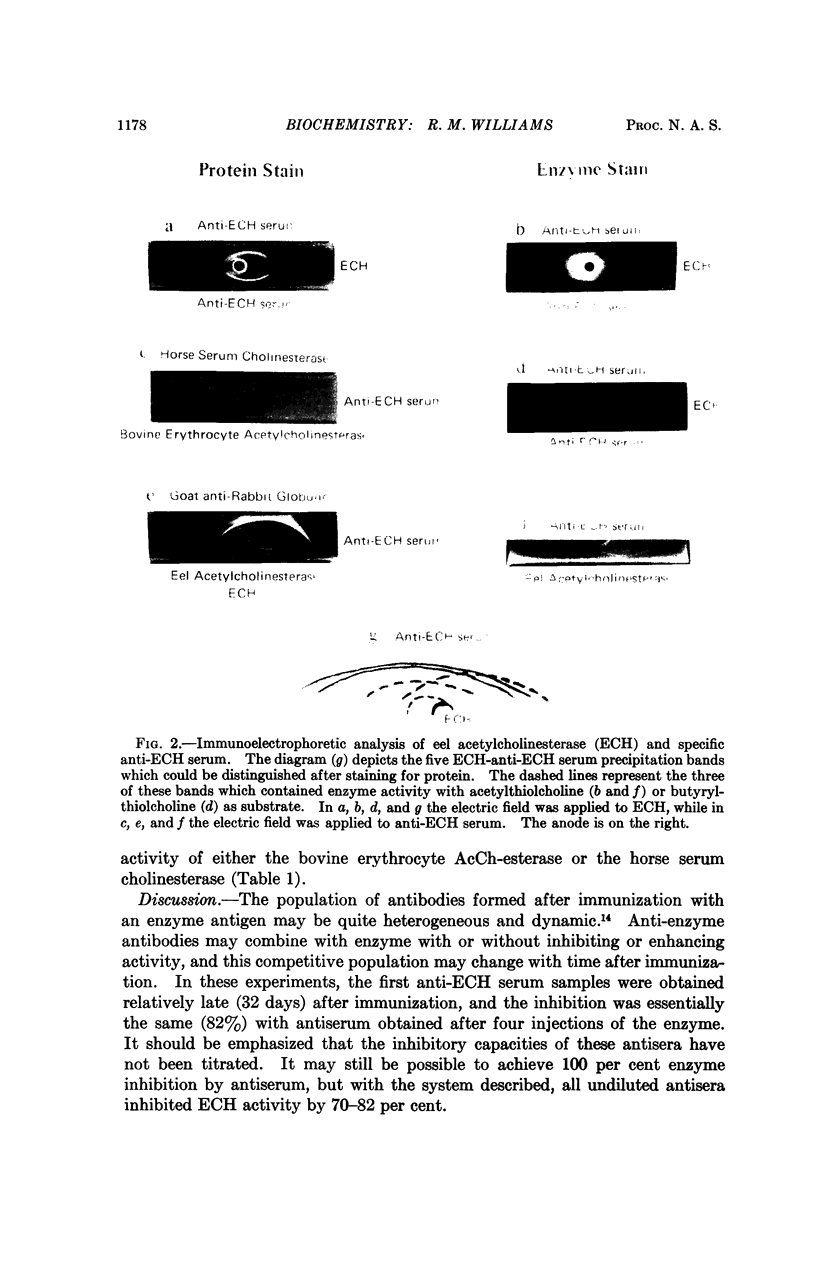
A rabbit antiserum against commercially available Electrophorus electricus acetylcholinesterase has been prepared. Five precipitation bands were distinguished by immunoelectrophoresis, but only three of these contained demonstrable enzyme activity. In an in vitro system, activity of the commercial enzyme or of highly purified acetylcholinesterase was inhibited by 70-82 per cent after incubation with antiserum. Antibody specificity was demonstrated by the absence of serological cross reactions or enzyme inhibition with bovine erythrocyte acetylcholinesterase or horse serum cholinesterase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom F. E., Barrnett R. J. Fine structural localization of acetylcholinesterase in electroplaque of the electric eel. J Cell Biol. 1966 Jun;29(3):475–495. doi: 10.1083/jcb.29.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Thiéry J., Tung Y., Kittel C. On the cooperativity of biological membranes. Proc Natl Acad Sci U S A. 1967 Feb;57(2):335–341. doi: 10.1073/pnas.57.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehninger A. L. The neuronal membrane. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1069–1080. doi: 10.1073/pnas.60.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenard J., Singer S. J. Alteration of the conformation of proteins in red blood cell membranes and in solution by fixatives used in electron microscopy. J Cell Biol. 1968 Apr;37(1):117–121. doi: 10.1083/jcb.37.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuzinger W., Baker A. L., Cauvin E. Acetylcholinesterase. II. Crystallization, absorption spectra, isoionic point. Proc Natl Acad Sci U S A. 1968 Feb;59(2):620–623. doi: 10.1073/pnas.59.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmansohn D. Chemical control of the permeability cycle in excitable membranes during electrical activity. Ann N Y Acad Sci. 1966 Jul 14;137(2):877–900. doi: 10.1111/j.1749-6632.1966.tb50207.x. [DOI] [PubMed] [Google Scholar]
- Nakane P K, Pierce G B., Jr Enzyme-labeled antibodies: preparation and application for the localization of antigens. J Histochem Cytochem. 1966 Dec;14(12):929–931. doi: 10.1177/14.12.929. [DOI] [PubMed] [Google Scholar]
- Van Orden D. E., Treffers H. P. A demonstration of the coprecipitation of beta-lipoproteins with specific precipitates of chicken antibodies and human serum albumin. J Immunol. 1968 Mar;100(3):664–674. [PubMed] [Google Scholar]
- Watanabe A., Tasaki I., Lerman L. Bi-ionic action potentials in squid giant axons internally perfused with sodium saltssalts. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2246–2252. doi: 10.1073/pnas.58.6.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker V. P. Synaptic transmission. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1081–1091. doi: 10.1073/pnas.60.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]




