Abstract
Ixodes scapularis salivary protein, Salp15, inhibits CD4+ T cell activation by binding to the most-extracellular domains of the CD4 molecule, potentially overlapping with the gp120-binding region. We now show that Salp15 inhibits the interaction of gp120 and CD4. Furthermore, Salp15 prevents syncytia formation between HL2/3 (a stable HeLa cell line expressing the envelope protein) and CD4-expressing cells. Salp15 prevented gp120-CD4 interaction at least partially through its direct interaction with the envelope glycoprotein. A phage display library screen provided the interacting residues in the C1 domain of gp120. These results provide a potential basis to define exposed gp120 epitopes for the generation of neutralizing vaccines.
Introduction
The human immunodeficiency virus (HIV) is the causative agent of the acquired immunodeficiency syndrome (AIDS). The infection of mammalian cells depend on specific interactions between envelope proteins of the virus and surface proteins of the host cell [1, 2]. The initial highly specific interaction between gp120 in the envelope of the virus and the T cell co-receptor CD4 [3-6] results in a conformational change in the former that allows the glycoprotein to interact with one of the chemokine co-receptors on the surface of the mammalian cell [7], CCR5 and CXCR4 [8, 9]. Upon the interaction between gp120 and the chemokine co-receptor, the virion becomes fusogenic with the participation of another viral protein, gp41 that ultimately leads to the fusion of viral and mammalian cell membranes and consequently, the introduction of the viral components inside the cell [10, 11].
The region of CD4 that specifically interacts with gp120 has been mapped to the second complementarity-determining region (CDR2) of the D1 domain [12, 13]. The amino acids that mediate this contact are located around Phe43 and contain positively charged residues in positions 46 and 59 [12, 13]. Since the fusion of HIV with the cell membrane consists of many organized events and intermolecular interactions, several potential targets for intervention are currently being studied [2, 14] aimed at the blockade of the interaction of gp120 with CD4 [15], or the fusion between the virus and the cell membrane. Indeed, the only entry-targeting inhibitor currently approved by the Food and Drug Administration (FDA) is a mimic of the helical region 2 of gp41 that prevents the fusion of the virus with the plasma membrane [16].
Ixodes scapularis salivary protein (Salp) 15 is an antigen that mediates at least partially the immunomodulatory action of tick saliva on host-acquired immune responses [17]. Inhibition mediated by Salp15 results from the repression of calcium fluxes triggered by T cell antigen receptor (TCR) ligation and a subsequent reduction in interleukin (IL)-2 production. Salp15 binds to the most extracellular domains (D1-D2) of the T cell co-receptor CD4 in both mouse and human cells [18] in a region that may overlap with the binding residues of gp120.
Materials and Methods
Recombinant proteins and synthetic peptides
His-tagged Salp15 and Salp15ΔP11 were purified using the Drosophila expression system (DES), as described [17]. Recombinant HIV-1IIIB HRP-conjugated gp120 and sCD4 (domains D1D2) were purchased from Immunodiagnostics, Inc. (Woburn, MA). The Salp15 overlapping peptides (P1-P11) were previously described [18]. The peptide CD4M9 (CNLARCQLRCKSLGLLGKCAGSFCACGP) [19, 20] was obtained from GeneScript Corporation (Piscataway, NJ). The overlapping 15-mer peptides corresponding to the HIV-1 consensus subtype B envelope protein were obtained from the NIH AIDS Research & Reference Reagent Program. Lysozyme and Keyhole limpet hemocyanin (KLH) were purchased from Sigmal Chemical Co. (Sant Louis, MO).
Microtiter binding and in vitro competition assays
Purified His-tagged Salp15, Salp15ΔP11, Salp15 peptides, or the CD4M9 peptide were coated overnight at 4 °C at the indicated concentrations in 0.1 M sodium carbonate buffer (pH 9.5) in 96 well plates. The peptide MOG35-55 from the myelin oligodendrocyte glycoprotein (GeneScript Corporation), Lysozyme and KLH were used as controls. The wells were washed, blocked with 10% fetal calf sera (FCS) in phosphate buffered saline (PBS) –PBS/FCS- for 1 h at room temperature and incubated with HRP-gp120 at the indicated concentrations. Binding was detected by using microwell peroxidase substrate (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD). The reaction was stopped by adding TMB stop solution (Kirkegaard & Perry Laboratories, Inc.). For competition experiments, 2 μM of purified sCD4 (Immunodiagnostics Inc.) or purified CD4M9 were coated in 96 well plates as above and incubated overnight at 4 °C. The wells were washed and blocked as above and incubated with either HRP-gp120 or HRP-gp120 that had been pre-incubated with Salp15 or P11 at the indicated concentrations. The reaction was developed and stopped as described above.
In vitro fusion assays
106 HL2/3 or HeLa cells were grown in 6 well plates and incubated with 50 μg/ml of Salp15, Salp15ΔP11 or KLH (control) overnight at 37 °C. The cells were then mixed with 106 HeLa-CD4 or 3T3.CD4.CCR5 cells (transfected with HIV-1 3′ LTR-luciferase) and incubated for an additional 24 and 48 h at 37 °C. After 24 h, the cells were stained with DAPI (Molecular Probes, Eugene, OR) and analyzed by confocal microscopy (Olympus Confocal microscope equipped with Fluorview 3.0 software, Center Valley, PA). Luciferase activity assay was performed after 48 h to quantitatively evaluate cell-cell fusion, in a TD20/20n luminometer (Turner BioSystems, Sunnyvale, CA). Anti-CD4 (OKT4) antibodies (eBioScience, San Diego, CA) were used as a control.
Phage display
A 7-amino acid random phage library (Ph7, New England Biolabs, Ipswich, MA) was screened with recombinant His-tagged Salp15, following the manufacturer's protocol. Briefly, 30-mm Petri dishes were coated with Salp15 (1 μM) in bicarbonate buffer. The plates were then incubated with 2 × 1011 phagi for 1 h and washed extensively. Interacting phagi were eluted in Tris-buffered saline (TBS) buffer containing 100 μg/ml of Salp15. The phagi were then amplified on E. coli and used to perform 2 further rounds of panning. The eluates from the third panning round were used to sequence isolated plaque-forming phage clones. The corresponding peptides were analyzed by BLAST search for homologies against bacterial, mammalian and viral protein databases, separately. The alignment of the sequence HVITPLW was also performed against the HIV sequence database (http://hiv.lanl.gov/content/index.html).
Results and Discussion
Because the regions in CD4 that interact with gp120 and Salp15 may overlap, we analyzed Salp15 as a potential HIV-1 gp120-CD4 blocking agent. We first determined the ability of increasing concentrations of recombinant Salp15 to compete for the binding of gp120 to CD4 in a microtiter assay in vitro. A dose-dependent competition of gp120 binding was observed when the wells were co-incubated with the salivary immunosuppressor (Figure 1A). In contrast, KLH (Figure 1A) or Lysozyme (not shown) had no effect on the binding.
Figure 1. Salp15 prevents gp120-CD4 interaction and syncytia formation.
(A) Microtiter assay showing the percentage of maximal binding between gp120 and CD4 in the presence of increasing concentrations of Salp15. KLH was used as a negative control. (B) Representative confocal micrograph of DAPI staining of CD4-expressing HeLa and gp120-expressing HL2/3 cells in the absence (middle panel) or the presence of 50 μg/ml of Salp15 (right panel). (C) Quantitative in vitro fusion assay showing the percent of maximal HIV-1 3′ LTR-driven luciferase activity in 3T3-CD4 and HL2/3 cells incubated in the presence or absence of 50 μg/ml of Salp15 for 48 h. The αCD4 mAb OKT4 was used as positive control. The results presented are the mean ± SE of three independent experiments.
Since Salp15 inhibited in vitro gp120-CD4 interaction, we hypothesized that Salp15 could prevent cell-cell fusion and the formation of syncytia in surrogate assays of HIV-1 infection. We used an experimental cell-cell fusion approach, in which different cell lines expressing both gp120 and CD4 are allowed to interact and fuse leading to the formation of syncytia. HL2/3 cells, a derivative HeLa cell line that expresses HIV-1 envelope proteins [21] were mixed at a 1:1 ratio with HeLa cells that express surface CD4 (HeLa-CD4 [22]). After 24 h, the cells were analyzed for the formation of multi-nucleated cells by staining with the nuclear stain DAPI by confocal microscopy. As expected, only HeLa-CD4 cells supported cell fusion and multi-nucleation (Fig 1B, left and middle panels). The addition of 50 μg/ml of Salp15 induced a decrease in cell fusion (Fig 1B, right panel).
We also preformed an in vitro fusion assay using a quantitative approach. HL2/3 cells were mixed at a 1:1 ratio with HeLa-CD4 (not shown) or 3T3.CD4.CCR5 cells [23] that had been transfected with the HIV-1 3′ LTR-luciferase plasmid [24, 25] in the absence or presence of 50 μg/ml of Salp15. Luciferase activity, indicative of cell fusion, was determined after 48 h. The presence of Salp15 decreased the LTR-induced transcription of the luc gene (Figure 1C), indicative that the tick antigen had prevented cell-cell fusion. As a control, a blocking monoclonal antibody against human CD4 showed the expected inhibition of LTR-mediated luciferase activity (Figure 1C). Theses results demonstrated that Salp15 prevents gp120-CD4 interaction as well as cell-cell fusion in surrogate assays of HIV infection.
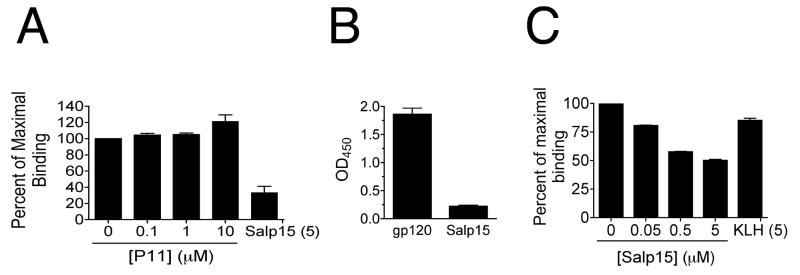
The C-terminal portion of Salp15 (amino acids 95-114 of the mature protein named P11, with the sequence GPNGQTCAEKNKCVGHIPGC -[17]) mediates its interaction with sCD4 [18] and recapitulates the immunosuppressive activity of Salp15 [26]. We thus tested the ability of purified P11 to compete CD4-gp120 binding. We used a microtiter assay with plate-bound sCD4 (1 μM) probed with HRP-gp120 (50 nM) in the presence of different concentrations of P11. The peptide was unable to compete with gp120 for its interaction with sCD4, in contrast to the whole protein (Figure 2A). These results suggested that the inhibitory effect of the salivary protein on the interaction between CD4 and gp120 was due to steric effects upon its interaction with the T cell co-receptor.
Figure 2. The residues in CD4 that are important for its interaction with gp120 do not participate in Salp15 binding.
(A) Microtiter assay showing the percentage of maximal binding between gp120 and CD4 in the presence of different concentrations of the Salp15 carboxyl-terminal peptide, P11. (B) In vitro binding assay of the mimetic peptide CD4M9 to soluble gp120 and Salp15 (C) Competition of the binding between gp120 and CD4M9 in the presence of different concentrations of Salp15, showed as percentage of the maximal binding. The results shown are representative of three to four independent experiments.
The CD4 residues Phe43 and Arg59 contribute to the contact between the T cell co-receptor and gp120 [27]. The replacement of these residues with Ala decreased the affinity to gp120 by ∼500 and ∼10 fold, respectively [28]. Scyllatoxin is a scorpion toxin with structural similarities in the hairpin region with the CDR2-like loop of CD4. A series of amino acid interchanges and point mutations resulted in peptides structurally similar to the parent CD4 molecule with the capacity to mimic the gp120 binding region of CD4 [29, 30]. CD4M9 had an IC50 of 0.1-1.0 μM, depending on viral strains, and inhibited the infection of CD4+ cells [30]. We coated a 96-well plate with 25 nM of soluble gp120 or 1 μM of Salp15 and incubated the wells with 2 μM of biotin-tagged CD4M9. CD4M9 interacted with gp120 but not with Salp15 (Fig 2B), indicating that Salp15 does not bind to the residues involved in the interaction of sCD4 and gp120. To test whether Salp15 could inhibit the interaction of gp120 and CD4M9, we performed a competition assay. CD4M9 was coated in a 96 wells plate and was incubated with either HRP-tagged gp120 alone or HRP-tagged gp120 in the presence of increasing concentrations of Salp15. Salp15 inhibited the binding of gp120 to CD4M9 (Fig 2C). These results showed that Salp15 binding to CD4 did not involve the residues of the co-receptor present in its mimic miniprotein. These results also suggested that the competition elicited by Salp15 is due, at least partially, to its interaction with gp120. However, the interaction of Salp15 with both CD4 and gp120 may be responsible for the observed effect, since the preincubation of 3T3-CD4 or HL2/3 cells with Salp15 prior to performing the fusion assays resulted in similar degrees of inhibition (data not shown).
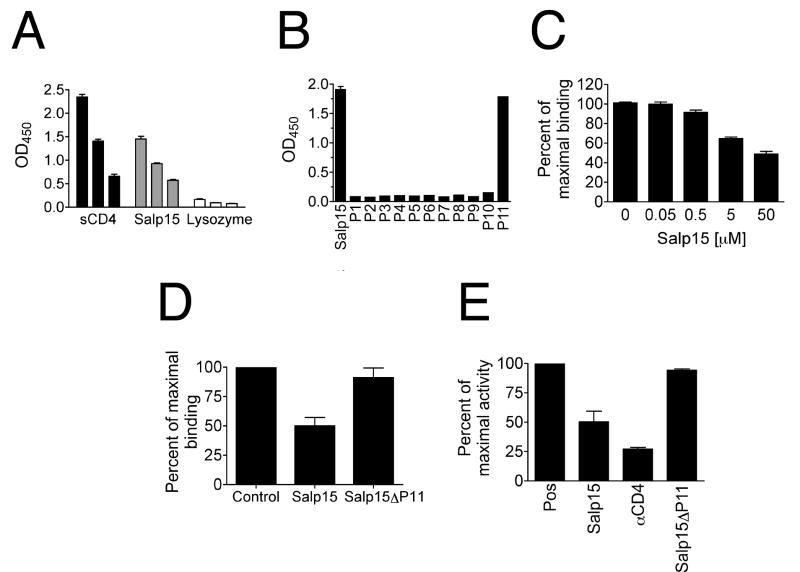
To test whether Salp15 is able to bind to gp120, we performed microtiter binding assays using Salp15 and synthetic 20 amino acid-long peptides with 10 amino acids overlaps encompassing the entire Salp15 protein sequence (P1-P11) [18]. Plate-bound Salp15 showed a dose-dependent binding to soluble HRP-gp120 (Figure 3A). Only the carboxyl terminal peptide (P11) of Salp15 [18] was able to bind to gp120 (Figure 3B). We could also compete the interaction of P11 with gp120 by adding increasing concentrations of free Salp15 (Figure 3C), suggesting a specific interaction. To further demonstrate the role of P11 in the interaction between Salp15 and gp120, we generated a deletion mutant that lacked the last 20 amino acids (Salp15ΔP11). Compared to Salp15, Salp15ΔP11 could not compete the binding between CD4 and gp120 in microtiter assays (Figure 3D) and was unable to recapitulate the syncytia-inhibiting activity shown by the full protein (Figure 3E). These results demonstrated that Salp15 binds gp120 through its carboxyl terminal amino acid residues.
Figure 3. Salp15 binds to gp120 through its carboxyl-terminal amino acid residues.
(A) Microtiter binding assay showing sCD4 and Salp15 binding to soluble HRP-tagged gp120 in a dose dependent manner. Lysozyme was used as negative control. (B) Binding of overlapping synthetic peptides of salp15 to HRP-tagged gp120. (C) Competition experiment showing percent of maximal binding between P11 and HRP-tagged gp120 in the presence of increasing concentrations of Salp15. A mutant version of Salp15 that lacks the carboxyl-terminal 20 amino acids (Salp15ΔP11) showed no effect on the percentage of maximal binding between gp120 and CD4 (D) or the maximal HIV-1 3′ LTR-induced luciferase activity in an in vitro fusion assay compared to the whole saliva protein (E). The results are expressed as the mean ± SE of three to four independent experiments.
In order to identify the residues in gp120 that interact with Salp15, we performed a screening of a phage display library. The sequences obtained corresponded to 2 distinct peptides, represented equally in the sequenced clones. None of the peptides corresponded to linear sequences located on the surface molecule CD4, suggesting that Salp15 binds to this protein in a non-linear region of the D1 domain [18]. The sequence HVITPLW was partially found (underlined) in the C1 domain of gp120 and showed good conservation among several isolates of HIV-1. A more thorough alignment using the HIV sequence database (http://hiv.lanl.gov/content/index), showed high levels of conservation in the sequence L/ITPL in viruses belonging to several subtypes with the exception of subtype O (Figure 4A and data not shown). In order to validate the binding to this region of gp120, we analyzed the binding of the C-terminal peptide P11 and Salp15 to overlapping 15-mer peptides corresponding to the HIV-1 Consensus Subtype B envelope protein. Both P11 (Figure 4B) and Salp15 (not shown) showed binding to the peptide #8792 (PCVKLTPLCVTLNCT). The C1/V1 region represented in peptide #8792 is shown in Figure 4C, and corresponds to the β2 sheet that faces the CD4 molecule in their binding state. These results demonstrate that Salp15 interacts with the HIV-1 envelope protein and shows neutralizing activity. Salp15 can therefore serve as a novel template for the identification of epitopes present in the envelope protein that can potentially yield neutralizing antibody generation.
Figure 4. Identification of the binding site of Salp15 in gp120.

(A) Partial alignment of the amino acid sequence of 30 HIV-1 isolates present in the panel of 60 primary strains of the virus [31] with the sequence of peptide #8792. Sequences in red represent a perfect match. (B) Microtiter binding assay using peptides that encompass the C1 and V1 domains of gp120 corresponding to the consensus sequence of HIV clade B. The peptide MOG35-55 (MOG) corresponding to the myelin oligodendrocyte glycoprotein protein was used as control. The results are representative of 3 independent experiments with similar results. (C) Location (red) of the sequence corresponding to peptide #8792 in gp120 (blue molecule) in relation to the interaction with CD4 (green). Both CD4 and gp120 were obtained from PBD 1GC1 to which the coordinates corresponding to antibody 17B were subtracted. For reference, the residues Phe43 and Arg59 are represented with side chains (pink). The sequence corresponding to peptide #8792 is shown below in red. LTPL is underlined. The sequence corresponding to the C1 domain is shown in bold letters, while those corresponding to the variable region, V1, are in italics.
Acknowledgments
This work was supported by the NIAID/NIH grant AI053064 to J.A.
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: HL2/3, HeLa-CD4 and 3T3-CD4 cells, the plasmid pBlue3′LTR-luc-A and the peptides corresponding to the HIV-1 Consensus Subtype B envelope protein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Doms RW. Unwelcome guests with master keys: how HIV enters cells and how it can be stopped. Top HIV Med. 2004;12:100–103. [PubMed] [Google Scholar]
- 2.Vermeire K, Schols D. Anti-HIV agents targeting the interaction of gp120 with the cellular CD4 receptor. Expert Opin Investig Drugs. 2005;14:1199–1212. doi: 10.1517/13543784.14.10.1199. [DOI] [PubMed] [Google Scholar]
- 3.Moore JP, Sattentau QJ, Klasse PJ, Burkly LC. A monoclonal antibody to CD4 domain 2 blocks soluble CD4-induced conformational changes in the envelope glycoproteins of human immunodeficiency virus type 1 (HIV-1) and HIV-1 infection of CD4+ cells. J Virol. 1992;66:4784–4793. doi: 10.1128/jvi.66.8.4784-4793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryu SE, Kwong PD, Truneh A, Porter TG, Arthos J, Rosenberg M, Dai XP, Xuong NH, Axel R, Sweet RW, et al. Crystal structure of an HIV-binding recombinant fragment of human CD4. Nature. 1990;348:419–426. doi: 10.1038/348419a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkinson RA, Piscitelli C, Teintze M, Cavacini LA, Posner MR, Lawrence CM. Structure of the Fab fragment of F105, a broadly reactive anti-human immunodeficiency virus (HIV) antibody that recognizes the CD4 binding site of HIV type 1 gp120. J Virol. 2005;79:13060–13069. doi: 10.1128/JVI.79.20.13060-13069.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu H, Myszka DG, Tendian SW, Brouillette CG, Sweet RW, Chaiken IM, Hendrickson WA. Kinetic and structural analysis of mutant CD4 receptors that are defective in HIV gp120 binding. Proc Natl Acad Sci U S A. 1996;93:15030–15035. doi: 10.1073/pnas.93.26.15030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briant L, Signoret N, Gaubin M, Robert-Hebmann V, Zhang X, Murali R, Greene MI, Piatier-Tonneau D, Devaux C. Transduction of activation signal that follows HIV-1 binding to CD4 and CD4 dimerization involves the immunoglobulin CDR3-like region in domain 1 of CD4. J Biol Chem. 1997;272:19441–19450. doi: 10.1074/jbc.272.31.19441. [DOI] [PubMed] [Google Scholar]
- 8.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 9.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 10.Eckert DM, Kim PS. Mechanisms of viral membrane fusion and its inhibition. Annu Rev Biochem. 2001;70:777–810. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
- 11.Gallo SA, Finnegan CM, Viard M, Raviv Y, Dimitrov A, Rawat SS, Puri A, Durell S, Blumenthal R. The HIV Env-mediated fusion reaction. Biochim Biophys Acta. 2003;1614:36–50. doi: 10.1016/s0005-2736(03)00161-5. [DOI] [PubMed] [Google Scholar]
- 12.Arthos J, Deen KC, Chaikin MA, Fornwald JA, Sathe G, Sattentau QJ, Clapham PR, Weiss RA, McDougal JS, Pietropaolo C, et al. Identification of the residues in human CD4 critical for the binding of HIV. Cell. 1989;57:469–481. doi: 10.1016/0092-8674(89)90922-7. [DOI] [PubMed] [Google Scholar]
- 13.Clayton LK, Hussey RE, Steinbrich R, Ramachandran H, Husain Y, Reinherz EL. Substitution of murine for human CD4 residues identifies amino acids critical for HIV-gp120 binding. Nature. 1988;335:363–366. doi: 10.1038/335363a0. [DOI] [PubMed] [Google Scholar]
- 14.Sutor GC, Dreikhausen U, Vahning U, Jurkiewicz E, Hunsmann G, Lundin K, Schedel I. Neutralization of HIV-1 by anti-idiotypes to monoclonal anti-CD4. Potential for idiotype immunization against HIV. J Immunol. 1992;149:1452–1461. [PubMed] [Google Scholar]
- 15.Santoro F, Vassena L, Lusso P. Chemokine receptors as new molecular targets for antiviral therapy. New Microbiol. 2004;27:17–29. [PubMed] [Google Scholar]
- 16.Matthews T, Salgo M, Greenberg M, Chung J, DeMasi R, Bolognesi D. Enfuvirtide: the first therapy to inhibit the entry of HIV-1 into host CD4 lymphocytes. Nat Rev Drug Discov. 2004;3:215–225. doi: 10.1038/nrd1331. [DOI] [PubMed] [Google Scholar]
- 17.Anguita J, Ramamoorthi N, Hovius JW, Das S, Thomas V, Persinski R, Conze D, Askenase PW, Rincon M, Kantor FS, Fikrig E. Salp15, an Ixodes scapularis salivary protein, inhibits CD4+ T cell activation. Immunity. 2002;16:849–859. doi: 10.1016/s1074-7613(02)00325-4. [DOI] [PubMed] [Google Scholar]
- 18.Garg R, Juncadella IJ, Ramamoorthi N, Ashish, Ananthanarayanan SK, Thomas V, Rincon M, Krueger JK, Fikrig E, Yengo CM, Anguita J. Cutting edge: CD4 is the receptor for the tick saliva immunosuppressor, Salp15. J Immunol. 2006;177:6579–6583. doi: 10.4049/jimmunol.177.10.6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowd CS, Leavitt S, Babcock G, Godillot AP, Van Ryk D, Canziani GA, Sodroski J, Freire E, Chaiken IM. Beta-turn Phe in HIV-1 Env binding site of CD4 and CD4 mimetic miniprotein enhances Env binding affinity but is not required for activation of co-receptor/17b site. Biochemistry. 2002;41:7038–7046. doi: 10.1021/bi012168i. [DOI] [PubMed] [Google Scholar]
- 20.Vita C, Drakopoulou E, Vizzavona J, Rochette S, Martin L, Menez A, Roumestand C, Yang YS, Ylisastigui L, Benjouad A, Gluckman JC. Rational engineering of a miniprotein that reproduces the core of the CD4 site interacting with HIV-1 envelope glycoprotein. Proc Natl Acad Sci U S A. 1999;96:13091–13096. doi: 10.1073/pnas.96.23.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciminale V, Felber BK, Campbell M, Pavlakis GN. A bioassay for HIV-1 based on Env-CD4 interaction. AIDS Res Hum Retroviruses. 1990;6:1281–1287. doi: 10.1089/aid.1990.6.1281. [DOI] [PubMed] [Google Scholar]
- 22.Chesebro B, Wehrly K. Development of a sensitive quantitative focal assay for human immunodeficiency virus infectivity. J Virol. 1988;62:3779–3788. doi: 10.1128/jvi.62.10.3779-3788.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng HK, Unutmaz D, KewalRamani VN, Littman DR. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 24.Jeeninga RE, Hoogenkamp M, Armand-Ugon M, de Baar M, Verhoef K, Berkhout B. Functional differences between the long terminal repeat transcriptional promoters of human immunodeficiency virus type 1 subtypes A through G. J Virol. 2000;74:3740–3751. doi: 10.1128/jvi.74.8.3740-3751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klaver B, Berkhout B. Comparison of 5′ and 3′ long terminal repeat promoter function in human immunodeficiency virus. J Virol. 1994;68:3830–3840. doi: 10.1128/jvi.68.6.3830-3840.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juncadella IJ, Garg R, Ananthnarayanan SK, Yengo CM, Anguita J. T-cell signaling pathways inhibited by the tick saliva immunosuppressor, Salp15. FEMS Immunol Med Microbiol. 2007;49:433–438. doi: 10.1111/j.1574-695X.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 27.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moebius U, Clayton LK, Abraham S, Harrison SC, Reinherz EL. The human immunodeficiency virus gp120 binding site on CD4: delineation by quantitative equilibrium and kinetic binding studies of mutants in conjunction with a high-resolution CD4 atomic structure. J Exp Med. 1992;176:507–517. doi: 10.1084/jem.176.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dowd CS, Leavitt S, Babcock G, Godillot AP, Van Ryk D, Canziani GA, Sodroski J, Freire E, Chaiken IM. Beta-turn Phe in HIV-1 Env binding site of CD4 and CD4 mimetic miniprotein enhances Env binding affinity but is not required for activation of co-receptor/17b site. Biochemistry. 2002;41:7038–7046. doi: 10.1021/bi012168i. [DOI] [PubMed] [Google Scholar]
- 30.Vita C, Drakopoulou E, Vizzavona J, Rochette S, Martin L, Menez A, Roumestand C, Yang YS, Ylisastigui L, Benjouad A, Gluckman JC. Rational engineering of a miniprotein that reproduces the core of the CD4 site interacting with HIV-1 envelope glycoprotein. Proc Natl Acad Sci U S A. 1999;96:13091–13096. doi: 10.1073/pnas.96.23.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown BK, Darden JM, Tovanabutra S, Oblander T, Frost J, Sanders-Buell E, de Souza MS, Birx DL, McCutchan FE, Polonis VR. Biologic and genetic characterization of a panel of 60 human immunodeficiency virus type 1 isolates, representing clades A, B, C, D, CRF01_AE, and CRF02_AG, for the development and assessment of candidate vaccines. J Virol. 2005;79:6089–6101. doi: 10.1128/JVI.79.10.6089-6101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]