Abstract
Cell differentiation is controlled by key transcription factors, and a major question is how they orchestrate cell-type-specific genetic programs. Muscle differentiation is a well studied paradigm in which the conserved Mef2 transcription factor plays a pivotal role. Recent genomic studies have identified a large number of mef2-regulated target genes with distinct temporal expression profiles during Drosophila myogenesis. However, the question remains as to how a single transcription factor can control such diverse patterns of gene expression. In this study we used a strategy combining genomics and developmental genetics to address this issue in vivo during Drosophila muscle development. We found that groups of mef2-regulated genes respond differently to changes in mef2 activity levels: some require higher levels for their expression than others. Furthermore, this differential requirement correlates with when the gene is first expressed during the muscle differentiation program. Genes that require higher levels are activated later. These results implicate mef2 in the temporal regulation of muscle gene expression, and, consistent with this, we show that changes in mef2 activity levels can alter the start of gene expression in a predictable manner. Together these results indicate that Mef2 is not an all-or-none regulator; rather, its action is more subtle, and levels of its activity are important in the differential expression of muscle genes. This suggests a route by which mef2 can orchestrate the muscle differentiation program and contribute to the stringent regulation of gene expression during myogenesis.
Keywords: muscle differentiation program, transcription factor levels
For several decades it has been appreciated that the controlled regulation of gene expression, including the coordinated activation of batteries of genes, lies behind cell differentiation programs (1–3). It is now clear that a principle tier of control of cell differentiation is through key transcription factors, and an important general question is how these factors coordinate the genetic program of such complex processes. A classic paradigm is muscle, in which the conserved Mef2 transcription factor is a major regulator of gene expression and differentiation (4). Mef2 was first identified in mammalian cell culture (5–7), but because mammals possess four closely related mef2 genes functional analyses during development are complicated. In contrast, Drosophila has a single mef2 gene and was the first organism to be used to show that mef2 is required for muscle development in vivo (8–10). This highlighted that the analysis of how mef2 functions is central to understanding how muscle is made. Important characteristics of the underlying genetic program include the temporal coordination of muscle gene expression (11–13) and the regulation of levels and relative stoichiometries of gene expression during myogenesis (14–19). Although these basic features have been known for many years, much remains to be understood about them. However, the identification of Mef2 as a key regulator of muscle gene expression and the more recent development of genomic methodologies provide the opportunity to dissect the mechanisms that underlie these phenomena.
Ideas for how a transcription factor might control programs of cell differentiation have been developed, for example, in the analysis of Hox gene function (20–23). It could directly activate many target genes required for the differentiation program, or it could function in a hierarchical system in which it directly regulates only a small number of genes, which then regulate the bulk of the required genes. In the case of Mef2, the former arrangement was suggested by the occurrence of the consensus DNA-binding site for Mef2 in the control regions of many muscle genes (4), together with the observation that ectopic expression of mef2 is capable of activating ectopic expression of a range of target genes (24–26). This mode of action has recently been strongly supported, and developed further, by two genomic ChIP studies that identified hundreds of mef2-regulated genes whose control elements bind Mef2 in vivo during Drosophila development (27, 28). Consistent with the expression of Mef2 from gastrulation to the end of embryogenesis (9), these genes display a range of expression profiles during muscle development. Among them, there are also groups of genes that are expressed together; for example, there is a burst of gene expression during the early phase of muscle differentiation. It is therefore apparent that to understand the genetic program of myogenesis it is necessary to understand how Mef2 can regulate an extensive array of target genes with diverse temporal expression patterns and, within this, how subsets of genes can be expressed together.
The importance of different levels of regulator proteins in patterning during early animal development is well documented (29, 30). However, much less is known about the possible quantitative requirements for transcription factors later in development when differentiation programs are operating. In muscle, two findings indicate that levels of key transcription factors contribute to the regulation of muscle development. First, the use of allelic combinations showed that skeletal muscle development in mice is sensitive to the levels of myogenin (31). Second, expression of different levels of a mef2 construct in a mef2-null background showed that distinct levels of Mef2 are required in Drosophila for different properties of a muscle (32). One explanation for these two findings is that different genes require different levels of the transcription factor for their expression. Here we have addressed this for Drosophila Mef2 by combining microarrays with Drosophila genetics to determine whether there are genes that respond differently to particular levels of mef2 activity in vivo. We found that some genes do indeed require higher levels for normal expression and that others only need lower levels. Moreover, this differential requirement for mef2 correlates with when the gene is first expressed during muscle development. These findings suggest one mechanism by which Mef2 can coordinate the expression of its many target genes during the muscle differentiation program.
Results
To investigate whether there are genes with different responses to particular levels of mef2 activity during muscle differentiation in vivo, we used microarrays in conjunction with a mef2 allelic series. The allelic series extends from a null mutation, mef222.21, through to the wild type via three hypomorphic alleles, mef2113, mef2424, and mef265 (10). The phenotype of each hypomorph corresponds to different levels of Mef2, because these phenotypes can be recapitulated by direct manipulation of Mef2 levels (32). mef2113 is the strongest of the hypomorph alleles corresponding to relatively low levels, and mef265 is the weakest corresponding to relatively high levels. This experimental design allowed us to determine the gene expression profile in developing embryos at five different levels of mef2 activity in the following order: wild type → 65 → 424 → 113 → 22.21 (Fig. 1). Throughout this article we use “levels of mef2 activity” to describe the total activity of the mef2 gene, which includes the level of expression and the activity of the expressed protein. For the microarrays, 30-min collections of wild-type and mutant embryos for each mef2 allele were individually staged, and pools of 150 embryos were then processed at mid stage 13. This corresponds to the early differentiation phase of muscle development and the expression of multiple muscle sarcomeric protein genes. Quadruplicate samples were assayed by using Affymetrix Genechips, and the results were analyzed as described in Materials and Methods.
Fig. 1.
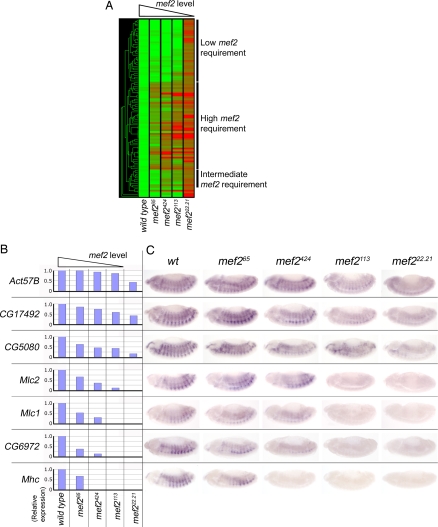
Different genes respond differently to changes in mef2 activity levels. (A) Hierarchical clustering of expression data for the 97 mef2-regulated genes selected as described. Columns show expression for each gene in wild-type, mef265, mef2424, mef2113, and mef222.21 embryos as indicated. Green represents genes with no change in expression from wild type, red represents genes with no detectable expression, and intermediate shades represent intermediate values. Labeled bars indicate gene cohorts with high, intermediate, and low requirements for mef2. (B) Histograms showing the expression array data for seven selected mef2-regulated genes (Act57B, CG17492, CG5080, Mlc2, Mlc1, CG6972, and Mhc) at stage 13 across the mef2 allelic series. (C) In situ hybridizations for the seven genes across the allelic series at stage 13 (anterior is to left and dorsal is to top in these and all other figures).
Different Muscle Genes Require Different Levels of mef2 Activity.
We assembled a list of 97 mef2-regulated genes. The availability of expression data across the mef2 allelic series enabled us to assign them with confidence. We included genes that were down-regulated by at least 2-fold in the mef2-null allele relative to wild type and whose expression shows a decreasing trend across the series (see Materials and Methods). The list contained many characterized muscle genes, e.g., Act57B, if, Mhc, Mlc1, Mlc2, Msp-300, up, nau, TpnC73F, TpnC47D, Scgα, and Tig. Hierarchical clustering of these 97 genes revealed a range of expression profiles across the mef2 allelic series, i.e., at different levels of mef2 activity (Fig. 1A). For example, genes at the top of the clustering output are expressed at almost wild-type levels in all of the hypomorph alleles and are significantly down-regulated only in the null mutant. They therefore need only relatively low levels of mef2 activity for normal expression. Other genes further down the figure display a relatively high requirement. They are significantly down-regulated even in the weakest hypomorph allele, mef265, which has the least reduced level of mef2 activity. There are also genes that display a variety of intermediate requirements for mef2. For example, some are expressed at normal levels in the wild type and mef265 allele but are down-regulated in the stronger hypomorph alleles. This expression profiling demonstrates that muscle genes do indeed respond differently to a given level of mef2 activity.
We then screened the 97 genes for those that had additional evidence implicating Mef2 in controlling their expression. We drew up a list of 16 genes with in situ hybridization results showing muscle-specific expression at the start of muscle differentiation (refs. 33 and 34 and data not shown) and that have one or more conserved Mef2 binding sites [supporting information (SI) Table 1]. From this list we selected a group of seven for detailed analysis that contained examples of high, intermediate, and low mef2-requirement genes identified in the hierarchical clustering. Each of these genes is ectopically expressed in response to ectopic expression of mef2 (SI Fig. 4) and also, for those present on the tiling array in a ChIP analysis, binds Mef2 in vivo (28). Taken together, these characteristics indicate that Mef2 is a major factor that determines the expression of this group of genes.
The pattern of expression of each of the seven genes was systematically analyzed by in situ hybridization with embryos from across the mef2 allelic series under carefully controlled conditions (see Materials and Methods). This both validated the microarray results and showed that each gene was expressed in the differentiating somatic muscle. The expression patterns (Fig. 1C) closely matched the trends of expression across the allelic series from the array data (Fig. 1B) and emphasize the different responses displayed by the seven genes and hence the data set as a whole. For example, both CG6972 and Act57B are strongly expressed in the wild type at stage 13. However, whereas the mef2113 allele has sufficient mef2 activity for Act57B to be expressed at almost wild-type levels, CG6972 expression is not detected in the mef2113 allele. Our findings suggest that these seven genes can be classified according to their requirement for mef2. Genes such as Act57B only require low levels of mef2 activity for normal expression, and genes such as CG6972 require higher levels. Thus, different Mef2 target genes respond very differently to a given level of mef2 activity in the same group of cells at the same stage of development. This is a significant step forward in understanding mef2 function in muscle differentiation.
The mef2 Requirement of Muscle Genes Correlates with the Start of Their Expression During Development.
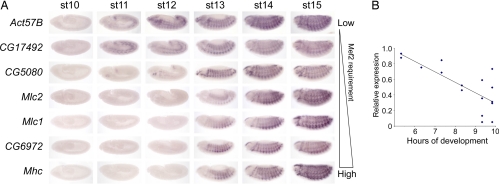
Having found that different muscle genes have different mef2 requirements, we then asked whether genes that respond to the level of mef2 activity in a particular way shared any characteristic. We investigated this by undertaking an in situ hybridization analysis of the seven genes analyzed in Fig. 1 to determine their gene expression profile during muscle development (Fig. 2A). This shows that the requirement of a gene for a certain mef2 activity level at stage 13 correlates with the start of its expression during development. Thus, genes such as Act57B and CG17492 that need only lower levels of mef2 activity, i.e., whose expression is hardly affected in the strong mef2113 hypomorph, are activated early in the muscle differentiation program (stage 11). Genes, such as CG6972 and Mhc, that are activated later in the program (stage 13) are those that require higher levels; i.e., their expression is affected even in the weak mef265 hypomorph. CG5080, a gene with an intermediate requirement for mef2, is first expressed between these two extremes at stage 12.
Fig. 2.
The mef2 requirement of target genes correlates with the start of their expression. (A) In situ hybridizations of wild-type embryos for Act57B, CG17492, CG5080, Mlc2, Mlc1, CG6972, and Mhc at different stages of development as indicated. Genes that only need low levels of mef2 activity (Act57B and CG17492) are at the top, and those that require the highest levels (CG6972 and Mhc) are at the bottom. (B) Graph, with fitted line from linear regression, showing the correlation between the requirement for mef2 and when the gene is first activated for 16 genes known to be expressed specifically in muscle at stage 13. The requirement for mef2 is taken as the expression of each gene at stage 13 in mef2424 embryos relative to wild type. The start of expression was compiled from in situ hybridizations (A) (refs. 33 and 34 and data not shown).
To determine whether this correlation is true for other genes, we compared the requirement for mef2 with the stage of first expression for our list of 16 genes. As a measure of mef2 requirement we used the expression at stage 13 in the mef2424 allele relative to wild type. When this is plotted against the time of onset of expression (Fig. 2A, data not shown, and refs. 33 and 34), again there is a clear trend (Fig. 2B). Genes that have the higher requirements for mef2 are activated at later stages. Together these findings suggest that mef2 influences the temporal expression of these genes and that this may be a widespread characteristic of muscle genes at this stage of development.
Manipulation of mef2 Activity Levels Can Modulate the Timing of Muscle Gene Expression.
If the time of the initiation of Mef2 target gene expression during muscle development depends on its response to a given level of mef2 activity, then changing the levels should alter the time of gene activation in a predictable manner. To test this we first looked at the effect of decreasing the level by using the mef2113 hypomorphic mutant and assessing gene expression relative to wild type in carefully staged embryos. There was a delay in the initiation of gene expression, as shown by in situ hybridization for CG6972, CG5080, and Act57B (Fig. 3A, compare the bottom two rows of embryos for each gene). These expression profiles were confirmed, and the relative transcript levels in these genetic backgrounds were measured, by using quantitative RT-PCR; this demonstrated a quantitatively significant delay in expression (Fig. 3A). Then we increased the level using the Gal4-UAS system to overexpress mef2 in the developing muscle cells using twist-Gal4. This resulted in premature initiation of expression of CG6972 and CG5080 (compare the top two rows in Fig. 3A for each). However, we did not find this for Act57B, and we interpret this as a requirement for another factor(s) in this case. In summary, using genetic manipulations, we could show that mef2 activity levels directly influence the temporal expression of Mef2 target genes in vivo during muscle development. We found that the start of expression of a given Mef2 target gene can be advanced by increases in mef2 activity levels and delayed by decreases in them. Taken together, these findings suggest a model whereby mef2 activity levels directly influence the initiation of target gene expression. One simple arrangement that could achieve this is the activation of expression of specific genes at particular thresholds of mef2 activity.
Fig. 3.
Increased or decreased levels of mef2 activity lead to premature or delayed expression of target genes, respectively. (A) In situ hybridization images of wild-type, twistGal4;twistGal4 > UAS-mef2, and mef2113 embryos for CG6972, CG5080, and Act57B at different stages of development as indicated. CG6972 and CG5080 expression is premature when mef2 is overexpressed. CG6972, CG5080, and Act57B expression is delayed when mef2 activity is reduced. (B) Quantitative RT-PCR analysis of CG6972, CG5080, and Act57B transcript levels at the indicated stages. Expression relative to stage 13 levels in the wild type is shown as means from triplicate experiments. Red stars denote values that differ by >1 SD in UAS-mef2 embryos relative to wild type, and blue stars denote values that differ by >1 SD in mef2113 embryos relative to wild type.
Discussion
An important question for understanding Mef2 function, and more generally the orchestration of genetic programs during development, is how hundreds of genes are regulated in a controlled way by a single transcription factor to coordinate the complex process of differentiation. In this study we have uncovered a facet of Mef2 function that offers one explanation for this. We found that Mef2 is not an all-or-none regulator. Rather, its action is more subtle, and levels of its activity differentially affect the expression of muscle genes. By combining Drosophila genetics with DNA microarrays, we found that groups of muscle genes have different expression profiles across a mef2 allelic series. The phenotypes of the mef2 hypomorphs in this allelic series correspond to different levels of Mef2 protein (32). Moreover, Western and immunohistochemistry analyses show that these alleles actually express different levels of the Mef2 protein, although there may also be additional effects on its activity (10). We have therefore referred to “levels of mef2 activity” to include both of these aspects. Regardless of how changes in the total levels of mef2 activity occur, our findings demonstrate that Mef2 can affect different muscle genes independently. Some require higher levels for their expression than others. This is a significant step forward in understanding the role of this transcription factor in the regulation of the genetic program of muscle differentiation. Furthermore, this differential requirement for mef2 correlates with when the gene is first expressed in this program, suggesting that levels of mef2 activity are implicated in temporal regulation. In support of this, we found that increased or decreased levels could lead to premature or delayed transcription of target genes, respectively.
A model for muscle development during Drosophila embryogenesis is suggested by these findings in which the level of mef2 activity increases and results in the sequential activation of Mef2 target genes: those that only need low levels would be expressed early in myogenesis, and those that require higher levels would be expressed later. An increase in the total level of mef2 activity during muscle development could in principle be achieved by two routes, and there is evidence for both. First, it could be more highly expressed and consistent with this, mef2 transcript levels increase during the early phase of muscle development (34). Second, there could be an increase in the effective activity of the Mef2 protein. One possible route is through the decrease in the expression of the Mef2 inhibitor Him during the period when many of the genes analyzed in this study are first expressed (35). Other regulators may also contribute to changes in Mef2 activity during Drosophila development because studies of mammalian Mef2 show that it is subject to an array of regulatory modifications (36–39).
The use of Mef2 in this way to regulate muscle genes during differentiation represents a simple mechanism to coordinate the relative timings and expression of these genes and offers an explanation for a number of features of the muscle differentiation program. For example, the existence of temporal patterns of muscle gene expression is long established (11–13), and our findings indicate how batteries of muscle genes might be expressed together. These would be genes that have a similar requirement for a particular level of mef2 activity. Our model can also explain the sequential activation of different genes, because those activated at low thresholds of mef2 activity would be expressed before genes that are activated at higher thresholds. Last, this temporal regulation by Mef2 may also contribute to the absolute level and relative stoichiometry of expression of sarcomeric proteins, which is known to be important in myogenesis (14–18).
Generally in animal development the importance of the level of regulators has been explored in early patterning events. However, an example later in development is the Caenorhabditis elegans PHA-4 transcription factor (40). It regulates the expression of a large number of target genes activated at different times throughout pharynx organogenesis, and a key feature in current understanding is an increase in PHA-4 levels that sequentially activates different targets. This parallels our findings with Mef2 in the muscle differentiation program. Nevertheless, other mechanisms may also contribute to temporal programs of muscle gene expression. For example, in a mammalian muscle cell culture model there is a MyoD-activated feed-forward circuit (41). Moreover, even though Pha4 has a dominant role in pharynx development, other factors modulate its action (42), and it is very likely that there are also additional inputs for Mef2 and muscle. Indeed, it has been suggested that the helix–loop–helix transcription factor Twist modulates Mef2 action, albeit on a cohort of genes expressed earlier than those we analyzed (28). Further progress on the orchestration of muscle development by Mef2 will require analysis of the mechanism(s) by which Mef2 differentially activates target gene expression, which in turn will necessitate studies of these other putative factors and the enhancer architecture of muscle genes.
In summary, our findings highlight a previously undescribed aspect to understanding muscle development and suggest one mechanism by which Mef2 can orchestrate multiple events in muscle differentiation. Rather than Mef2 working as a simple on/off switch of muscle differentiation, our work leads to a model in which the level of mef2 activity increases during muscle differentiation and results in the sequential activation of muscle genes.
Materials and Methods
Drosophila Genetics.
The stocks used were mef265 (10), mef2424 (10), mef2113 (10), mef222.21 (8), UAS-mef2 (8), da-Gal4, 69B-Gal4, and twi-Gal4; twi-Gal4 (43). For mutant selection by the absence of GFP in the microarray and quantitative RT-PCR analyses, the mef2 alleles were balanced over CyO P(Gal4-twi)P(UAS-2eGFP) (44). For mutant selection by absence of lacZ for in situ hybridization, the mef2 alleles were balanced over CyO ftzlacZ. All Gal4-UAS crosses were at 25°C. The wild-type stock for the microarray analysis and the in situ hybridizations in Fig. 1 was dp cn a px sp (the stock used for the mutagenesis that produced mef265, mef2424, and mef2113 and kindly provided by Janis O'Donnell, University of Alabama, Tuscaloosa, AL). The wild type for the in situ hybridization analyses in Figs. 2 and 3 and SI Fig. 4 was Oregon R.
Microarray Analysis.
After two 1-h prelays, 30-min embryo collections were aged at 25°C for 6.5 h before sorting. Mutant embryos were individually selected by the absence of the GFP balancer chromosome. Both mutant and wild-type embryos were accurately staged at mid 12 by using autofluorescence to visualize the germ band to ensure that all were within a 30-min window. Embryos were allowed to develop until mid stage 13, dechorionated, inspected to ensure that normal development had continued, and immediately homogenized in TRIzol before storage at −80°C. RNA was isolated, labeled, hybridized to Affymetrix Genechip 1 arrays, and scanned by the Flychip Drosophila microarray resource (www.flychip.org.uk). Data were supplied in MAS5.0 normalized form and analyzed by using Genespring software (Agilent Technologies). A total of 97 mef2-regulated genes (available on request) were selected by using the following criteria: (i) four of four measurements flagged present in wild-type embryos; (ii) >2-fold down-regulation in the mef222.21 allele relative to wild type or flagged absent in the mef222.21 allele; (iii) expressed with a decreasing trend across the allelic series (decreases, or increases by <1.5-fold, between each successively stronger allele); (iv) mutant alleles show statistically significant differences relative to wild type by one-way ANOVA (P < 0.05). Hierarchical clustering used the Gene Tree function of Genespring with the standard correlation similarity measure.
Phylogenetic Footprinting.
Precomputed pairwise Drosophila melanogaster–Drosophila virilis (SLAGAN) alignments were viewed by using VISTA (45) (http://genome.lbl.gov/vista/index.shtml). Mef2 sites were identified by using the degenerate consensus YTAWWWWTAR (8).
In Situ Hybridization.
For RNA probe synthesis, the following cDNA clones were obtained from the Drosophila Genomics Resource Center: CG5080, clone LD34147; CG17492, clone GH28686; CG6972, clone RH25557; Mlc1, clone RE07220; Mlc2, clone RE35841; Mhc, clone LD31809; Act57B, clone LD04994. In situ hybridizations and antibody stainings were undertaken as previously described (26). The responses to mef2 activity levels were analyzed by comparing embryos processed in parallel using common reagents and the same incubation times. At least 10 embryos for each genetic condition at each stage were analyzed, and representative images are shown.
Quantitative RT-PCR.
Embryos from 1-h collections were individually inspected and sorted to ensure that all were the correct stage. For mutant selection they were also sorted for the absence of GFP fluorescence at mid stage 12. Embryos were allowed to develop at 25°C to the appropriate stage and rinsed before snap freezing. Embryos were homogenized, and total RNA was isolated by using an RNeasy protect mini kit (Qiagen). First-strand cDNA was primed with poly d(T) and used SuperScript III (Invitrogen) under the manufacturer's standard conditions. Quantitative PCR used SYBR green (Bio-Rad) in the manufacturer's standard conditions in a Chromo4 instrument (MJ Research). Cycle conditions were as follows: 95°C for 15 min, 35 × 95°C for 30 s, 60°C for 30 s, 72°C for 30 s, and 82°C for 30 s. Measurements in triplicate were made by using rp49 as the reference, and mean results were plotted as 2-ΔΔC(T) relative to the peak level of expression in the wild type (46).
Supplementary Material
ACKNOWLEDGMENTS.
We thank J. O'Donnell, A. Michelson (National Institutes of Health, Bethesda), S. Abmayr (Stowers Institute, Kansas City, MO), M. Baylies (Sloan–Kettering Institute, New York), G. Ranganayakulu (University of Texas Southwestern Medical Center, Dallas), and R. Schulz (M. D. Anderson Cancer Center, Houston) for kindly providing Drosophila stocks; Flychip for expert processing of the microarrays; and P. Kille, D. Liotta, and D. Hancock for discussions. This research was supported by the Biotechnology and Biological Sciences Research Council, the John Ryder Memorial Trust, and the European Union FP6 Network of Excellence MYORES.
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE9889).
This article contains supporting information online at www.pnas.org/cgi/content/full/0711255105/DC1.
References
- 1.Britten RJ, Davidson EH. Gene regulation for higher cells: A theory. Science. 1969;165:349–357. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- 2.Davidson EH, Britten RJ. Molecular aspects of gene regulation in animal cells. Cancer Res. 1974;34:2034–2043. [PubMed] [Google Scholar]
- 3.Garcia-Bellido A. Genetic control of wing disc development in Drosophila. Ciba Found Symp. 1975:161–182. doi: 10.1002/9780470720110.ch8. [DOI] [PubMed] [Google Scholar]
- 4.Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol. 1998;14:167–196. doi: 10.1146/annurev.cellbio.14.1.167. [DOI] [PubMed] [Google Scholar]
- 5.Gossett LA, Kelvin DJ, Sternberg EA, Olson EN. A new myocyte-specific enhancer-binding factor that recognizes a conserved element associated with multiple muscle-specific genes. Mol Cell Biol. 1989;9:5022–5033. doi: 10.1128/mcb.9.11.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollock R, Treisman R. Human SRF-related proteins: DNA-binding properties and potential regulatory targets. Genes Dev. 1991;5:2327–2341. doi: 10.1101/gad.5.12a.2327. [DOI] [PubMed] [Google Scholar]
- 7.Yu YT, et al. Human myocyte-specific enhancer factor 2 comprises a group of tissue-restricted MADS box transcription factors. Genes Dev. 1992;6:1783–1798. doi: 10.1101/gad.6.9.1783. [DOI] [PubMed] [Google Scholar]
- 8.Bour BA, et al. Drosophila MEF2, a transcription factor that is essential for myogenesis. Genes Dev. 1995;9:730–741. doi: 10.1101/gad.9.6.730. [DOI] [PubMed] [Google Scholar]
- 9.Lilly B, et al. Requirement of MADS domain transcription factor D-MEF2 for muscle formation in Drosophila. Science. 1995;267:688–693. doi: 10.1126/science.7839146. [DOI] [PubMed] [Google Scholar]
- 10.Ranganayakulu G, et al. A series of mutations in the D-MEF2 transcription factor reveal multiple functions in larval and adult myogenesis in Drosophila. Dev Biol. 1995;171:169–181. doi: 10.1006/dbio.1995.1269. [DOI] [PubMed] [Google Scholar]
- 11.Devlin RB, Emerson CP., Jr Coordinate regulation of contractile protein synthesis during myoblast differentiation. Cell. 1978;13:599–611. doi: 10.1016/0092-8674(78)90211-8. [DOI] [PubMed] [Google Scholar]
- 12.Hastings KE, Emerson CP., Jr cDNA clone analysis of six co-regulated mRNAs encoding skeletal muscle contractile proteins. Proc Natl Acad Sci USA. 1982;79:1553–1557. doi: 10.1073/pnas.79.5.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckingham M, et al. Expression of muscle genes in the mouse embryo. Symp Soc Exp Biol. 1992;46:203–217. [PubMed] [Google Scholar]
- 14.Karlik CC, Fyrberg EA. An insertion within a variably spliced Drosophila tropomyosin gene blocks accumulation of only one encoded isoform. Cell. 1985;41:57–66. doi: 10.1016/0092-8674(85)90061-3. [DOI] [PubMed] [Google Scholar]
- 15.Mogami K, O'Donnell PT, Bernstein SI, Wright TR, Emerson CP., Jr Mutations of the Drosophila myosin heavy-chain gene: Effects on transcription, myosin accumulation, and muscle function. Proc Natl Acad Sci USA. 1986;83:1393–1397. doi: 10.1073/pnas.83.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beall CJ, Sepanski MA, Fyrberg EA. Genetic dissection of Drosophila myofibril formation: Effects of actin and myosin heavy chain null alleles. Genes Dev. 1989;3:131–140. doi: 10.1101/gad.3.2.131. [DOI] [PubMed] [Google Scholar]
- 17.Warmke JW, Kreuz AJ, Falkenthal S. Co-localization to chromosome bands 99E1-3 of the Drosophila melanogaster myosin light chain-2 gene and a haplo-insufficient locus that affects flight behavior. Genetics. 1989;122:139–151. doi: 10.1093/genetics/122.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cripps RM, et al. Transformation of Drosophila melanogaster with the wild-type myosin heavy-chain gene: Rescue of mutant phenotypes and analysis of defects caused by overexpression. J Cell Biol. 1994;126:689–699. doi: 10.1083/jcb.126.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marco-Ferreres R, Arredondo JJ, Fraile B, Cervera M. Overexpression of troponin T in Drosophila muscles causes a decrease in the levels of thin-filament proteins. Biochem J. 2005;386:145–152. doi: 10.1042/BJ20041240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weatherbee SD, Halder G, Kim J, Hudson A, Carroll S. Ultrabithorax regulates genes at several levels of the wing-patterning hierarchy to shape the development of the Drosophila haltere. Genes Dev. 1998;12:1474–1482. doi: 10.1101/gad.12.10.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovegrove B, et al. Coordinated control of cell adhesion, polarity, and cytoskeleton underlies Hox-induced organogenesis in Drosophila. Curr Biol. 2006;16:2206–2216. doi: 10.1016/j.cub.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 22.Hersh BM, et al. The UBX-regulated network in the haltere imaginal disc of D. melanogaster. Dev Biol. 2007;302:717–727. doi: 10.1016/j.ydbio.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hueber SD, et al. Comparative analysis of Hox downstream genes in Drosophila. Development. 2007;134:381–392. doi: 10.1242/dev.02746. [DOI] [PubMed] [Google Scholar]
- 24.Lin MH, Bour BA, Abmayr SM, Storti RV. Ectopic expression of MEF2 in the epidermis induces epidermal expression of muscle genes and abnormal muscle development in Drosophila. Dev Biol. 1997;182:240–255. doi: 10.1006/dbio.1996.8484. [DOI] [PubMed] [Google Scholar]
- 25.Stronach BE, Renfranz PJ, Lilly B, Beckerle MC. Muscle LIM proteins are associated with muscle sarcomeres and require dMEF2 for their expression during Drosophila myogenesis. Mol Biol Cell. 1999;10:2329–2342. doi: 10.1091/mbc.10.7.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor MV. A novel Drosophila, mef2-regulated muscle gene isolated in a subtractive hybridization-based molecular screen using small amounts of zygotic mutant RNA. Dev Biol. 2000;220:37–52. doi: 10.1006/dbio.2000.9608. [DOI] [PubMed] [Google Scholar]
- 27.Junion G, et al. Mapping Dmef2-binding regulatory modules by using a ChIP-enriched in silico targets approach. Proc Natl Acad Sci USA. 2005;102:18479–18484. doi: 10.1073/pnas.0507030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandmann T, et al. A temporal map of transcription factor activity: mef2 directly regulates target genes at all stages of muscle development. Dev Cell. 2006;10:797–807. doi: 10.1016/j.devcel.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Gurdon JB, Dyson S, St Johnston D. Cells' perception of position in a concentration gradient. Cell. 1998;95:159–162. doi: 10.1016/s0092-8674(00)81747-x. [DOI] [PubMed] [Google Scholar]
- 30.Ashe HL, Briscoe J. The interpretation of morphogen gradients. Development. 2006;133:385–394. doi: 10.1242/dev.02238. [DOI] [PubMed] [Google Scholar]
- 31.Vivian JL, Gan L, Olson EN, Klein WH. A hypomorphic myogenin allele reveals distinct myogenin expression levels required for viability, skeletal muscle development, and sternum formation. Dev Biol. 1999;208:44–55. doi: 10.1006/dbio.1998.9182. [DOI] [PubMed] [Google Scholar]
- 32.Gunthorpe D, Beatty KE, Taylor MV. Different levels, but not different isoforms, of the Drosophila transcription factor DMEF2 affect distinct aspects of muscle differentiation. Dev Biol. 1999;215:130–145. doi: 10.1006/dbio.1999.9449. [DOI] [PubMed] [Google Scholar]
- 33.Michelson AM, Abmayr SM, Bate M, Arias AM, Maniatis T. Expression of a MyoD family member prefigures muscle pattern in Drosophila embryos. Genes Dev. 1990;4:2086–2097. doi: 10.1101/gad.4.12a.2086. [DOI] [PubMed] [Google Scholar]
- 34.Tomancak P, et al. Systematic determination of patterns of gene expression during Drosophila embryogenesis. Genome Biol. 2002;3:RESEARCH0088. doi: 10.1186/gb-2002-3-12-research0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liotta D, et al. The Him gene reveals a balance of inputs controlling muscle differentiation in Drosophila. Curr Biol. 2007;17:1409–1413. doi: 10.1016/j.cub.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ornatsky OI, et al. Post-translational control of the MEF2A transcriptional regulatory protein. Nucleic Acids Res. 1999;27:2646–2654. doi: 10.1093/nar/27.13.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao M, et al. Regulation of the MEF2 family of transcription factors by p38. Mol Cell Biol. 1999;19:21–30. doi: 10.1128/mcb.19.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shalizi A, et al. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science. 2006;311:1012–1017. doi: 10.1126/science.1122513. [DOI] [PubMed] [Google Scholar]
- 39.Flavell SW, et al. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- 40.Gaudet J, Mango SE. Regulation of organogenesis by the Caenorhabditis elegans FoxA protein PHA-4. Science. 2002;295:821–825. doi: 10.1126/science.1065175. [DOI] [PubMed] [Google Scholar]
- 41.Penn BH, Bergstrom DA, Dilworth FJ, Bengal E, Tapscott SJ. A MyoD-generated feed-forward circuit temporally patterns gene expression during skeletal muscle differentiation. Genes Dev. 2004;18:2348–2353. doi: 10.1101/gad.1234304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Updike DL, Mango SE. Temporal regulation of foregut development by HTZ-1/H2A.Z and PHA-4/FoxA. PLoS Genet. 2006;2:e161. doi: 10.1371/journal.pgen.0020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baylies MK, Bate M. twist: A myogenic switch in Drosophila. Science. 1996;272:1481–1484. doi: 10.1126/science.272.5267.1481. [DOI] [PubMed] [Google Scholar]
- 44.Halfon MS, et al. New fluorescent protein reporters for use with the Drosophila Gal4 expression system and for vital detection of balancer chromosomes. Genesis. 2002;34:135–138. doi: 10.1002/gene.10136. [DOI] [PubMed] [Google Scholar]
- 45.Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004;32:W273–W279. doi: 10.1093/nar/gkh458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR, the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.