Abstract
We have identified a novel β amyloid precursor protein (βAPP) mutation (V715M-βAPP770) that cosegregates with early-onset Alzheimer’s disease (AD) in a pedigree. Unlike other familial AD-linked βAPP mutations reported to date, overexpression of V715M-βAPP in human HEK293 cells and murine neurons reduces total Aβ production and increases the recovery of the physiologically secreted product, APPα. V715M-βAPP significantly reduces Aβ40 secretion without affecting Aβ42 production in HEK293 cells. However, a marked increase in N-terminally truncated Aβ ending at position 42 (x-42Aβ) is observed, whereas its counterpart x-40Aβ is not affected. These results suggest that, in some cases, familial AD may be associated with a reduction in the overall production of Aβ but may be caused by increased production of truncated forms of Aβ ending at the 42 position.
A subset of early-onset cases of Alzheimer’s disease (AD) is due to autosomal dominant mutations identified on the β amyloid precursor protein (βAPP), presenilin 1, and presenilin 2, the gene products of chromosomes 21, 14, and 1, respectively (1–3). To date, the common phenotype of these familial AD (FAD)-linked mutations was the exacerbation of the production of Aβ and, particularly, its readily aggregatable and pathogenic 42-aa-long species (for reviews see refs. 4 and 5). Here we report on the identification of a novel βAPP mutation (V715M-βAPP) likely responsible for probable early-onset AD that triggers unusual alterations of βAPP processing. Thus, Aβ production appears drastically lowered whereas the physiological product of βAPP processing, APPα, is increased. Our data suggest that the overall amount of Aβ or increase of Aβ42 secretion is not, per se, always sufficient to explain all FAD-linked neuropathologies. The important increase of N-terminally truncated Aβ products ending at the 42 position produced by V715M-βAPP-expressing cells indicates that these x-42 species likely contribute to the development of the neurodegenerative disease in V715M-βAPP-bearing patients.
EXPERIMENTAL PROCEDURES
Antibodies and Epitope Mapping.
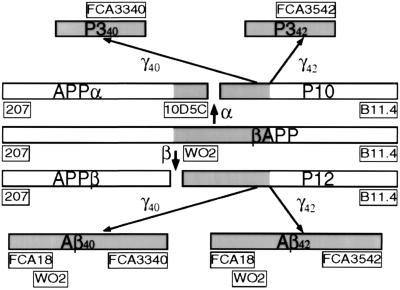
All the antibodies used in the present work and the corresponding epitopes that are recognized are shown in Fig. 1.
Figure 1.
Organization of βAPP and mapping of epitopes recognized by antibodies. α, α-secretase; β, β-secretase; γ40, γ-secretase acting at the 40th aa of Aβ; γ42, γ-secretase acting at the 42nd aa of Aβ. Antibodies are shown in boxes.
Pedigree Description.
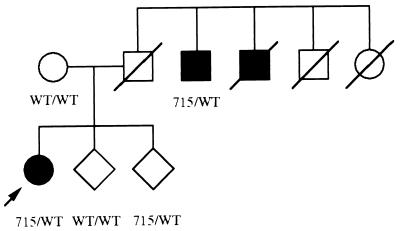
Family 074 is a two-generation family of Italian origin with three affected subjects fulfilling the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association criteria for probable Alzheimer’s disease (6). The proband is a 44-year-old woman with a progressive and insidious history of memory decline over a 3-year period. Her father was dead by age 40 from an accident. Two paternal uncles also developed dementia at ages 60 and 52, respectively (see the pedigree drawing in Fig. 2). The duration of survival from symptom onset to death is 14 years in the first affected relative. The second patient is still alive after 13 years of symptom duration. The ApoE genotypes of the proband and his affected uncle were 3/3 and 2/4, respectively. In all affected subjects, memory problems are associated with apraxia, aphasia, and agnosia. Neurological examination otherwise is normal.
Figure 2.
Partial pedigree of the 074 family with the V715M mutation. Solid symbols indicate affected individuals. Arrow denotes the propositus. WT, wild type; 715, V715M mutation.
Genetic Analysis.
In proband and available relatives, exon 17 of the APP gene was sequenced by using the intronic primers described by Fidani et al. (7) with an M13 reverse sequence and an M13–21 sequence added to the 5′ ends of the sense and antisense primers, respectively. The PCR conditions were similar to those described previously (8). PCR products were purified by electrophoresis on low-melt agarose gel and sequenced directly on both strands using the PRISM Ready Reaction Dye Primer sequencing kit (Applied Biosystems; Perkin–Elmer) and an Applied Biosystems model 373A automated sequencer. DNA analysis revealed a heterozygote GTG → ATG substitution at codon 715 (βAPP770 numbering), changing the predicted amino acid valine to methionine in affected subjects.
Mutagenesis.
Oligonucleotide-directed mutagenesis was performed according to the uracylated, single-strand strategy directly on pcDNA3 containing wild-type (wt)-βAPP751. The antisense oligonucleotide used to obtain the V696M-βAPP751 (corresponding to the V715M-βAPP770) was 5′-ATGACGATCATTGTCGCTA-3′. The sequencing of whole βAPP cDNA confirmed the only presence of the expected mutation.
Cell Culture, Transfections, and Detection of βAPP.
HEK293 cells were cultured as described previously (9) and stably transfected by calcium phosphate precipitation with pcDNA3, empty or containing wt-βAPP, Sw-βAPP, or the V715M-βAPP. The presence of the V715M and Swedish mutations was confirmed after sequence analysis of PCR products (by means of adequate primers surrounding the mutation region) of cDNA obtained by reverse transcription from total cellular RNA prepared from transfectant cells.
TSM1 neuronal cells were established and cultured as described (10, 11) and transiently transfected with lipofectamine reagent (GIBCO/BRL) with pcDNA3, empty or containing wt-βAPP, Sw-βAPP, or V715M-βAPP.
Cells were homogenized and analyzed for their βAPP content by means of WO2 (0.12 μg/ml) as described previously (11, 12).
Metabolic Labeling and Detection of Secreted Aβ40, Aβ42, and APPα.
Cells were metabolically labeled in the presence of phosphoramidon (10 μM) and analyzed for Aβ42 and Aβ40 production by sequential immunoprecipitation with a 350-fold dilution of FCA3542 and FCA3340 (see Fig. 1). Tris-tricine gel analyses, radioautography, and then densitometric analyses were performed by PhosphorImager (Fuji) as described previously (13). Total sAPP (APPα/β) was quantified after immunoprecipitation with a 1,000-fold dilution of mAb207 (see Fig. 1), SDS/PAGE, protein transfer on nitrocellulose, and direct radioautography. APPα was quantified from the same nitrocellulose by Western blot analysis with a 200-fold dilution of mAb 10D5C (see Fig. 1) as described previously (14).
Detection of Total Intracellular Aβ, p10, and p12.
Cells were labeled metabolically as above and then scraped, rinsed in 1× PBS (Quantum, Durham, NC), and lysed in 1× RIPA buffer containing detergents (10 mM Tris⋅HCl/150 mM NaCl/5 mM EDTA/0.1% SDS/0.5% deoxycholic acid/1% Nonidet P-40). Cellular lysates were centrifuged and the resulting supernatants were incubated overnight with a 350-fold dilution of FCA18 (see Fig. 1) and protein A-Sepharose (total Aβ and p12) or with a 1,000-fold dilution of B11.4 antibody [detection of p10 and p12 (see Fig. 1)]. Immunoprecipitates were resuspended with loading buffer, heated at 95°C, and then submitted to a 16.5% Tris-tricine electrophoresis and analyzed as in ref. 11.
Detection of Total Aβ by Western Blot Analysis.
Conditioned media were treated overnight with a 350-fold dilution of FCA18 in the presence of protein A-Sepharose, and immunoprecipitated proteins were submitted to Tris-tricine gels and Western blotted as above. The nitrocellulose membrane was heated in boiling PBS for 5 min to enhance the signal and capped with 5% skim milk in PBS containing 0.05% Tween 20 (PBS-Tween buffer) for 15–30 min. Membranes then were exposed overnight with WO2 antibody (1 μg/ml) in 1% skim milk PBS-Tween buffer, and total Aβ was quantified by enhanced chemiluminescence as described for βAPP.
RESULTS
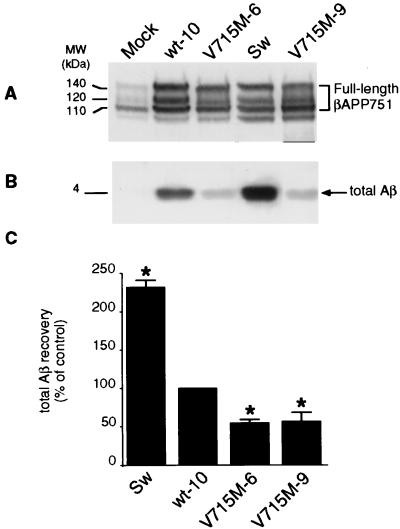
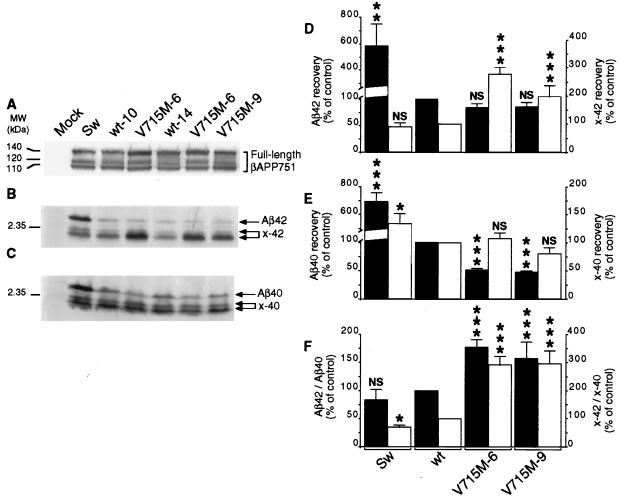
Overexpression of V715M-βAPP in independent HEK293 clones (Fig. 3A) leads to a 43–46% decrease in total Aβ immunoreactivity when compared with wt-βAPP-expressing cells (Fig. 3 B and C). Metabolic labeling and immunoprecipitation indicated a very similar 46–56% reduction in the recovery of radiolabeled Aβ (data not shown). This very unusual FAD-linked inhibition of Aβ production prompted us to examine the Aβ species, the formation of which could be influenced by the mutation. By means of recently developed selective Aβ antibodies (15), we demonstrate that wt-βAPP- and V715M-βAPP-expressing cells secrete virtually equal amounts of Aβ42 (Fig. 4 B and D) and that the decrease of total secreted Aβ is due to the lowering of the Aβ40 species (49–52% of decrease, P < 0.001; see Fig. 4 C and E). Interestingly, it should be noted that although the total amount of Aβ is lowered significantly, the ratio of Aβ42 to Aβ40 is increased by the V715M mutation (157–177% of control, P < 0.001; see Fig. 4F). Control experiments performed in identical conditions indicate that HEK293 cells expressing the Sw-βAPP secrete higher amounts of Aβ than wt-βAPP-expressing cells (232% of control, P < 0.001; see Fig. 3B) and that the Swedish mutation drastically increases the secretion of both Aβ42 (Fig. 4 B and D) and Aβ40 (Fig. 4 C and E) without significantly affecting the Aβ42/Aβ40 ratio (Fig. 4F).
Figure 3.
Effect of the V715M-βAPP mutation on the secretion of total Aβ by HEK293 cells. Stably transfected HEK293 cells overexpressing wild-type βAPP (wt-10 clone), V715M-βAPP (two clones, V715M-6 and V715M-9), or Swedish mutated (Sw)-βAPP were obtained and cultured as described in Experimental Procedures. βAPP expression in cell lysates was revealed with WO2 (A). Total secreted Aβ (B) was immunoprecipitated (with FCA18 antibody), submitted to SDS/PAGE and Western blot analysis (with mAbWO2), and then analyzed as described in Experimental Procedures. Bars in C correspond to densitometric analyses of secreted Aβ (normalized to the amount of endogenous βAPP content) and are the means ± SEM of three to four independent determinations. Mock indicates HEK293 cells stably transfected with the empty pcDNA3 vector. ∗, P < 0.001 (versus wt-10 cells).
Figure 4.
Effect of the V715M-βAPP mutation on the secretion of Aβ40 and Aβ42 and their x-40/42-related products by HEK293 cells. Stably transfected HEK293 cells are as in Fig. 3 and metabolically labeled as described in Experimental Procedures. Expression of βAPP revealed as in Fig. 3 is shown A. Secreted Aβ42/x-42 (B) and Aβ40/x-40 (C) were obtained after sequential immunoprecipitation by means of FCA3542 and FCA3340, respectively, and quantified after radioautography and densitometric analysis. Bars in D and E represent the densitometric analysis, normalized to the amount of endogenous βAPP content, of Aβ42 or Aβ40 (solid bars) and x-42 or x-40 (open bars) and are expressed as the percentage of those obtained with wt-βAPP751-expressing cells taken as 100 (note the distinct scales of ordinates). Values are the means ± SEM of three to eight independent experiments. F illustrates the ratios of Aβ42/Aβ40 (solid bars) and x-42/x-40 (open bars). Mock indicates HEK293 cells stably transfected with the empty vector pcDNA3. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001; NS, nonstatistically significant (versus corresponding wt).
Our resolved SDS/PAGE analysis allows the detection of a doublet protein of lower molecular weights reacting with FCA3542 (Fig. 4B) and FCA3340 (Fig. 4C) but not with FCA18 [not shown, but see Figs. 3 and 5 for secreted and intracellular Aβ detection by means of the N-terminally directed FCA18 (see Fig. 1) that does not reveal any truncated Aβ-related species at lower molecular weights]. Therefore, although not definitely identified, these Aβ-related products likely correspond to the previously reported N-terminally truncated Aβ11–40 and Aβ17–40 and their 11/17-Aβ42 counterparts. Two independent V715M-βAPP-expressing HEK293 clones elicit selective increases of x-42 (197–227% of control, P < 0.001; see Fig. 4 B and D) but not x-40 (Fig. 4 C and E) N-terminally truncated Aβ species. This is illustrated further by the drastic augmentation of the x-42/x-40 ratio (Fig. 4F). It is noteworthy that the production of the x-42 species was not affected by the Swedish mutation (Fig. 4 B and D).
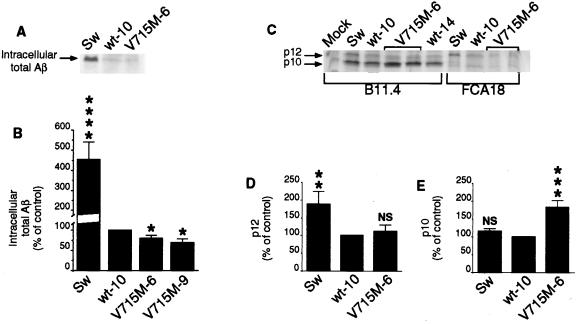
Figure 5.
Effect of the V715M-βAPP mutation on the intracellular formation of total Aβ, p10, and p12. The indicated transfectants were obtained and metabolically labeled as in Figs. 3 and 4. Cells were lysed and centrifuged, and then supernatants were treated with FCA18 (A and C) or B11.4 (C) antibodies and analyzed as in Fig. 4. Quantification of total intracellular Aβ (B), p12 (D), and p10 (E) was performed as in Fig. 4. Values are expressed as the percentage of those obtained with wt-βAPP-expressing cells (taken as 100) and are the means ± SEM of three to four independent experiments. ∗, P < 0.1; ∗∗, P < 0.05; ∗∗∗, P < 0.01; ∗∗∗∗, P < 0.001; NS, nonstatistically significant (versus corresponding wt-10).
We examined further the influence of the V715M mutation on the intracellular production of Aβ as well as on the recovery of p12 and p10, the C-terminal proteolytic products of βAPP derived from a unique cleavage by β- or α-secretases, respectively (for review, see ref. 4). Intracellular detection of Aβ is low but clearly quantitative in wt-βAPP-expressing cells. Two independent clones expressing V715M-βAPP produce statistically significantly lower amounts of intracellular Aβ (79 and 69% of the Aβ produced by wt-βAPP-expressing cells) whereas the Swedish mutation-bearing cells exhibit 4-fold more Aβ (P < 0.001) than the latter cells (Fig. 5 A and B).
The β-secretase-derived product p12 was monitored by means of B11.4 (anti-C terminus of βAPP; see Fig. 1) and FCA18 (that recognizes only intact free N terminus of Aβ; see Fig. 1 and ref. 15). Therefore, the genuine p12 should react with both antibodies. Indeed, an intracellular protein immunoprecipitated by B11.4 and FCA18 and migrating at the expected 12-kDa molecular mass was detected (Fig. 5C), the production of which was not influenced significantly by the V715M mutation (Fig. 5D). As expected, the β-secretase cleavage-derived p12 formation is augmented by the Swedish mutation (188% of control, P < 0.05; see Fig. 5 C and D). A 10-kDa protein also was detected with B11.4 but not with FCA18 (Fig. 5C). The latter data indicating the lack of the N terminus of Aβ, together with the molecular mass, indicate that this fragment likely corresponds to the α-secretase-derived product, p10. The production of p10 is not affected by the Swedish mutation but is augmented by the V715M substitution (183% of control, P < 0.01; see Fig. 5E).
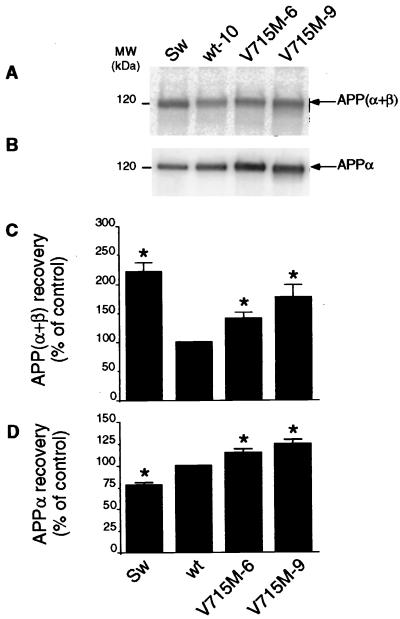
Both Swedish (221% of control, P < 0.001) and V715M (140–177% of control, P < 0.001) mutations increase the recovery of total secreted sAPP (APPα + APPβ), the N-terminal βAPP-derived products of α- and β-secretase cleavages, respectively (Fig. 6 A and C). However, the use of an immunological probe (10D5C) interacting with the C terminus of APPα but not with APPβ (see Fig. 1) allows us to establish that APPα secretion is diminished by the Swedish mutation (P < 0.001; see Fig. 6 B and D) whereas the V715M mutation augments the secretion of APPα (120–126% of control, P < 0.001; see Fig. 6 B and D), as it was shown for its C-terminal counterpart, p10 (see Fig. 5C), thereby confirming the stimulatory influence of V715M on the α-secretase cleavage of βAPP.
Figure 6.
Effect of the V715M-βAPP mutation on the secretion of total sAPP and APPα by HEK293 cells. The indicated transfectants were obtained and metabolically labeled as in Figs. 3 and 4. Cells media were immunoprecipitated with the 207 antibody. Quantification of total sAPP [i.e., APPα + APPβ (A and C)] was performed after SDS/PAGE, Western blot analysis, and direct radioautography as in Fig. 4. APPα (B and D) was analyzed from the same nitrocellulose after hybridization with 10D5C as described in Experimental Procedures. Values are expressed as the percentage of those obtained with wt-βAPP-expressing cells (taken as 100) and are the means ± SEM of three to five independent experiments. ∗, P < 0.001 (versus corresponding wt).
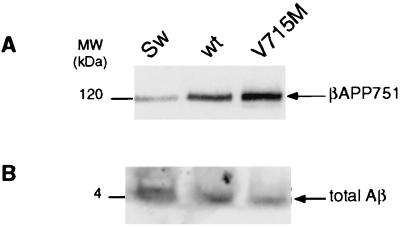
Several lines of evidence indicate that the mechanisms of βAPP maturation and the influence of regulators on this processing could be cell-specific. In this context, we took advantage of a recently established neuronal cell line (10) to examine the influence of the V715M mutation in such a cell type. The whole amount of Aβ produced by neurons expressing wt-βAPP after transient transfection precludes to delineate the effect on Aβ40 and Aβ42 formation but allowed us to assess the influence of the V715M mutation on total Aβ. Fig. 7 indicates that the neuronal TSM1 cell line secretes Aβ (Fig. 7B). After normalization with respect to the transiently transfected cDNAs (Fig. 7A), we established that the production of Aβ is increased by the Swedish mutation (482% ± 57 of control, n = 4) and lowered by the V715M mutation (75% ± 7 of control, n = 8).
Figure 7.
Effect of the V715M-βAPP mutation on the secretion of total Aβ by cultured neurons. TSM1 neuronal cells were obtained (10), cultured, and transiently transfected with wt-, Sw-, or V715M-βAPP as described in Experimental Procedures. βAPP content was analyzed after Western blotting by means of mAbWO2 (A). Total secreted Aβ was analyzed after immunoprecipitation with FCA18 and Western blot analysis of immunoprecipitated proteins with mAbWO2 as described in Experimental Procedures (B).
DISCUSSION
Examination of the pedigree F074 indicates that the mutation cosegregates with the disease. Thus, the affected uncle bears the mutation whereas unaffected relatives do not, except for an at-risk 37-year-old sibling of the proband (see Fig. 2). According to the above considerations, together with the typical clinical profile and age of the proband, it appears reasonable to consider that the V715M mutation probably leads to early-onset FAD in this pedigree. It is noteworthy that the mutation is not a common polymorphism because exon 17 of the βAPP gene has been sequenced in more than 100 subjects without finding such an alteration.
Several chromosome 21-linked mutations responsible for AD have been identified on the βAPP gene. A Swedish mutation consists of a double-substitution KM → NL (adjacent to the N terminus of Aβ; ref. 16). Several reports consistently indicated that the Swedish mutation leads to the exacerbation of the production of total Aβ (16–23), by increasing both Aβ40 and, to a lesser extent, Aβ42 (24). This is accompanied by a decreased production of APPα (21) and corresponding N-terminally truncated Aβs (25). The overproduction of Aβ also takes place intracellularly in several cell systems (19, 23). This overall Swedish mutation-linked overproduction of Aβ is documented further by the presence of senile plaques in the brains of transgenic mice and affected patients.
Additional mutations located close to the C terminus of Aβ have been documented. Three mutations, substituting the valine residue at position 717 (Aβ770 numbering) for a glycine, phenylalanine, or isoleucine, have been reported (26–28). These substitutions do not affect significantly the overall production of Aβ (24) but consistently increase the formation of the 42-aa-long Aβ species, thereby increasing the ratio of Aβ42 to total Aβ (24, 29). Recently, a novel mutation, I716V-βAPP770, has been reported (30) that also leads to increased production of Aβ42 in transfected cells. The stimulatory effect of these C-terminal mutations on the γ42-secretase cleavage recently has been documented further by an in vitro mutagenesis approach. Thus, mutations introduced at positions 43 and 46 of C-terminal βAPP constructs (corresponding to positions 714 and 717 of βAPP770) all led to increased production of Aβ42 (31).
The new V715M mutation reported in the present work clearly elicits a distinct phenotypic alteration of βAPP processing because the total amount of Aβ, particularly Aβ40, is drastically lowered whereas that of Aβ42 is not affected in HEK293 cells. This could indicate that the ratio of Aβ42 to total Aβ produced is likely a more significant clue of a pathological state than the absolute amount of Aβ42 or total Aβ detectable. Alternatively and more likely, the importance of x-42 species in the neuropathological process perhaps has been underestimated. Because the main modification of βAPP processing triggered by the V715M mutation is the selective, drastic increase in the x-42Aβ-related fragments, it can be postulated that this Aβ-related species is a main contributor of the genesis of senile plaques.
It is interesting to note that the V715M mutation appears to affect the α-secretase cleavage. This is indicated by the fact that a statistically significant, increased recovery of both APPα and its C-terminal counterpart, p10, is observed in HEK293 cells. Whether this is due to the direct influence of the mutation on the α-secretase affinity/catalytic properties or to the misrouting of the mutated βAPP to a route including a cell compartment enriched in α-secretase remains to be established. The combined overproductions of APPα and the x-42 species indicate that the mutation also influences the γ-secretase site of cleavage. That the x-40 production is not affected suggests the occurrence of two distinct γ-secretases, with the γ42-secretase drastically influenced by the mutation that would not affect the γ40-secretase. The hypothesis of two distinct γ-secretases agrees well with recent studies (32–35).
It should be added that we have introduced the V715M mutation on the C-terminal β-secretase-derived p12 fragment (see Fig. 1). Transient transfection of this cDNA construction in HEK293 cells leads to drastic lowering of secreted Aβ recovery (data not shown). This suggests that the mechanistic influence of the mutation is likely on the kinetic parameters of γ-secretases rather than on a putative, intracellular misrouting of βAPP.
Although the patient bearing the V715M mutation exhibited clinical alterations reminiscent of Alzheimer’s disease, we do not know whether this mutation elicits the classical neurohistological cortical stigmata. Transgenesis analysis of mice overexpressing this mutated βAPP, in progress in the laboratory, should allow us to examine this point and to support further the possibility that overproduction of x-42 species could be important for the genesis of senile plaques.
Acknowledgments
We would like to thank Dr. D. Schenk (Athena Neuroscience) for providing us with 10D5C antibodies. We also would like to sincerely thank Dr. Bart de Strooper (Leuven, Belgium) for the generous supply of B11.4. We are grateful to Dr. K. Beyreuther (Heidelberg, Germany) for providing us with WO2. We thank Drs. B. Greenberg and M. Savage for the kind supply of the 207 antibody. Drs. Chun and Allelix, Biopharmaceutical Inc. (Missisauga, Canada), are thanked for providing the TSM1 cell line. We thank J. Kervella for secretarial assistance. C.D. was supported by a grant from the Conseil Général de Haute Normandie. This work was supported by the Centre National de la Recherche Scientifique and the Institut National de la Santé et de la Recherche Médicale.
ABBREVIATIONS
- AD
Alzheimer’s disease
- FAD
familial AD
- wt
wild type
- βAPP
β amyloid precursor protein
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Selkoe D J. J Biol Chem. 1996;271:18295–18298. doi: 10.1074/jbc.271.31.18295. [DOI] [PubMed] [Google Scholar]
- 2.Hutton M, Hardy J. Human Mol Gen. 1997;6:1639–1646. doi: 10.1093/hmg/6.10.1639. [DOI] [PubMed] [Google Scholar]
- 3.Haass C. Neurons. 1997;18:687–690. doi: 10.1016/s0896-6273(00)80309-8. [DOI] [PubMed] [Google Scholar]
- 4.Checler F. J Neurochem. 1995;65:1431–1444. doi: 10.1046/j.1471-4159.1995.65041431.x. [DOI] [PubMed] [Google Scholar]
- 5.Checler, F. (1999) Mol. Neurobiol., in press. [DOI] [PubMed]
- 6.McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D. & Stadlan, E. (1984) Neurology 939–944. [DOI] [PubMed]
- 7.Fidani L, Rooke K, Chartier-Harlin M, Hughes D, Tanzi R, Mullan M, Roques P, Rossor M, Hardy J, Goate A. Hum Mol Genet. 1992;1:165–168. doi: 10.1093/hmg/1.3.165. [DOI] [PubMed] [Google Scholar]
- 8.Campion D, Brice A, Hannequin D, Charbonnier F, Dubois B, Martin C, Michon A, Penet C, Bellis M, Calenda A, Martinez M, Agid Y, Clerget-Darpoux F, Frébourg T. J Med Genet. 1996;33:661–664. doi: 10.1136/jmg.33.8.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marambaud P, Wilk S, Checler F. J Neurochem. 1996;67:2616–2619. doi: 10.1046/j.1471-4159.1996.67062616.x. [DOI] [PubMed] [Google Scholar]
- 10.Chun J, Jaenisch R. Mol Cell Neurosci. 1996;7:304–321. doi: 10.1006/mcne.1996.0023. [DOI] [PubMed] [Google Scholar]
- 11.Marambaud P, Chevallier N, Ancolio K, Checler F. Mol Med. 1998;4:715–723. [PMC free article] [PubMed] [Google Scholar]
- 12.Ancolio K, Marambaud P, Dauch P, Checler F. J Neurochem. 1997;69:2494–2499. doi: 10.1046/j.1471-4159.1997.69062494.x. [DOI] [PubMed] [Google Scholar]
- 13.Marambaud P, Ancolio K, Lopez-Perez E, Checler F. Mol Med. 1998;4:146–156. [PMC free article] [PubMed] [Google Scholar]
- 14.Marambaud P, Lopez-Perez E, Wilk S, Checler F. J Neurochem. 1997;69:2500–2505. doi: 10.1046/j.1471-4159.1997.69062500.x. [DOI] [PubMed] [Google Scholar]
- 15.Barelli H, Lebeau A, Vizzavona J, Delaere P, Chevallier N, Drouot C, Marambaud P, Ancolio K, Buxbaum J D, Khorkova O, et al. Mol Med. 1997;3:695–707. [PMC free article] [PubMed] [Google Scholar]
- 16.Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, Winblad B, Lannfelt L. Nat Genet. 1992;1:345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- 17.Cai X-D, Golde T E, Younkin S G. Science. 1993;259:514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- 18.Johnston J A, Cowburn R F, Norgren S, Wiehager B, Venizelos N, Winblad B, Vigo-Pelfrey C, Schenk D, Lannfelt L, O’Neill C. FEBS Lett. 1994;354:274–278. doi: 10.1016/0014-5793(94)01137-0. [DOI] [PubMed] [Google Scholar]
- 19.Martin B L, Schrader-Fisher G, Busciglio J, Duke M, Paganetti P, Yankner B A. J Biol Chem. 1995;270:26727–26730. doi: 10.1074/jbc.270.45.26727. [DOI] [PubMed] [Google Scholar]
- 20.Perez R G, Squazzo S L, Koo E H. J Biol Chem. 1996;271:9100–9107. doi: 10.1074/jbc.271.15.9100. [DOI] [PubMed] [Google Scholar]
- 21.Thinakaran G, Teplow D B, Siman R, Greenberg B, Sisodia S S. J Biol Chem. 1996;271:9390–9397. doi: 10.1074/jbc.271.16.9390. [DOI] [PubMed] [Google Scholar]
- 22.Reaume A G, Howland D S, Trusko S P, Savage M J, Lang D M, Greenberg B D, Siman R, Scott R W. J Biol Chem. 1996;271:23380–23388. doi: 10.1074/jbc.271.38.23380. [DOI] [PubMed] [Google Scholar]
- 23.Forman M S, Cook D G, Leight S, Doms R W, Lee V M Y. J Biol Chem. 1997;272:32247–32253. doi: 10.1074/jbc.272.51.32247. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki N, Cheung T T, Cai X-D, Odaka A, Otvos L, Jr, Eckman C, Golde T E, Younkin S G. Science. 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 25.Citron M, Oltersdorf T, Haass C, McConlogue L, Hung A Y, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe D J. Nature (London) 1992;360:672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- 26.Goate A, Chartier-Harlin M C, Mullan M, Brown J, Crawford F, Fidami L, Giuffra L, Haynes A, Irving N, James L, et al. Nature (London) 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 27.Chartier-Harlin M C, Crawford F, Houlden H, Warren A, Hughes D, Fidani L, Goate A, Rossor M, Roques P, Hardy J, Mullan M. Nature (London) 1991;353:844–846. doi: 10.1038/353844a0. [DOI] [PubMed] [Google Scholar]
- 28.Murrell J, Farlow M, Ghetti B, Benson M D. Science. 1991;254:97–99. doi: 10.1126/science.1925564. [DOI] [PubMed] [Google Scholar]
- 29.Maruyama K, Tomita T, Shinozaki K, Kume H, Asada H, Saido T C, Ishiura S, Iwatsubo T, Obata K. Biochem Biophys Res Commun. 1996;227:730–735. doi: 10.1006/bbrc.1996.1577. [DOI] [PubMed] [Google Scholar]
- 30.Eckman C B, Mehta N D, Crook R, Perez-Tur J, Prihar G, Pfeiffer E, Graff-Radford N, Hinder P, Yager D, Zenk B, et al. Hum Mol Gen. 1997;12:2087–2089. doi: 10.1093/hmg/6.12.2087. [DOI] [PubMed] [Google Scholar]
- 31.Lichtenthaler S F, Ida N, Multhaup G, Masters C L, Beyreuther K. Biochemistry. 1997;36:15396–15403. doi: 10.1021/bi971071m. [DOI] [PubMed] [Google Scholar]
- 32.Klafki H W, Abramowski D, Swoboda R, Paganetti P A, Staufenbiel M. J Biol Chem. 1996;271:28655–28659. doi: 10.1074/jbc.271.45.28655. [DOI] [PubMed] [Google Scholar]
- 33.Citron M, Diehl T S, Gordon G, Biere A L, Seubert P, Selkoe D J. Proc Natl Acad Sci USA. 1996;93:13170–13175. doi: 10.1073/pnas.93.23.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cook D G, Forman M S, Sung J C, Leight S, Kolson D L, Iwatsubo T, Lee V M Y, Doms R W. Nat Med. 1997;3:1021–1023. doi: 10.1038/nm0997-1021. [DOI] [PubMed] [Google Scholar]
- 35.Hartmann T, Bieger S C, Brühl B, Tienari P J, Ida N, Allsop D, Roberts G W, Masters C L, Dotti C G, Unsicker K, Beyreuther K. Nat Med. 1997;3:1016–1020. doi: 10.1038/nm0997-1016. [DOI] [PubMed] [Google Scholar]