Abstract
Context: Although GH promotes growth and protein anabolism, which are ATP-dependent processes, the GH effect on mitochondrial regulation remains to be determined.
Objective: Our objective was to determine the acute effect of GH on mitochondrial oxidative capacity in skeletal muscle of healthy subjects.
Design and Setting: The study was a randomized crossover design at an academic medical center.
Participants: Nine healthy men and women completed the study.
Intervention: GH (150 μg/h) or saline was infused for 14 h on separate days, and muscle biopsies were obtained.
Main Outcome Measures: Outcome measures included mitochondrial function, gene expression, and protein metabolism.
Results: The 4-fold increase in plasma GH caused elevations in plasma IGF-I, insulin, glucose, and free fatty acids and a shift in fuel selection, with less carbohydrate (−69%) and leucine (−43%) oxidation and 29% more fat oxidation. Muscle mitochondrial ATP production rate and citrate synthase activity were increased 16–35% in response to GH. GH also resulted in higher abundance of muscle mRNAs encoding IGF-I, mitochondrial proteins from the nuclear (cytochrome c oxidase subunit 4) and mitochondrial (cytochrome c oxidase subunit 3) genomes, the nuclear-derived mitochondrial transcription factor A, and glucose transporter 4. Although GH increased whole-body protein synthesis (nonoxidative disposal of leucine), no effect on synthesis rate of muscle mitochondrial proteins was observed.
Conclusions: These results demonstrate that acute GH action promotes an increase in mitochondrial oxidative capacity and abundance of several mitochondrial genes. These events may occur through direct or indirect effects of GH on intracellular signaling pathways but do not appear to involve a change in mitochondrial protein synthesis rate.
A 14-hour growth hormone infusion in normal subjects, inducing a four-fold increase plasma levels, increases mitochondrial oxidative capacity and abundance of several mitochondrial genes, but does not appear to alter the rate of mitochondrial protein synthesis.
Human GH promotes organ growth and is an important regulator of fuel metabolism in both health and disease. GH receptors have been identified in most tissues including muscle, adipose tissue, liver, heart, kidney, brain, and pancreas (1,2). The recognized actions of GH include protein anabolism, promotion of lipolysis, and resistance to insulin-induced glucose metabolism in liver and peripheral tissues (1,2,3). Several studies demonstrated that both acute and chronic GH infusion reduces urea synthesis, promotes protein synthesis, and typically results in reduction of protein breakdown in humans (4,5,6,7,8,9,10). These anabolic actions are particularly evident when GH is replaced in patients with GH deficiency or when given therapeutically in catabolic conditions like fasting, surgery, burns, or illness (1,11).
Growth, protein synthesis, and many components of fuel metabolism are ATP-requiring processes that may increase energetic demands on mitochondria, but GH effects on mitochondrial function are not fully established. There is some evidence that GH action may be important for mitochondrial regulation in skeletal muscle. A case study of a patient with acromegaly demonstrated that structural abnormalities of muscle mitochondria were resolved after surgical treatment, although no functional results were provided (12). Lange et al. (13) reported that when older women combined GH injections with aerobic exercise training for 12 wk, they had a greater increase in activity of muscle mitochondrial oxidative enzymes than exercising women who received a placebo injection. However, that study did not include a group taking GH without exercise, so it is not known whether GH could regulate mitochondria oxidative capacity independent of the exercise program. In contrast, when nonexercising rats were given daily GH injections for 2 wk, there was no change in muscle mitochondrial respiration rate (14). The last dose of GH in that study was given 1–2 d before the muscle measurements, however, so any acute effects of GH action may not have been detectable. To our knowledge, no studies have reported whether administration of GH regulates muscle mitochondrial function in humans. The present study was designed to test the hypothesis that GH would induce an increase in mitochondrial oxidative capacity and expression of oxidative genes and to define the effects of GH on muscle protein synthesis, including mitochondrial proteins. We investigated the acute effect of GH by infusing GH or saline for 14 h in young healthy volunteers in the postabsorptive state in a randomized crossover study design. Muscle biopsies were used to measure mitochondrial ATP production rate, mitochondrial protein synthesis rate, and the abundance of several gene transcripts that regulate to the muscle oxidative phenotype.
Subjects and Methods
Participants
Nine healthy volunteers (five men, four women) completed the study. Average characteristics (mean ± sd) of the group were age 33 ± 10 yr, body mass index 25.2 ± 3.2 kg/m2, body fat-free mass 50.8 ± 14.6 kg, and body fat 26.4 ± 7.4%. Body composition was determined using dual-energy x-ray absorptiometry. Health status was assessed by medical history, physical exam, blood chemistries (including liver enzymes, creatinine, electrolytes, and glucose), complete blood count, urinalysis, and electrocardiogram. Inclusion criteria included age (18–45 yr) and body mass index (20–30 kg/m2). Exclusion criteria included diabetes, chronic renal, liver, or other metabolic/endocrine disorders, or use of tobacco or medications that could affect metabolism. None of the participants were taking medications at the time of the study. Female participants were studied during the luteal phase of their menstrual cycle. The Institutional Review Board of Mayo Foundation approved the study protocol. All procedures were performed in accordance with the guidelines in The Declaration of Helsinki and were clearly explained to each study volunteer before obtaining informed oral and written consent.
Protocol and procedures
Each participant was studied once with saline infusion and once with GH infusion in a randomized crossover design. The two trials were separated by an average of 10 wk with regular lifestyle patterns maintained in the interim. For 3 d before each study, strenuous physical activity was avoided and a weight-maintaining diet (55:30:15% of energy as carbohydrate, fat, and protein, respectively) was provided by the Metabolic Kitchen at the Mayo Clinic General Clinical Research Center (GCRC). On the evening before each study, participants were admitted for an overnight stay in the GCRC. After the evening meal (1800 h), no food was consumed until completion of the study the next day.
A polyethylene catheter was placed in an antecubital arm vein for infusion of either saline or GH (150 μg/h; 2.1 ± 0.1 μg/kg·h) beginning at 2200 h and continuing for 14 h. At 0400 h the next morning, priming doses of l-[1,2-13C]leucine (6.9 μmol/kg, 97 atom percent excess; MA Trace, Woburn, MA) and [13C]bicarbonate (2.4 μmol/kg; 99 atom percent excess; Cambridge Isotopes, Andover, MA) were given, and the [1,2-13C]leucine was continued at 6.9 μmol/kg·h until the end of the study (1200 h). Isotope solutions were prepared under sterile conditions and were determined to be bacteria and pyrogen free before their administration. At 0600 h, a second catheter was placed in the hand opposite the infusion site and used to obtain arterialized blood each hour using the heated hand vein method (15). Expired breath was sampled hourly, coinciding with blood collections. Muscle biopsies of the vastus lateralis were obtained under local anesthesia (16) at 0700 and 1200 h, each obtained from a different leg within the study day. Both biopsies of each study day were used for protein synthesis and mRNA measurements. Mitochondrial and signal protein analyses were performed only on the second biopsy. A portion of the muscle was kept on ice in saline-soaked gauze for mitochondrial studies, and the remainder was rapidly frozen in liquid nitrogen and stored at −80 C until analysis.
Resting energy expenditure was determined by indirect calorimetry (DeltaTrac; SensorMedics, Yorba Linda, CA) for 45 min beginning at approximately 0800 h. The last 20 min of this measurement were used for data analysis. Urinary nitrogen content was measured using a Beckman GM7 Analox Microstat (Beckman Instruments, Fullerton, CA).
Muscle mitochondrial function
Mitochondria were isolated by centrifugation from fresh muscle tissue, and ATP production capacity was assessed using a bioluminescent method (17,18). Briefly, mitochondria were added to cuvettes containing luciferin/luciferase (BioTherma, Haninge, Sweden), 0.3 mm ADP, and one of six substrate combinations. Substrates used were (in mm) 10 glutamate plus 1 malate (GM), 1 pyruvate plus 0.05 palmitoyl-l-carnitine plus 10 α-ketoglutarate plus malate (PPKM), 10 α-ketoglutarate (KG), 20 succinate plus 0.1 rotenone (SR), 1 pyruvate plus 1 malate (PM), and 0.05 palmitoyl-l-carnitine plus 1 malate (PCM). ATP production was measured simultaneously for all reactions in triplicate at 25 C in a BioOrbit 1251 luminometer. Each reaction was calibrated using an internal ATP standard. A separate piece of muscle (20 mg) was used to measure the activity of citrate synthase and β-hydroxyacyl coenzyme A dehydrogenase (BHAD) using spectrophotometric assays (17).
Quantification of mRNA
Abundance of selected mRNAs in muscle was measured with a real-time quantitative PCR system (ABI Prism 7700; PE Biosystems, Foster City, CA). RNA was extracted by TRIZOL method (Life Technologies, Gaithersburg, MD), treated with DNase (Life Technologies), and reverse-transcribed using TaqMan reverse transcription reagents (PE Biosystems). Transcripts measured included IGF-I, because it is a major effector of GH action, and the mitochondrial components cytochrome c oxidase subunit 3 (COX3) and 4 (COX4), and uncoupling protein 3 (UCP3). Nuclear transcription factors involved in regulation of muscle oxidative genes were also measured: peroxisome-proliferator receptor-γ coactivator 1α (PGC-1α), nuclear respiratory factor 1 (NRF1), estrogen-related receptor-α (ERR-α), mitofusin 2 (MFN2), and mitochondrial transcription factor-α (TFAM). Two additional genes associated with muscle oxidative capacity were measured: glucose transporter 4 (GLUT4) and the slow-twitch isoform of the contractile protein myosin heavy chain (MHCI). Samples were run in triplicate with coamplification of the target gene and 28S rRNA (as a housekeeping gene) and quantified by normalizing the target signal for the 28S rRNA signal. For each gene, the average transcript abundance in the saline trial was assigned a value of 1.0, and all individual values from both the saline and GH trials were linearly transformed relative to this value and expressed in arbitrary units (AU).
Sequences for the following primers and probes have been previously published: COX3 (18), COX4, PGC-1α, NRF1, TFAM, GLUT4 (19), MHCI, and 28S rRNA (20). The primers used for IGF-I (GenBank accession number X57025) were forward CCAGCGCCACACCGA and reverse CTCCCTCTACTTGCGTTCTTCAA, and the probe sequence was ATGCCCAAGACCCAGAAGGAAGTACA. Corresponding information for the other genes is as follows: UCP3 (GenBank AF011449) forward primer CTCAAGGAGAAGCTGCTGGACTA, reverse primer GCTCCAAAGGCAGAGACAAAGT, and probe ACCTGCTCACTGACAACTTCCCCTGC; ERR-α (GenBank NM004451) forward primer AGATTGTGGTCACCATCAGCTG, reverse primer TCCACACGCTCTGCAGTACTG, and probe CCAAGAGCATCCCAGGCTTCTCA; and MFN2 (GenBank NM014874) forward primer GGCTCGGAGGCACATGAA, reverse primer CGGTGCTCTTCCCATTGC, and probe CGTCCGGCCAAAAAAAGCCA.
Western blotting
Sufficient tissue was available in five of the nine subjects to measure the phosphorylation status of two key signaling molecules that regulate protein synthesis. Frozen muscle samples were prepared, separated by polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membranes as previously described (21). After blocking in nonfat milk, membranes were incubated overnight at 4 C with primary antibodies directed against the total or phosphorylated (Ser 2448) forms of the mammalian target of rapamycin (mTOR; Cell Signaling Technology, Danvers, MA), and the total or phosphorylated (Thr 37/46) form of translation elongation binding protein 4E-BP1 (Cell Signaling). After incubation with horseradish peroxidase-conjugated secondary antibodies and the ECL-Plus detection system (Amersham Biosciences, Piscataway, NJ), images were captured on Biomax XAR film (Kodak Scientific, New Haven, CT) and analyzed using Kodak Molecular Imaging software. Data are expressed as the ratio of phosphorylated to total protein signal for each subject. The group average in the saline trial was then assigned a value of 1.0, and all individual values from both the saline and GH trials were linearly transformed relative to this value and expressed in AU.
Hormone and metabolite assays
Glucose was measured with a Beckman Glucose Analyzer (Beckman Instruments, Porterville, CA). Nonesterified fatty acids (NEFA) were measured using an enzymatic colorimetric assay (NEFA C; Wako Chemicals USA, Richmond, VA). Plasma levels of amino acids were measured by an HPLC system (HP 1090, 1046 fluorescence detector and cooling system) with precolumn o-phthaldehyde derivatization (22). Hormone assays were performed by the Mayo Clinic Clinical Chemistry Laboratory. Insulin and human GH were measured with two-site immunoenzymatic assays (Access system; Beckman Instruments, Chaska, MN). Glucagon and C-peptide were measured by direct RIAs (Linco Research, St. Louis, MO). After separation from their binding proteins with a simple organic solvent, total IGF-I and IGF-II were measured with two-site immunoradiometric assays (Diagnostic Systems Laboratories, Webster, TX). IGF-binding protein (IGFBP)-1 and -3 were also measured with two-site immunoradiometric assays, whereas IGFBP-2 was measured by a double-antibody RIA (Diagnostic Systems Laboratories).
Plasma amino acid kinetics
The enrichment level of [1,2-13C]leucine in plasma was determined using a gas chromatograph/mass spectrometer (HP5973; Hewlett-Packard Instruments, Avondale, CA) by multiple ion monitoring at m/z 342/344 under positive ion methane chemical ionization conditions. [1,2-13C]Ketoisocaproate in plasma was determined as its quinoxalinol-trimethylsilyl derivative under electron ionization conditions using an HP5988 gas chromatograph/mass spectrometer (23). Isotopic enrichment of breath 13CO2 was measured by isotope ratio mass spectrometry (24). Average steady-state enrichment values from 0700–1200 h (corresponding to the muscle biopsy times) were used to calculate whole-body rates of leucine flux, oxidation, and nonoxidative disposal using standard equations (24).
Muscle protein synthesis
A portion of each muscle sample was used for the isolation of mitochondrial and sarcoplasmic protein fractions by differential centrifugation (16). A separate piece of tissue was used to prepare total mixed muscle proteins and to isolate free tissue fluid amino acids (25). The muscle protein fractions were hydrolyzed overnight in 0.6 m HCl in the presence of cation exchange resin (AG-50; Bio-Rad, Hercules, CA) and purified the next day using a column of the same resin. The amino acids were dried and then derivatized as their trimethyl acetyl methyl ester. [13C]Leu enrichment in muscle proteins was determined using a gas chromatograph-combustion-isotope ratio mass spectrometer (δ Plus; Finigan MAT, Bremen, Germany) as described (26). Tissue fluid amino acids were derivatized as their t-butyldimethylsilyl ester and analyzed using an HP5973 gas chromatograph/mass spectrometer under electron ionization conditions (26). The fractional synthetic rate of muscle proteins was calculated from the increment in protein-bound enrichment between biopsies, using muscle tissue fluid enrichment as the precursor pool (25).
Statistical analysis
Summary data are reported as mean ± sem. Differences between the saline and GH trials were analyzed using paired t tests. P values < 0.05 were considered statistically significant.
Results
Plasma metabolites and hormones
GH infusion resulted in significant elevations in circulating GH, glucose, NEFA, insulin, C-peptide, and free IGF-I, whereas there were reductions in glucagon, IGFBP-1, and IGFBP-3 during the last 5 h of the study (Table 1). There were also trends for increased total IGF-I and reduced IGF-II. There were no differences between trials for cortisol or IGFBP-2. The total concentration of measured amino acids in plasma was not different between trials (2.47 ± 0.11 and 2.40 ± 0.12 mmol/liter for saline and GH, respectively, P = 0.525), nor was there a difference for individual amino acids, including leucine (157 ± 10 and 150 ± 10 μmol/liter for saline and GH, respectively, P = 0.471).
Table 1.
Plasma metabolites and hormones (mean ± sem)
| Saline | GH | % Differencea | P value | |
|---|---|---|---|---|
| Glucose (mmol/liter) | 5.10 ± 0.13 | 5.41 ± 0.15 | 6 | 0.004 |
| Fatty acids (mmol/liter) | 0.452 ± 0.036 | 0.892 ± 0.063 | 97 | 0.001 |
| Insulin (pmol/liter) | 34 ± 6 | 57 ± 6 | 68 | 0.001 |
| C-peptide (nmol/liter) | 0.42 ± 0.04 | 0.63 ± 0.03 | 48 | 0.001 |
| Cortisol (μg/dl) | 14.8 ± 1.1 | 14.2 ± 1.9 | −4 | 0.689 |
| Glucagon (pg/ml) | 141 ± 8 | 135 ± 8 | −4 | 0.007 |
| GH (μg/liter) | 2.3 ± 1.0 | 12.2±1.1 | 425 | 0.001 |
| IGF-I total (ng/ml) | 314 ± 37 | 367 ± 33 | 17 | 0.096 |
| IGF-I free (ng/ml) | 0.87 ± 0.10 | 1.74 ± 0.26 | 101 | 0.004 |
| IGF-II (ng/ml) | 717 ± 70 | 651 ± 49 | −9 | 0.090 |
| IGFBP-1 (ng/ml) | 37.9 ± 6.7 | 20.5 ± 5.9 | −46 | 0.030 |
| IGFBP-2 (ng/ml) | 372 ± 60 | 344 ± 54 | −7 | 0.211 |
| IGFBP-3 (ng/ml) | 3621 ± 170 | 3444 ± 143 | −5 | 0.019 |
Values shown are the average of blood samples collected at 0700 and 1200 h, corresponding to 9 and 14 h of infusion with either saline or GH.
GH vs. saline.
Indirect calorimetry
GH infusion did not significantly alter resting energy expenditure or urinary nitrogen excretion rate, although there was a reduction in the nonprotein respiratory quotient (Table 2), indicating a shift in fuel metabolism away from carbohydrate utilization and toward greater reliance on fat. Calculated carbohydrate oxidation (grams per hour) was 69% lower, whereas fat oxidation tended to be higher (29%, P = 0.056) in the GH trial compared with saline.
Table 2.
Indirect calorimetry (mean ± sem)
| Saline | GH | % Differencea | P value | |
|---|---|---|---|---|
| VO2 (ml/min) | 256 ± 18 | 245 ± 17 | −4 | 0.318 |
| VCO2 (ml/min) | 207 ± 17 | 184 ± 12 | −11 | 0.063 |
| Urinary urea nitrogen excretion (g/h) | 0.38 ± 0.04 | 0.36 ± 0.04 | −6 | 0.717 |
| Nonprotein RQ | 0.81 ± 0.02 | 0.75 ± 0.02 | −8 | 0.035 |
| Energy expenditure (kcal/min) | 1.22 ± 0.09 | 1.15 ± 0.08 | −6 | 0.208 |
| Fat oxidation (g/h) | 5.02 ± 0.47 | 6.49 ± 0.65 | 29 | 0.056 |
| CHO oxidation (g/h) | 6.43 ± 1.41 | 1.98 ± 0.78 | −69 | 0.027 |
CHO, Carbohydrate; RQ, respiratory quotient (VCO2/VO2) after adjustment for protein loss.
GH vs. saline.
Muscle mitochondrial function
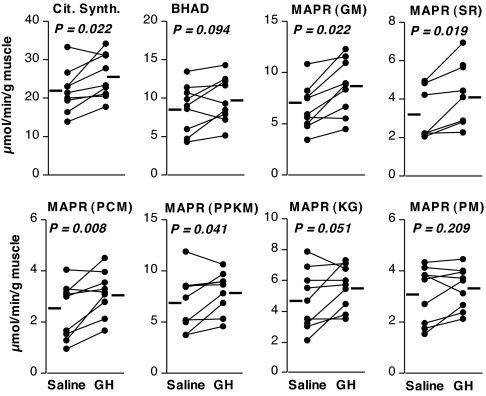
Enzymatic activity of citrate synthase in muscle homogenates was significantly increased (16%), and BHAD showed a trend to increase (13%) in the GH trial vs. saline (Fig. 1). Likewise, GH infusion resulted in an 8–35% higher mitochondrial ATP production rate, reaching statistical significance or a strong trend (P ≤ 0.051) for five of the six substrates tested, as shown in Fig. 1.
Figure 1.
Effect of GH on muscle oxidative capacity. Enzymatic activity of citrate synthase (Cit. Synth.) and BHAD and mitochondrial ATP production rate (MAPR) are shown. Paired data from individual participants are shown as connected circles, with treatment means denoted by the adjacent bars. For MAPR, mitochondria were incubated with glutamate plus malate (GM), succinate plus rotenone (SR), palmitoyl carnitine plus malate (PCM), pyruvate plus palmitoyl carnitine plus ketoglutarate plus malate (PPKM), ketoglutarate (KG), or pyruvate plus malate (PM). P values are for paired t test comparisons between treatments.
Abundance of mRNAs
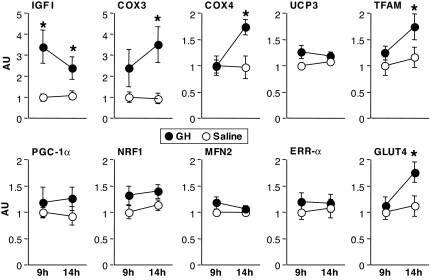
Compared with saline, GH infusion resulted in a 240 and 119% increase in IGF-I mRNA abundance at 9 and 14 h, respectively (Fig. 2). None of the other mRNAs were significantly different between trials at the 9-h measurement time. By 14 h, however, both COX3, encoded by the mitochondrial DNA, and COX4, encoded by the nuclear DNA, were increased by 342 and 78%, respectively, during the GH trial. However, GH did not alter mRNA content for another mitochondrial membrane protein, UCP3. Among the nuclear transcription factors measured, only TFAM increased (34%) with GH infusion. GLUT4 mRNA abundance increased 52% with GH infusion. MHCI mRNA increased from 1.00 ± 0.23 AU in the saline trial to 1.43 ± 0.25 AU in the GH trial but did not reach statistical significance (P = 0.25).
Figure 2.
Abundance of mRNA transcripts for selected genes measured at 9 and 14 h of GH or saline infusion. Values for each gene are expressed in AU after normalizing for 28S rRNA signal and adjusting the mean of the saline trial at 9 h to 1. Results are shown for IGF-I, COX3 and -4, UCP3, TFAM, PGC-1α, NRF1, MFN2, ERRα, and GLUT4. *, GH greater than saline, P < 0.05.
Western blotting
The relative Ser 2448 phosphorylation level of mTOR was 1.00 ± 0.13 during saline and increased in each of the five subjects measured to 1.76 ± 0.16 during the GH trial (P = 0.020). Phosphorylation of Thr 37/46 on 4E-BP1 was 1.00 ± 0.22 during saline and increased in three of the five subjects to 1.42 ± 0.41 during the GH trial, but this was not a statistically significant difference (P = 0.457).
Amino acid kinetics
Whole-body amino acid kinetics are shown in Table 3. Leucine oxidation was reduced during GH infusion, and nonoxidative leucine disposal was increased. There was a trend for reduced leucine flux that did not reach statistical significance. As shown in Table 3, the infusion of GH did not result in significant changes in the fractional synthetic rate of muscle proteins.
Table 3.
Whole body amino acid kinetics and fractional synthetic rates of muscle proteins (mean ± sem)
| Saline | GH | % Differencea | P value | |
|---|---|---|---|---|
| Whole-body kinetics (μmol/h·kg body weight) | ||||
| Leucine rate of appearance | 103.6 ± 4.0 | 96.9 ± 2.6 | −7 | 0.091 |
| Leucine oxidation | 40.9 ± 2.3 | 23.5 ± 1.3 | −43 | 0.001 |
| NOLD | 62.7 ± 3.7 | 73.4 ± 3.0 | 17 | 0.018 |
| Muscle protein synthesis (%/h) | ||||
| Mixed protein | 0.078 ± 0.010 | 0.072 ± 0.007 | −7 | 0.587 |
| Mitochondrial protein | 0.102 ± 0.014 | 0.108 ± 0.013 | 6 | 0.815 |
| Sarcoplasmic protein | 0.077 ± 0.007 | 0.067 ± 0.013 | −13 | 0.596 |
NOLD, Nonoxidative leucine disposal.
GH vs. saline.
Discussion
The current study demonstrated that a 14-h infusion of GH causing physiological elevation of GH in healthy people resulted in increased skeletal muscle mitochondrial oxidative capacity, as shown by increased mitochondrial ATP production rate, increased citrate synthase activity, and a trend for higher BHAD activity. GH infusion also resulted in higher muscle content of mRNA transcripts encoding oxidative proteins in mitochondria (COX 3 and COX4), a nuclear transcription factor that regulates mitochondrial biogenesis (TFAM), and the glucose transport protein GLUT4. These effects of GH action on muscle oxidative capacity were accompanied by a shift in whole-body fuel utilization reflecting reduced carbohydrate and leucine oxidation and a trend toward enhanced fat oxidation, although whole-body energy expenditure was not changed.
To our knowledge, this is the first report of an acute effect of GH infusion on muscle mitochondrial function and gene transcripts in healthy human subjects. A prior study suggested that GH injections for 12 wk may add to the effect of aerobic exercise to increase muscle oxidative capacity in older women (13), but the effect of GH alone has not been reported. The current study indicated that GH enhanced transcript abundance of genes involved in mitochondrial biogenesis, including TFAM, the key nuclear transcription factor regulating mitochondrial transcription and replication. These findings are similar to recent reports that showed that acute insulin infusion, like GH, stimulates mitochondrial oxidative capacity and transcript levels of several mitochondrial genes (18,21,27,28), raising the possibility that the actions of these hormones may overlap.
The existing literature, however, suggests that insulin and GH probably regulate mitochondrial function though different pathways. For example, GH infusion caused an increase in circulating insulin in the present study, but the concentration remained at least 5-fold lower than used in studies reporting a stimulatory effect of insulin on muscle mitochondrial ATP production (18,21,27,28). GH action is known to cause insulin resistance (1,2,3,10), which could further diminish any effects of the modest increase in insulin during GH infusion. In the present study, GH resulted in increased mitochondrial function despite evidence of insulin resistance as indicated by higher insulin, glucose, and fatty acids. This observation is an important point for distinguishing the regulatory actions of GH and insulin because it was demonstrated that the stimulatory effect of insulin on mitochondrial ATP production is blunted in subjects with clinical insulin resistance (18,21,27,28) and in healthy individuals in whom insulin resistance is acutely induced by raising plasma lipid concentration (27). In the present study, plasma fatty acid concentration was nearly doubled during GH infusion compared with saline and was accompanied by a shift in fuel metabolism toward greater reliance on fat. This increase in fatty acids did not appear to limit mitochondrial function, and the mitochondrial data indicate that GH increased the capacity to produce ATP with the fatty acid substrate palmitoyl carnitine or the tricarboxylic acid cycle intermediates α-ketoglutarate (and glutamate, which is exchanged with α-ketoglutarate) and succinate. GH action may therefore activate proteins in the β-oxidation or tricarboxylic acid cycles (e.g. BHAD and citrate synthase enzymes) or other components of the mitochondrial fuel delivery and oxidation machinery. In contrast, GH did not stimulate ATP production with pyruvate plus malate. This may be due to regulation of the pyruvate dehydrogenase complex, a known control point for fat and glucose oxidation (29,30). During insulin-resistant conditions, including fatty acid elevation, pyruvate dehydrogenase activity is inhibited by an increase in pyruvate dehydrogenase kinase 4 (PDK4) (29). It has been shown that GH induces PDK4 expression in adipose cells through a signal transducer and activator of transcription 5 (STAT5) binding site in the PDK4 promoter region (31,32). The only study, of which we are aware, to examine whether this also occurs in muscle found that a single bolus dose of GH given to healthy young men resulted in STAT5 phosphorylation (activation) 30–60 min later but did not change STAT5 binding to DNA (32). However, this single dose of GH may not be sufficient to induce the characteristic insulin resistance and altered fuel selection. It is also possible that changes in DNA binding events in muscle may require more than 60 min to occur. Nevertheless, the mechanism through which GH action regulates muscle mitochondrial substrate oxidation remains to be elucidated.
GH action resulted in increased expression of mRNAs that encode mitochondrial proteins COX3 and COX4 and the major nuclear-derived transcription factor that regulates replication and transcription of the mitochondrial genome, TFAM (33). These responses are similar to the acute effects of insulin infusion (18,21,34) and the effects of acute or chronic aerobic exercise training (19,35). Exercise is a potent stimulus of GH release (36), and thus it is possible that GH action contributes to many exercise-induced adaptations, including increased mitochondrial biogenesis and fat utilization. We explored the potential role of the nuclear transcription factor PGC-1α in mediating the changes in mitochondria because PGC-1α is a key regulator of muscle mitochondrial biogenesis and several related proteins that ultimately determine muscle oxidative phenotype (37,38,39,40). PGC-1α exerts its effects by promoting the transcription of, and working in conjunction with, other transcription factors, including NRF-1, ERR-α, MFN2, and TFAM, all of which have been reported to increase in muscle in response to endurance exercise (19,35,37,38,41). PGC-1α has also been reported to exert positive regulatory control on the GLUT4 gene (39), which was increased by GH in the present study and increases in response to exercise (19,38). There were no significant differences in PGC-1α mRNA or the other transcription factors measured, besides TFAM, at either 9 or 14 h of GH infusion. Therefore, if the PGC-1α transcriptional pathway is responsible for the downstream increases in COX3, COX4, TFAM, and GLUT4 mRNA during GH infusion, then either there were transient changes in transcript levels occurring before our first measurement (9 h) or the regulation occurred through changes in protein content or DNA binding. In response to acute exercise, it was reported that PGC-1α and ERR-α mRNA abundance is transiently increased for only a few hours (35,37). Whether that is also true in response to GH elevation needs to be determined. Alternately, regulation of the mitochondrial pathways may occur through direct actions of GH (perhaps the STAT5 pathway), or secondarily by increasing IGF-I and fatty acids. Chronic fatty acid elevation, for example, was shown to increase the muscle protein content of citrate synthase, subunits of COX, and related mitochondrial proteins and did not appear to be regulated by PGC-1α, which was unchanged (42). Whether the fatty acid-mediated events also occur within the time frame of our overnight GH infusion remains to be shown.
The increase in GLUT4 mRNA in the GH trial appears to contradict the muscle and whole-body changes favoring increased fat utilization, including the insulin resistance for glucose metabolism shown to occur with GH treatment (2,10). It was previously shown that GH infusion for 6 h in healthy young men did not alter muscle GLUT4 protein content (3). There was also no change in muscle GLUT4 mRNA or protein in rats treated with GH for 4 wk (43). Thus, the mechanism and functional impact of the increased GLUT4 mRNA in the present study is not yet clear.
We found that GH had no effect on muscle protein synthesis, including mitochondrial proteins. We previously showed that insulin infusion could selectively increase synthesis of mitochondrial proteins in skeletal muscle, thus potentially contributing to insulin-mediated stimulation of mitochondrial oxidative capacity (18,44). This is the first report, to our knowledge, on the effect of GH on synthesis of subfractions of the total muscle protein such as mitochondrial or sarcoplasmic proteins. This measurement technique has been used to demonstrate changes in synthesis of muscle proteins with aging or exercise (20,45), so it seems unlikely that an effect of GH would not be detected. A limitation, however, is that the measurements represent the average synthesis rates of multiple individual mitochondrial or sarcoplasmic proteins. It is possible that GH has a selective effect on synthesis of specific proteins, particularly those proteins with increased mRNA abundance. Interestingly, the lack of effect of GH on muscle protein synthesis rate occurred despite an increased activation (phosphorylation) of mTOR, a key energy-sensing anabolic signal molecule that contributes to the regulation of transcription and translation. However, the lack of change in the phosphorylation of 4E-BP1, a downstream effector of mTOR action, indicates that the pathway leading to increased protein synthesis rate was not fully activated. mTOR and 4E-BP1 are rapidly stimulated by insulin, amino acids, and exercise (46,47), but their response to GH in human muscle was unknown. Because we made our measurements at rest during the postabsoptive state, it is possible that the addition of exercise (13) and/or protein meals (46,47) while GH is elevated could have a synergistic effect on activating mTOR, downstream signaling pathways, and muscle protein synthesis.
The lack of change in muscle protein synthesis in response to GH infusion is consistent with most previous studies in which healthy humans received GH acutely (3–10 h) or up to 3 months (6,9,10,48) and protein metabolism measurements were performed in the postabsorptive state. The daily equivalency of GH doses in those studies were similar to the amount administered in the current investigation, and the resulting serum GH was similar to that observed after prolonged exercise or clinically stressful conditions (1,36). In comparison, studies in which GH increased muscle protein synthesis in healthy volunteers used larger doses (1.7- to 4-fold higher than the present study) either alone (49) or in combination with high-dose insulin (50). In contrast to the results in muscle, GH exerted an anabolic effect on whole-body amino acid metabolism, as shown by reduced leucine oxidation, increased nonoxidative leucine disposal (an index of protein synthesis), and a trend for a lower rate of leucine appearance (a measure of protein breakdown). These findings on whole-body kinetics are consistent with previous reports and collectively demonstrate that a major effect of short-term administration of GH on protein metabolism in healthy subjects occurs in nonmuscle tissues (1,6,10).
Because we measured total GH action using a continuous infusion of GH, we recognize that the observed changes may differ if a more physiological (i.e. pulsatile) pattern of GH delivery is used. We also note that changes in muscle mitochondrial function and mRNA expression could result from either primary effects of GH or secondary changes in metabolites and hormones, such as IGF-I. IGF-I has similar effects as GH on whole-body protein turnover but may have an additional stimulatory effect on protein synthesis in muscle (51,52,53,54). We are not aware of any studies that tested whether IGF-I exerts control of muscle mitochondrial function, gene expression, or protein synthesis. GH action may also be related to increased lipolysis. There is evidence that GH-mediated suppression of protein breakdown occurs through the elevation of fatty acids (5). As already noted, elevated fatty acids, induced by a 4-wk high-fat diet in rats, can stimulate an increase in many of the muscle mitochondrial proteins that were shown to change with GH infusion in the present study (42). The relative importance of primary and secondary GH actions requires further careful investigation.
A potential area of application is assessing whether GH may have beneficial effects on muscle mitochondrial function in elderly people because circulating GH concentration, the magnitude of exercise-mediated GH release, and muscle mitochondrial ATP production capacity are reduced with age (17,36,55,56). However, it remains to be determined how long the changes in mitochondrial function and gene expression persist after cessation of GH infusion and how this may affect muscle performance. The observed effects may have little or no benefit to physically active people for whom regular exercise may provide equal or greater stimulus for mitochondrial biogenesis. Still, there may be some benefits for select clinical populations, such as GH-deficient individuals.
In conclusion, the current study demonstrates that overnight infusion of GH stimulates muscle mitochondrial metabolism by increasing the mRNA levels of specific genes and raising the capacity for oxidative ATP generation in healthy subjects. This response in mitochondrial functional capacity is consistent with the shift in fuel utilization away from carbohydrate toward fat use and appears to involve the activation of specific enzymes but does not appear to involve a change in mitochondrial protein synthesis rate.
Acknowledgments
We thank Jane Kahl, Dawn Morse, Kate Klaus, and Bushra Ali for their technical assistance with sample analysis. We also thank the members of the GCRC dietary, nursing, and support staff for their help in carrying out these studies.
Footnotes
This work was supported by Grants RO1-DK41973 (to K.S.N.), T32-DK07352 (to K.R.S.), and MO1-RR00585 from the National Institutes of Health. Additional support was provided by the Mayo Foundation and the Murdock-Dole Professorship (to K.S.N.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online November 13, 2007
Abbreviations: AU, Arbitrary units; BHAD, β-hydroxyacyl coenzyme A dehydrogenase; COX3, cytochrome c oxidase subunit 3; ERR-α, estrogen-related receptor-α; GCRC, General Clinical Research Center; GLUT4, glucose transporter 4; IGFBP, IGF-binding protein; MFN2, mitofusin 2; MHCI, slow-twitch isoform of the contractile protein myosin heavy chain; mTOR, mammalian target of rapamycin; NEFA, nonesterified fatty acids; NRF1, nuclear respiratory factor 1; PDK4, pyruvate dehydrogenase kinase 4; PGC-1α, peroxisome-proliferator receptor-γ coactivator 1α; STAT5, signal transducer and activator transcription 5; TFAM, mitochondrial transcription factor-α; UCP3, uncoupling protein 3.
References
- Moller N, Norrelund H 2003 The role of growth hormone in the regulation of protein metabolism with particular reference to conditions of fasting. Horm Res 59(Suppl 1):62–68 [DOI] [PubMed] [Google Scholar]
- Davidson MB 1987 Effect of growth hormone on carbohydrate and lipid metabolism. Endocr Rev 8:115–131 [DOI] [PubMed] [Google Scholar]
- Jessen N, Djurhuus CB, Jorgensen JO, Jensen LS, Moller N, Lund S, Schmitz O 2005 Evidence against a role for insulin-signaling proteins PI 3-kinase and Akt in insulin resistance in human skeletal muscle induced by short-term GH infusion. Am J Physiol Endocrinol Metab 288:E194–E199 [DOI] [PubMed] [Google Scholar]
- Norrelund H, Djurhuus C, Jorgensen JO, Nielsen S, Nair KS, Schmitz O, Christiansen JS, Moller N 2003 Effects of GH on urea, glucose and lipid metabolism, and insulin sensitivity during fasting in GH-deficient patients. Am J Physiol Endocrinol Metab 285:E737–E743 [DOI] [PubMed] [Google Scholar]
- Norrelund H, Nair KS, Nielsen S, Frystyk J, Ivarsen P, Jorgensen JO, Christiansen JS, Moller N 2003 The decisive role of free fatty acids for protein conservation during fasting in humans with and without growth hormone. J Clin Endocrinol Metab 88:4371–4378. [DOI] [PubMed] [Google Scholar]
- Copeland KC, Nair KS 1994 Acute growth hormone effects on amino acid and lipid metabolism. J Clin Endocrinol Metab 78:1040–1047 [DOI] [PubMed] [Google Scholar]
- Buijs MM, Romijn JA, Burggraaf J, De Kam ML, Cohen AF, Frolich M, Stellaard F, Meinders AE, Pijl H 2002 Growth hormone blunts protein oxidation and promotes protein turnover to a similar extent in abdominally obese and normal-weight women. J Clin Endocrinol Metab 87:5668–5674 [DOI] [PubMed] [Google Scholar]
- Healy ML, Gibney J, Russell-Jones DL, Pentecost C, Croos P, Sonksen PH, Umpleby AM 2003 High dose growth hormone exerts an anabolic effect at rest and during exercise in endurance-trained athletes. J Clin Endocrinol Metab 88:5221–5226 [DOI] [PubMed] [Google Scholar]
- Welle S, Thornton C, Statt M, McHenry B 1996 Growth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years old. J Clin Endocrinol Metab 81:3239–3243 [DOI] [PubMed] [Google Scholar]
- Nygren J, Thorell A, Brismar K, Essen P, Wernerman J, McNurlan MA, Garlick PJ, Ljungqvist O 2002 Glucose flux is normalized by compensatory hyperinsulinaemia in growth hormone-induced insulin resistance in healthy subjects, while skeletal muscle protein synthesis remains unchanged. Clin Sci 102:457–464 [DOI] [PubMed] [Google Scholar]
- Gamrin L, Essen P, Hultman E, McNurlan MA, Garlick PJ, Wernerman J 2000 Protein-sparing effect in skeletal muscle of growth hormone treatment in critically ill patients. Ann Surg 231:577–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah JS, Chua SP, Ho CL 1975 Ultrastructure of the skeletal muscles in acromegaly: before and after hypophysectomy. Am J Med Sci 269:183–187 [DOI] [PubMed] [Google Scholar]
- Lange KH, Isaksson F, Juul A, Rasmussen MH, Bülow J, Kjær M 2000 Growth hormone enhances effects of endurance training on oxidative muscle metabolism in elderly women. Am J Physiol Endocrinol Metab 279:E989–E996 [DOI] [PubMed] [Google Scholar]
- Peyreigne C, Raynaud E, Fedou C, Prefaut C, Brun JF, Mercier J 2002 Does growth hormone treatment alter skeletal muscle mitochondrial respiration in rats? Horm Res 58:287–291 [DOI] [PubMed] [Google Scholar]
- Copeland KC, Kenney FA, Nair KS 1992 Heated dorsal hand vein sampling for metabolic studies: a reappraisal. Am J Physiol Endocrinol Metab 263:E1010–E1014 [DOI] [PubMed] [Google Scholar]
- Rooyackers OE, Balagopal P, Nair KS 1997 Measurement of synthesis rates of specific muscle proteins using needle biopsy samples. Muscle Nerve Suppl 5:S93–S96 [PubMed] [Google Scholar]
- Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS 2005 Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA 102:5618–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stump CS, Short KR, Bigelow ML, Schimke JC, Nair KS 2003 Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci USA 100:7996–8001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RR, Coenen-Schimke JM, Nair KS 2003 Impact of aerobic training on age-related changes in insulin action and muscle oxidative capacity. Diabetes 52:1888–1896 [DOI] [PubMed] [Google Scholar]
- Balagopal P, Schimke JC, Ades P, Adey D, Nair KS 2001 Age effect on transcript levels and synthesis rate of MHC and response to resistance exercise. Am J Physiol Endocrinol Metab 280:E203–E208 [DOI] [PubMed] [Google Scholar]
- Asmann YW, Stump CS, Short KR, Coenen-Schimke JM, Guo Z, Bigelow ML, Nair K 2006 Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and non-diabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes 55:3309–3319 [DOI] [PubMed] [Google Scholar]
- Jones B, Gilligan J 1983 Amino acid analysis by o-pthaldehyde pre-column derivitization and reversed phase HPLC. Am Biotechnol Lab 12:45–51 [Google Scholar]
- Matthews DE, Schwartz HP, Yang RD, Motil KJ, Young VR, Bier DM 1982 Relationship of plasma leucine and α-ketoisocaproate during a l-[1-C-13]leucine infusion in man: a method for measuring human intracellular leucine tracer enrichment. Metabolism 31:1105–1112 [DOI] [PubMed] [Google Scholar]
- Nair KS, Ford GC, Ekberg K, Fernqvist-Forbes E, Wahren J 1995 Protein dynamics in whole body and in splanchnic and leg tissues in type I diabetic patients. J Clin Invest 95:2926–2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungqvist OH, Persson M, Ford GC, Nair KS 1997 Functional heterogeneity of leucine pools in human skeletal muscle. Am J Physiol Endocrinol Metab 273:E564–E570 [DOI] [PubMed] [Google Scholar]
- Balagopal P, Ford GC, Ebenstein DB, Nadeau DA, Nair KS 1996 Mass spectrometric methods for determination of [13C] leucine enrichment in human muscle protein. Anal Biochem 239:77–85 [DOI] [PubMed] [Google Scholar]
- Brehm A, Krssak M, Schmid AI, Nowotny P, Waldhausl W, Roden M 2006 Increased lipid availability impairs insulin-stimulated ATP synthesis in human skeletal muscle. Diabetes 55:136–140 [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Shulman GI 2005 Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med 2(9):e233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokkalingam K, Jewell K, Norton L, Littlewood J, van Loon LJC, Mansell P, Macdonald IA, Tsintzas K 2007 High-fat/low-carbohydrate diet reduces insulin-stimulated carbohydrate oxidation but stimulates nonoxidative glucose disposal in humans: an important role for skeletal muscle pyruvate dehydrogenase kinase 4. J Clin Endocrinol Metab 92:284–292 [DOI] [PubMed] [Google Scholar]
- Spriet LL, Heigenhauser GJ 2002 Regulation of pyruvate dehydrogenase (PDH) activity in human skeletal muscle during exercise. Exerc Sport Sci Rev 30:91–95 [DOI] [PubMed] [Google Scholar]
- White UA, Coulter AA, Miles TK, Stephens JM 2007 The STAT5A-mediated induction of pyruvate dehydrogenase kinase 4 expression by prolactin or growth hormone in adipocytes. Diabetes 56:1623–1629 [DOI] [PubMed] [Google Scholar]
- Jorgensen JO, Jessen N, Pedersen SB, Vestergaard E, Gormsen L, Lund S, Billestrup N 2006 GH receptor signaling in skeletal muscle and adipose tissue in human subjects following exposure to an intravenous GH bolus. Am J Physiol Endocrinol Metab 291:E899–E905 [DOI] [PubMed] [Google Scholar]
- Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, Clayton DA 1998 Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet 28:231–236 [DOI] [PubMed] [Google Scholar]
- Huang X, Eriksson KF, Vaag A, Lehtovirta M, Hansson M, Laurila E, Kanninen T, Olesen BT, Kurucz I, Koranyi L, Groop L 1999 Insulin-regulated mitochondrial gene expression is associated with glucose flux in human skeletal muscle. Diabetes 48:1508–1514 [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Saltin B, Neufer PD 2002 Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol 340:831–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibney J, Healy ML, Sonksen PH 2007 The Growth hormone/insulin-like growth factor-I axis in exercise and sport. Endocr Rev 28:603–624 [DOI] [PubMed] [Google Scholar]
- Cartoni R, Leger B, Hock MB, Praz M, Crettenand A, Pich S, Ziltener J-L, Luthi F, Deriaz O, Zorzano A, Gobelet C, Kralli A, Russell AP 2005 Mitofusins 1/2 and ERRα expression are increased in human skeletal muscle after physical exercise. J Physiol 567:349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO 2002 Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 16:1879–1886 [DOI] [PubMed] [Google Scholar]
- Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JL, Kelly DP, Spiegelman BM 2001 Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci USA 98:3820–3825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM 2002 Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibers. Nature 418:797–800 [DOI] [PubMed] [Google Scholar]
- Mootha VK, Handschin C, Arlow D, Xie X, St. Pierre J, Sihag S, Yang W, Altshuler D, Puigserver P, Patterson N, Willy PJ, Schulman IG, Heyman RA, Lander ES, Spiegelman BM 2004 Errα and Gabpa/b specify PGC-1α-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc Natl Acad Sci USA 101:6570–6575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Roves P, Huss JM, Han DH, Hancock CR, Iglesias-Gutierrez E, Chen M, Holloszy JO 2007 Raising plasma fatty acid concentration induces increased biogenesis of mitochondria in skeletal muscle. Proc Natl Acad Sci USA 104:10709–10713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli R, Cittadini A, Chow JC, Hirshman MF, Smith RJ, Douglas PS, Horton ES 1996 Chronic growth hormone treatment in normal rats reduces post-prandial skeletal muscle plasma membrane GLUT1 content, but not glucose transport or GLUT4 expression and localization. Biochem J 315:959–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boirie Y, Short KR, Ahlman B, Charlton M, Nair KS 2001 Tissue-specific regulation of mitochondrial and sarcoplasmic protein synthesis rates by insulin. Diabetes 50:2652–2658 [DOI] [PubMed] [Google Scholar]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS 2004 Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab 286:E92–E101 [DOI] [PubMed] [Google Scholar]
- Bolster DR, Kubica N, Crozier S, Williamson PA, Farrell PA, Kimball SR, Jefferson LS 2003 Immediate response of mammalian target of rapamycin (mTOR)-mediated signaling following acute resistance exercise in rat skeletal muscle. J Physiol 553:213–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS 2004 Amino acids as regulators of gene expression. Nutr Metab 1:http://www.nutritionandmetabolism.com/content/1/1/3. Last accessed Feb 10, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarasheski KE, Zachwieja JJ, Angelopoulos TJ, Bier DM 1993 Short-term growth hormone treatment does not increase muscle protein synthesis in experienced weight lifters. J Appl Physiol 74:3073–3076 [DOI] [PubMed] [Google Scholar]
- Fryburg DA, Barrett EJ 1993 Growth hormone acutely stimulates skeletal muscle but not whole-body protein synthesis in humans. Metabolism 42:1223–1227 [DOI] [PubMed] [Google Scholar]
- Wolf RF, Heslin MJ, Newman E, Pearlstone DB, Gonenne A, Brennan MF 1992 Growth hormone and insulin combine to improve whole-body and skeletal muscle protein kinetics. Surgery 112:284–292 [PubMed] [Google Scholar]
- Butterfield GE, Thompson J, Rennie MJ, Marcus R, Hintz RL, Hoffman AR 1997 Effect of rhGH and rhIGF-I treatment on protein utilization in elderly women. Am J Physiol Endocrinol Metab 272:E94–E99 [DOI] [PubMed] [Google Scholar]
- Bark TH, McNurlan MA, Lang CH, Garlick PJ 1998 Increased protein synthesis after acute IGF-I or insulin infusion is localized to muscle in mice. Am J Physiol Endocrinol Metab 275:E118–E123 [DOI] [PubMed] [Google Scholar]
- Lang CH, Vary TC, Frost RA 2003 Acute in vivo elevation of insulin-like growth factor (IGF) binding protein-1 decreases plasma free IGF-I and muscle protein synthesis. Endocrinology 144:3922–3933 [DOI] [PubMed] [Google Scholar]
- Russel-Jones DL, Umpleby AM, Hennessy TR, Bowes SB, Shojaee-Moradie F, Hopkins KD, Jackson NC, Kelly JM, Jones RH, Sonksen PH 1994 Use of a leucine clamp to demonstrate that IGF-I activity stimulates protein synthesis in normal humans. Am J Physiol Endocrinol Metab 267:E591–E598 [DOI] [PubMed] [Google Scholar]
- Conley KE, Jubrias SA, Esselman PC 2000 Oxidative capacity and ageing in human muscle. J Physiol 526:203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Befoy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI 2003 Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300:1140–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]