Abstract
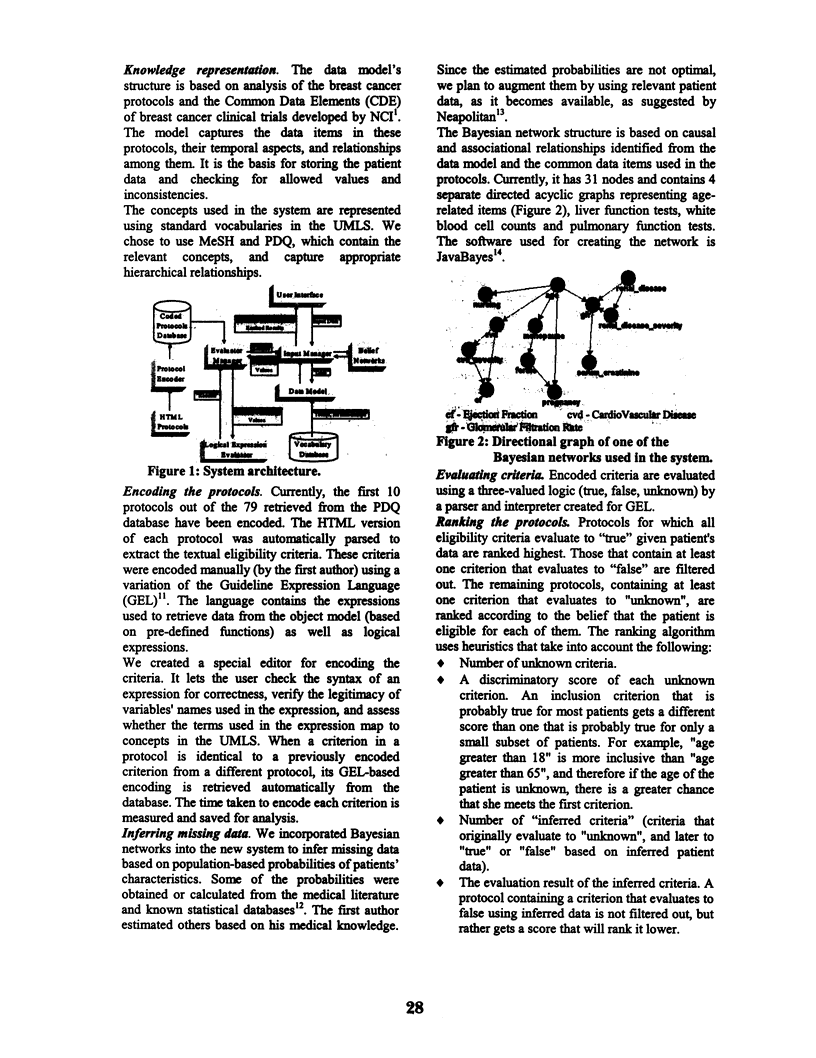
We describe our work on creating a system that selects appropriate clinical trials by automating the evaluation of eligibility criteria. We developed a data model of eligibility for breast cancer clinical trials, upon which the criteria were encoded. Standard vocabularies are utilized to represent concepts used in the system, and retrieve their hierarchical relationships. The system incorporates Bayesian networks to handle missing patient information. Protocols are ranked by the belief that the patient is eligible for each of them. In a preliminary evaluation, we found good agreement (kappa 0.86) between the system and an independent physician in selection of protocols, but poor agreement (kappa 0.24) in protocol ranking. We conclude that our approach is feasible, and potentially useful in assisting both physicians and patients in the task of selecting appropriate trials.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breitfeld P. P., Weisburd M., Overhage J. M., Sledge G., Jr, Tierney W. M. Pilot study of a point-of-use decision support tool for cancer clinical trials eligibility. J Am Med Inform Assoc. 1999 Nov-Dec;6(6):466–477. doi: 10.1136/jamia.1999.0060466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennari J. H., Reddy M. Participatory design and an eligibility screening tool. Proc AMIA Symp. 2000:290–294. [PMC free article] [PubMed] [Google Scholar]
- McCray A. T. Better access to information about clinical trials. Ann Intern Med. 2000 Oct 17;133(8):609–614. doi: 10.7326/0003-4819-133-8-200010170-00013. [DOI] [PubMed] [Google Scholar]
- Ohno-Machado L., Wang S. J., Mar P., Boxwala A. A. Decision support for clinical trial eligibility determination in breast cancer. Proc AMIA Symp. 1999:340–344. [PMC free article] [PubMed] [Google Scholar]
- Papaconstantinou C., Theocharous G., Mahadevan S. An expert system for assigning patients into clinical trials based on Bayesian networks. J Med Syst. 1998 Jun;22(3):189–202. doi: 10.1023/a:1022667800953. [DOI] [PubMed] [Google Scholar]
- Ries L. A., Wingo P. A., Miller D. S., Howe H. L., Weir H. K., Rosenberg H. M., Vernon S. W., Cronin K., Edwards B. K. The annual report to the nation on the status of cancer, 1973-1997, with a special section on colorectal cancer. Cancer. 2000 May 15;88(10):2398–2424. doi: 10.1002/(sici)1097-0142(20000515)88:10<2398::aid-cncr26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Rubin D. L., Gennari J. H., Srinivas S., Yuen A., Kaizer H., Musen M. A., Silva J. S. Tool support for authoring eligibility criteria for cancer trials. Proc AMIA Symp. 1999:369–373. [PMC free article] [PubMed] [Google Scholar]
- Tu S. W., Kemper C. A., Lane N. M., Carlson R. W., Musen M. A. A methodology for determining patients' eligibility for clinical trials. Methods Inf Med. 1993 Aug;32(4):317–325. [PubMed] [Google Scholar]


