Abstract
The ovarian hormones, estradiol (E) and progesterone (P) facilitate the expression of sexual behavior in female rats. E and P mediate many of these behavioral effects by binding to their respective intracellular receptors in specific brain regions. Nuclear receptor coactivators, including Steroid Receptor Coactivator-1 (SRC-1) and CREB Binding Protein (CBP), dramatically enhance ligand-dependent steroid receptor transcriptional activity in vitro. Previously, our lab has shown that SRC-1 and CBP modulate estrogen receptor (ER)-mediated induction of progestin receptor (PR) gene expression in the ventromedial nucleus of the hypothalamus (VMN) and hormone-dependent sexual receptivity in female rats. Female sexual behaviors can be activated by high doses of E alone in ovariectomized rats, and thus are believed to be ER-dependent. However, the full repertoire of female sexual behavior, in particular, proceptive behaviors such as hopping, darting and ear wiggling, are considered to be PR-dependent. In the present experiments, the function of SRC-1 and CBP in distinct ER- (Exp. 1) and PR- (Exp. 2) dependent aspects of female sexual behavior was investigated. In Exp. 1, infusion of antisense oligodeoxynucleotides to SRC-1 and CBP mRNA into the VMN decreased lordosis intensity in rats treated with E alone, suggesting that these coactivators modulate ER-mediated female sexual behavior. In Exp. 2, antisense to SRC-1 and CBP mRNA around the time of P administration reduced PR-dependent ear wiggling and hopping and darting. Taken together, these data suggest that SRC-1 and CBP modulate ER and PR action in brain and influence distinct aspects of hormone-dependent sexual behaviors. These findings support our previous studies and provide further evidence that SRC-1 and CBP function together to regulate ovarian hormone action in behaviorally-relevant brain regions.
Keywords: Steroid hormone, SRC-1, CBP, Hypothalamus, Reproductive behavior, Lordosis, Proceptive behavior, Nuclear receptor coactivator
Introduction
The ovarian steroid hormones estradiol (E) and progesterone (P) act in the brain to regulate female sexual behavior in rodents (Boling and Blandau, 1939; Yanase and Gorski, 1976; Pfaff, 1980; Clemens and Weaver, 1985; Blaustein and Erskine, 2002). In female rats, estradiol is necessary for sexual receptivity, which is characterized by the lordosis posture (Kow and Pfaff, 1975; Barfield and Chen, 1977; Pleim et al., 1989). Estradiol, followed by progesterone, is required for optimal expression of female sexual behavior (Hardy and DeBold, 1971; Pfaff, 1980; Rubin and Barfield, 1983; Edwards and Pfeifle, 1983). However, administration of high doses of estradiol alone can elicit receptivity in female rats (Davidson et al., 1968; Komisaruk and Diakow, 1973; Gilman and Hitt, 1978; Hlinak and Madlafousek, 1981; Edwards and Pfeifle, 1983; Fernandez-Guasti et al., 1991). Furthermore, lordosis increases in frequency with increasing E doses (Whalen, 1974; Gilman and Hitt, 1978; Hlinak and Madlafousek, 1981).
Progesterone appears to be essential for specific reproductive behaviors in E-primed female rodents (Whalen, 1974; Fadem et al., 1979; Pfaff, 1980; Tennent et al., 1980; Rubin and Barfield, 1983; Edwards and Pfeifle, 1983; Hlinak and Madlafousek, 1983; Clemens and Weaver, 1985; Erskine, 1989; Mani et al., 1997). An example of P-dependent female sexual behavior is proceptivity, which serves to solicit interaction with the male (Hardy and DeBold, 1971; Whalen, 1974; Gilman and Hitt, 1978; Fadem et al., 1979; Clemens and Weaver, 1985; Erskine, 1989; Ogawa et al., 1994). These proceptive behaviors include hopping and darting and ear-wiggling. While low levels of proceptivity may be elicited in females by high pharmacological doses of estradiol (Gilman et al., 1978; Fadem et al., 1979; Hlinak et al., 1981; Edwards et al., 1983), a variety of studies show that proceptivity is P-dependent within physiological doses of exogenous hormone (Hardy et al., 1971; Whalen, 1974; Fadem et al., 1979; Tennent et al., 1980; Hlinak and Madlafousek, 1981, 1983; Edwards and Pfeifle, 1983; Erskine, 1989). In further support of proceptivity being P-dependent, P increases proceptivity in a dose-dependent manner (Whalen, 1974; Tennent et al., 1980; Hlinak and Madlafousek, 1983), while E does not (Whalen, 1974). Finally, studies using antisense to progestin receptor (PR) mRNA reveal that proceptivity is PR-dependent (Ogawa et al., 1994; Mani et al., 1994).
Estradiol and progesterone regulate the expression of female sexual behaviors by acting in specific brain regions, including the medial preoptic area (mPOA), ventromedial nucleus of the hypothalamus (VMN), ventral tegmental area, amygdala, and midbrain central gray (Yanase et al., 1976; Gorski, 1976; Luttge and Hughes, 1976; Barfield and Chen, 1977; Franck and Ward, 1981; Tennent et al., 1982; Pleim et al., 1989; Mani and O'Malley, 2002). The VMN is considered to be an important site for the expression of hormone-dependent female sexual behaviors (Barfield et al., 1977; Davis et al., 1982; Pleim et al., 1989). Lesions of the VMN inhibit the display of lordosis (Pfaff and Sakuma, 1979a), while electrical stimulation of this region enhances lordosis (Pfaff and Sakuma, 1979b). Moreover, the VMN appears to be a critical site for hormone-dependent sexual receptivity. Estradiol implants (Pfaff and Sakuma, 1979a; Pleim et al., 1989), or P implants in E-primed ovariectomized rats (Pleim et al., 1991), in this region facilitate lordosis.
Estradiol and progesterone elicit many of their behavioral effects by binding to their respective receptors in specific brain regions. Receptors for estrogens (ER) and PR, as well as receptors for androgens and glucocorticoids, are members of a large superfamily of nuclear transcriptional activators (Tsai and O'Malley, 1994; Mangelsdorf et al., 1995). Upon binding hormone, the receptor changes conformation, allowing dissociation of heat shock proteins and dimerization of two activated receptors (DeMarzo et al., 1991). The activated dimer complex preferentially binds to hormone response elements of target genes to alter the rate of transcription (Beato and Sánchez-Pacheco, 1996). One classic example of steroid-dependent transcription is E-induction of PR in brain and other tissues (MacLusky and McEwen, 1978; Blaustein and Feder, 1979; Parsons et al., 1980; Pleim et al., 1989; Lauber et al., 1991). Estradiol-bound ER dimers bind to the estrogen response element of the PR gene (Savouret et al., 1991; Kraus et al., 1994) to induce PR expression. This ER-mediated transactivation of the PR gene occurs in brain areas known to regulate female sexual behavior, including the mPOA, VMN, arcuate nucleus and midbrain central gray (MacLusky and McEwen, 1978; Blaustein and Feder, 1979; Parsons et al., 1982; Pleim et al., 1989; Lauber et al., 1991; Simerly, 1993). Moreover, E induction of PR in the VMN is correlated with high levels of female sexual behavior in rats (Pleim et al., 1989).
Steroid receptor transcriptional activity is dramatically enhanced by nuclear receptor coactivators in vitro (for reviews, see Glass et al., 1997; McKenna et al., 1999; Robyr et al., 2000). Nuclear receptor coactivators are thought to promote steroid-dependent gene expression by bridging the steroid receptor complex with the basal transcription machinery and inducing chromatin remodeling via their intrinsic histone acetyltransferase activity (Bannister and Kouzarides, 1996; Spencer et al., 1997; Liu et al., 1999).
The p160 family of nuclear receptor coactivators includes Steroid Receptor Coactivator-1 (SRC-1/NCoA-1) (Oñate et al., 1995), SRC-2 (NCoA-2/GRIP-1/TIF-2) (Voegel et al., 1996; Hong et al., 1997) and SRC-3 (RAC3/AIB1/pCIP/ACTR/ TRAM1) (Anzick et al., 1997; Li et al., 1997; Xu et al., 2000a, b). In vitro, SRC-1 enhances ligand-dependent transcriptional activity of a variety of nuclear receptors, including ER and PR (Oñate et al., 1995). SRC-1 is expressed in many regions of the rat brain, including the hypothalamus, hippocampus, cerebellum, paraventricular nucleus, thalamus and amygdala (Meijer et al., 2000; and for review see Tetel, 2000; Molenda et al., 2003).
CREB Binding Protein (CBP) was initially described as a transcriptional activator of cAMP-response element binding protein (CREB) (Chrivia et al., 1993; Kwok et al., 1994), but more recently has been found to be important in ligand-dependent transcription by nuclear receptors, including ER and PR (Smith et al., 1996; Xu et al., 2000a,b). In vitro studies indicate that SRC-1 and CBP act together to enhance full ER and PR transcriptional activity and function (Smith et al., 1996; Tetel et al., 1999; Xu et al., 2000a,b; Liu et al., 2001; Kim et al., 2001). Immunohisto-chemical studies reveal that CBP protein is expressed throughout the brain, and found in high levels in the hypothalamus, preoptic area, thalamus, amygdala, hippocampus, cortex and cerebellum (Stromberg et al., 1999; Molenda et al., 2002; Auger et al., 2002).
Our lab and others have found that SRC-1 (Molenda et al., 2002; Auger et al., 2000; Apostolakis et al., 2002; Charlier et al., 2005) and CBP (Molenda et al., 2002; Auger et al., 2002) are important for ER action in the brain. SRC-1 and CBP are critical in hormone-dependent sexual differentiation of sexual behaviors in rats (Auger et al., 2000, 2002). In addition, SRC-1 is important for the expression of hormone-dependent male sexual behaviors in Japanese quail (Charlier et al., 2005). Work by others has shown that antisense to SRC-1 decreases estrogen-induced PR in the VMN of female rats (Apostolakis et al., 2002). In our previous work, infusion of antisense oligodeoxynucleotides (ODNs) to both SRC-1 and CBP mRNA decreases ER-mediated PR gene expression in the behaviorally-relevant VMN (Molenda et al., 2002). Interestingly, infusion of antisense to either SRC-1 or CBP mRNA alone did not alter PR expression in the VMN (Molenda et al., 2002). These findings (Molenda et al., 2002), which are supported by in vitro studies (Smith et al., 1996; Kim et al., 2001), suggest that SRC-1 and CBP act together to modulate ER-dependent transcription.
Given that SRC-1 and CBP have been found to function together to enhance ER- and PR-dependent transcription in vitro (Smith et al., 1996; Liu et al., 2001; Kim et al., 2001) and our previous work (Molenda et al., 2002), the present studies investigated the role of both SRC-1 and CBP in female sexual behaviors in the rat. While our earlier study found that SRC-1 and CBP modulated sexual receptivity in hormone (E+P)-primed adult female rats (Molenda et al., 2002), it did not distinguish whether these coactivators were influencing the action of ER, PR, or both. Therefore, the present experiments investigated the function of SRC-1 and CBP in distinct ovarian steroid receptor-dependent reproductive behaviors: ER-dependent receptivity and PR-dependent proceptivity.
Methods
Animals
Adult female (175–200 g) and male (300–350 g) Sprague–Dawley rats from Charles River Breeding Laboratories (Wilmington, MA) were housed singly in a 14:10 light–dark cycle, with lights off at 10 a.m. Animals were given food and water ad libitum. Female rats were anesthetized with a combination of chloral hydrate (170 mg/kg) and pentobarbital (35 mg/kg), ovariectomized and then placed in a stereotaxic apparatus for implantation of 28 gauge bilateral cannulae aimed just dorsal to the VMN (AP, −3.14 mm; ML, 1.0 mm; DV, −8.0 mm Paxinos and Watson, 1998), as described previously (Molenda et al., 2002). This cannula placement allows infusion of oligodeoxynucleotides into the VMN without destroying VMN cell bodies or fibers (Molenda et al., 2002). Only animals in which infusion cannulae descended to a depth within 0.3 mm of the fornix, and medial to the fornix, were included in immunocytochemical and behavioral analyses. During a 1-week recovery period to allow clearing of endogenous hormones, the animals were handled and dummy cannulae removed and replaced to prevent clogging of guide cannulae. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Massachusetts, Amherst and Skidmore College.
Antisense oligonucleotides
Antisense oligodeoxynucleotides (ODNs) (Oligos Etc., Wilsonville, OR) (21-mer) specific to SRC-1 (5′ CTG-TCC-CCA-AGG-CCA-CTC-ATG 3′, targeting both SRC-1a and SRC-1e isoforms) and CBP (5′ CAG-CAA-GTT-CTC-GGC-CAT-CTT 3′) mRNA contain limited phosphorothioate-linkages and were designed to span the translation start site to prevent translation of these coactivators (Molenda et al., 2002). Control oligodeoxynucleotides (Oligos, Etc., Wilsonville, OR) containing the same bases in scrambled sequence were confirmed to not cross-hybridize with other rodent gene sequences by a BLAST search of NCBI (http://www.ncbi.nlm.nih.gov).
Experiment 1: Nuclear receptor coactivator function in ER-dependent receptivity
Prior to the experiment, it was critical to establish a dose of estradiol benzoate (EB) that would yield consistent levels of estradiol-dependent sexual receptivity. In pilot studies, ovariectomized females (n = 4–5 rats/group) were tested with EB doses of 3, 5, 7 or 10 μg subcutaneously (Hardy and DeBold, 1971, 1972; Komisaruk and Diakow, 1973) 48 and 24 h prior to behavior. To standardize male rats' sexual stimulation across experimental females, all female rats were vaginally masked by placing tape over the vaginal opening and securing it to the base of the tail during prescreening and behavioral tests (Castro-Vazquez and Carreno, 1985). Each lordosis response was measured on a scale from 0 (no dorsoflexion) to 3 (maximal dorsoflexion) (Hardy and DeBold, 1972; Powers and Valenstein, 1972). The 7 μg dose of EB resulted in consistent levels of receptivity (rats displaying a lordosis intensity response of 2 on at least 3 out of 5 mounts) and was chosen as the EB dose for this experiment.
In Exp. 1, animals (n = 42) were allowed to recover from ovariectomy and cannulations for 1 week. Females were then injected with 7 μg of EB on two consecutive days. Twenty-eight hours following the last EB treatment, females were prescreened for sexual receptivity with an intact, sexually experienced male. The lordosis response was measured on the 0–3 intensity scale described above. For each female, lordosis intensity (LI, total points scored/number of mounts) and the lordosis quotient [(LQ, number of lordosis responses/number of mounts)×100] was calculated. Only females that were sexually receptive, as indicated by displaying a lordosis intensity rating of 2 or better, on 3 out of 5 mounts, remained in the study. Thirteen rats were eliminated from the study because they did not meet the receptivity prescreening criteria.
One week following prescreening, each female was administered one ODN infusion at 0900 h on days 1, 2 and 3. The experimental group of females was administered bilateral infusions of 1 μg of SRC-1 antisense ODN and 1 μg of CBP antisense ODN in 1 μl of saline into the VMN (n = 14). The control group received bilateral infusions of 1 μg of each of the scrambled control ODNs in 1 μl of saline (n = 15). Infusion cannulae were left in place for 1 min after infusions to prevent the ODNs from being drawn up into the guide cannulae. On days 2 and 3, all females were injected with 7 μg of EB.
Twenty-eight hours after the last EB injection, females were tested for receptivity with an intact sexually experienced male in a Plexiglass arena (53 cm diameter). Testing was performed under a dim red light during the dark phase of the light:dark cycle, with each female receiving 10 mounts by the male. For each mount, the lordosis response and rejection behaviors (kicking, biting, or fighting) were recorded by two experimenters, who were blind with regard to treatment group. For each female, LI and LQ were calculated. Because a variety of previous studies report that proceptive behaviors are P-dependent (Hardy and DeBold, 1971; Whalen, 1974; Fadem et al., 1979; Tennent et al., 1980; Hlinak and Madlafousek, 1981, 1983; Edwards and Pfeifle, 1983; Erskine, 1989), proceptive behaviors were not analyzed in this experiment (Exp. 1). The number of testing chamber midline crossings was also recorded as a measure of general motor activity. All behavior sessions were videotaped for confirmation of behavioral scoring.
Experiment 2: Nuclear receptor coactivator function in PR-dependent proceptive behaviors
In order to confirm that proceptive behaviors were P-dependent in our experimental paradigm, we tested E-primed females treated with P or vehicle in a pilot experiment. Rats were ovariectomized and 1 week later primed with either 2 μg EB + 300 μg P 44 h later (n = 13), or EB (2 μg) + oil vehicle (n = 14) (Molenda et al., 2002). In this pilot experiment, the LI, LQ, the number of proceptive behaviors, including ear wiggles and hopping and darting, and rejection behaviors, were quantified over the 10-mount testing period.
For Exp. 2, a new group of female rats (n = 65) was ovariectomized and cannulated as described in Experiment 1. One week later, all females were prescreened for sexual receptivity. Rats were primed with 2 μg of EB and 44 h later with 300 μg of P. Four hours after P, females were tested for sexual receptivity with an intact male. Only receptive females remained in the study. Using the prescreening criteria described in Exp. 1, 13 animals were eliminated from this experiment. One week later, rats were administered 2 μg EB at 600 h on day 1 of the experiment. At 1100 h and 2200 h on day 2, experimental females were given bilateral infusions of 1 μg of SRC-1 antisense ODN and 1 μg of CBP antisense ODN in 1 μl of saline (n = 27), while control animals were given bilateral infusions of 1 μg of each of the scrambled control ODNs (n = 25). On day 3, animals were injected with 300 μg of P and tested 4 and 10 h later for sexual behavior with an intact male as described above. The number of ear wiggles, hops, darts and rejections were counted over the 10-mount testing period (Tennent et al., 1980; Vathy et al., 1989), and LI and LQ were calculated for each female. The number of times the rats crossed the testing chamber midline was also recorded as a measure of locomotor activity. Cannulae placements were checked for each animal, and only animals in which infusion cannulae descended to a depth within 0.3 mm of the fornix, and medial to the fornix, were included in immunocytochemical and behavioral analyses.
Immunohistochemistry
In this experiment we targeted PR action using an antisense ODN technique to investigate the function of SRC-1 and CBP in P-facilitated sexual behaviors. Therefore, it was critical to ensure that the timing of the antisense infusions would not interfere with estradiol induction of PR in the VMN, which peaks around 24 h following estradiol treatment (Parsons et al., 1980). To confirm that treatment with antisense ODNs did not interfere with estradiol- induction of PR in Exp. 2, immunohistochemistry for PR expression in the VMN was done as described previously (Molenda et al., 2002). Animals were administered 2 μg of EB and infused bilaterally with antisense to SRC-1 and CBP mRNA (n = 14) or control ODNs (n = 13) as described above. Forty-eight hours following E, animals were injected i.p. with an overdose of an anesthetic mixture (425 mg/kg chloral hydrate and 89 mg/kg pentobarbital) and perfused intracardially with saline for 1 min, followed by 2% acrolein in 0.1 M phosphate buffer for 14 min at a fixative flow rate of 25 ml/min. Brains were removed and stored in 20% sucrose solution for 48 h at 4°C and transverse sections were cut from the POA through the hypothalamus at 40μm on a freezing microtome. Brain sections were stored in cryoprotectant at −20°C until immunohistochemistry was performed.
Sections were initially rinsed in 0.05 M Tris-buffered saline (TBS), followed by a pretreatment of 1% sodium borohydride for 10 min to remove residual aldehydes. Tissue was then rinsed in TBS and incubated in a solution of 1% H2O2, 20% normal goat serum and 1% bovine serum albumin in TBS for 20 min to decrease non-specific staining and reduce endogenous peroxidase activity. Sections were incubated for 48 h in 1:1000 dilution of a rabbit polyclonal primary antiserum generated against the DNA binding domain of human PR (A0098, DAKO, Denmark) in TBS containing 0.02% sodium azide (NaN3), 1% normal goat serum, 0.1% gelatin and Triton-X (pH 7.6 at 4°C). Tissue was rinsed in TBS containing NaN3, gelatin and Triton-X prior to incubation in a biotinylated goat anti-rabbit antiserum (3 μg/ml, Vector Laboratories, Burlingame, CA) in TBS containing NaN3 and Triton X-100 and 1.5% normal goat serum for 90 min. Tissue was rinsed in TBS containing NaN3, gelatin and Triton X-100 followed by rinsing in TBS. Sections were then incubated for 90 min in TBS containing 1% avidin DH: biotinylated horseradish peroxidase H complex (Vectastain ABC Elite Kit, Vector) followed by rinsing in TBS. Finally, sections were exposed to 0.05% diaminobenzine (DAB) with 3% hydrogen peroxide with TBS for approximately 3 min. The sections were rinsed in TBS and then mounted on microscope slides and coverslipped using DePeX mounting medium (Electron Microscopy Sciences, Fort Washington, PA).
PR-immunoreactive cells in one matched section of the VMN (Plate 32 Paxinos and Watson, 1998) were analyzed under 200× magnification using a Leitz Dialux 20 microscope (Leitz, Wetzler, Germany) with an MTI CCD72 camera (Dage MTI, Michigan City, MI) connected to a Macintosh G3 computer. The number of immunoreactive cells was quantified using the NIH Image 1.62 program as described previously (Molenda et al., 2002). Briefly, the microscope was placed in Kohler illumination, and the gain and black levels of the camera were adjusted so that the pixel density of a blank section of the slide measured 2–10 units and black measured 252 units. The threshold for detection of specific immunoreactivity was determined as a function of background. Cells were considered immunopositive at a density four times that of the background threshold and if they were greater than 10 pixels, and less than 200 pixels, in total area.
Interestingly, SRC-2 is overexpressed in the brains of SRC-1 knockout mice, suggesting that SRC-2 could compensate for the loss of SRC-1 (Xu et al., 1998). Therefore, we investigated whether there was an increase in SRC-2 expression during an acute decrease in SRC-1 and CBP protein levels in the VMN following antisense administration. Immunostaining for SRC-2 was performed as described above for PR with the following changes. Tissue was incubated in a mouse monoclonal primary antibody (1.5 μg/ml, BD Biosciences, Palo Alto, CA), generated against amino acids 962 to 1067 of human SRC-2 (TIF2), followed by a biotinylated goat anti mouse secondary antiserum (3 μg/ml, Vector Laboratories, Inc., Burlingame, CA).
Western blot analysis of nuclear receptor coactivator protein expression
To determine if antisense to SRC-1 and CBP mRNA infused into the VMN around the time of progesterone administration decreased coactivator expression, animals were ovariectomized and cannulated as described previously. Animals were administered EB (2 μg) on day 1, followed by two infusions on day 2 with antisense to SRC-1 and CBP mRNA on one side of the VMN, and scrambled control ODNs on the contralateral side. The following day, animals were administered P (300 μg) and sacrificed 4 h later by decapitation. The brains were removed and the hypothalami were dissected out and cut in half along the third ventricle to divide antisense- and control-treated sides of the VMN. Each half was flash frozen on dry ice, placed in a microcentrifuge tube, and stored at −80°C until homogenization. Tissue samples were homogenized in lysis buffer (10 mM Tris, 10% glycerol, 400 mM NaCl, 1 mM DTT, 1 mM EDTA, pH 7.4) with a 1:10 dilution of protease inhibitor cocktail (2 mM AEBSF, 1 mM EDTA, 130 μM Bestatin, 14 μM E-64, 1 μM Leupeptin, 0.3 μM Aprotinin, Sigma, Saint Louis, MO). Samples were then incubated on ice for 30 min, and then centrifuged for 30 min. at 4°C at 40,000 rpm. Following centrifugation, supernatants were frozen at −80°C.
Samples were assayed by Western blot analysis for detection of SRC-1 (~160 kDa, Oñate et al., 1995) and CBP (~265 kDa, Chrivia et al., 1993) proteins. Briefly, nitrocellulose membranes containing eluted proteins were washed in 0.1 M TBS containing 0.05% Tween-20 (TBS-T) and blocked for 1 h in TBS-T with 5% nonfat dry milk. The membranes were then incubated in a rabbit polyclonal antibody generated against amino acids 350–690 of mouse SRC-1 (1:1000, M-341, Santa Cruz Biotechnology, Santa Cruz, CA). Following the incubation, membranes were washed in TBS-T. Membranes were then incubated in a horseradish peroxidase linked donkey anti-rabbit secondary antibody (1:10,000, Amersham Biosciences, Buckinghamshire, England) for 1 h, at room temperature. Membranes were then rinsed in TBS-T. Immunoreactive bands were detected with an enhanced chemiluminescence kit (ECL Plus; New England Biolabs), and membranes exposed to film (Blue Sensitive X-ray film, Laboratory Products Sales, Rochester, NY). Membranes were stripped for 3 h at 70°C in stripping buffer (2% sodium laurel sulfate, 62.5 mM Tris HCl, 100 mM 2-mercaptoethanol, H2O, pH 6.7), and then reprobed for the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using a mouse monoclonal primary antibody (1:100,000, Chemicon International, Inc., Temecula, CA) followed by a sheep anti-mouse secondary antiserum (1:10,000, Amersham Biosciences, Buckinghamshire, England). Membranes were stripped again and reprobed for CBP using a rabbit polyclonal primary antibody generated against amino acids 162–176 of human CBP peptide (1:4000, PA1-847, Affinity BioReagents, Golden, CO), followed by a donkey anti-rabbit secondary antibody (1:7500).
Films were placed on a light box (Fotodyne, New Berlin, WI) and photographed with an Olympus Camedia digital camera (Olympus Optical Co.). Images were imported into the NIH ImageJ 1.36 analysis program (National Institutes of Health) on a Dell Optiplex GX140 computer and analyzed for integrated density (area of band×mean optical density) of immunoreactive bands.
Statistics
Analyses of behavioral scores, immunoreactivity for each brain area, and integrated density of immunoreactive bands from Western blots were done using two-tailed t tests unless indicated otherwise (SigmaStat 2.03, SPSS, San Rafael, CA). Statistics are reported as the mean and the standard error of the mean. Differences were considered significant at a probability of less than 0.05.
Results
Exp. 1: ER-dependent female sexual receptivity
Our lab has previously demonstrated that reduction of SRC-1 and CBP decreased the intensity of sexual receptivity in E and P primed rats (Molenda et al., 2002). However, this previous study did not determine if these coactivators were altering ER or PR action in the brain to affect behavior. Therefore, the hypothesis that nuclear receptor coactivators modulate the expression of ER-dependent receptive behavior in animals administered estradiol alone was tested.
In animals administered EB alone, bilateral infusion of antisense ODNs to both SRC-1 and CBP mRNA in the VMN reduced the lordosis quotient compared to scrambled control ODNs (Fig. 1A). Treatment with antisense to SRC-1 and CBP mRNA also decreased the intensity of lordosis in female rats compared to rats treated with scrambled control ODNs (Fig. 1B). The number of times the animals crossed the midline of the testing chamber did not differ between rats treated with antisense to SRC-1 and CBP (23.17 ± 4.08; n = 6) and scrambled control-treated rats (28.71 ± 3.03; n = 7; P = 0.29), suggesting that the decrease in receptivity in the antisense treated animals was not due to decreases in overall locomotor behavior. These data extend our previous findings and provide further evidence that SRC-1 and CBP regulate ER action in the VMN. Moreover, these results suggest these nuclear receptor coactivators function in the VMN to modulate the expression of ER-dependent female sexual behavior.
Fig. 1.
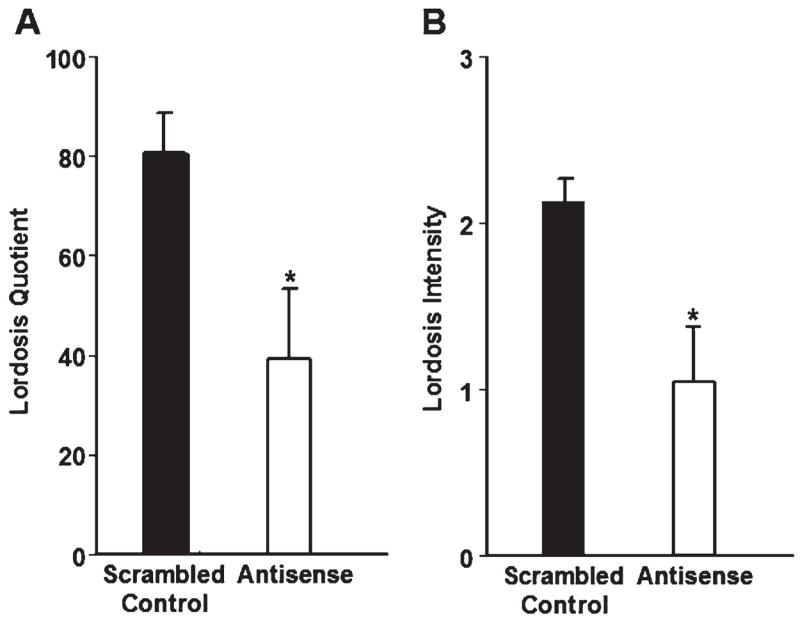
(A) Animals infused with antisense ODNs to both SRC-1 and CBP mRNA (n = 11) in the VMN had a decrease in lordosis quotient compared with scrambled control ODN-treated animals (n = 10). *P < 0.03. (B) Infusion of antisense ODNs to both SRC-1 and CBP mRNA in the VMN decreased lordosis intensity compared with scrambled control ODN-treated animals. *P < 0.01.
Three antisense- and five scrambled control-treated rats were eliminated from data analyses due to cannula placement that did not meet criteria described in the Methods section. Antisense treated rats with inappropriately placed cannulae did not differ from scrambled control-treated animals with correct cannula placement on any behavioral measures (data not shown). While it should be pointed out that the small number of antisense-treated rats with missed cannula placement makes these results difficult to interpret, these findings suggest that antisense is acting in a region-specific manner to affect female sexual behavior.
Exp. 2: PR-dependent proceptive behavior
In vitro studies suggest that SRC-1 and CBP act together to modulate progestin receptor activity and function (Smith et al., 1996; Tetel et al., 1999; Xu et al., 2000a,b; Liu et al., 2001). We tested the hypothesis that SRC-1 and CBP function together in PR-dependent proceptive behaviors, including ear-wiggling and hopping and darting. In order to test this, it was first determined in pilot studies if proceptivity in female rats was P-dependent within our experimental paradigm, as has been reported previously. In the pilot study, females treated with 2 μg EB and 300 μg P displayed ear-wiggling behaviors (7.92 ± 3.00) and hops and darts (2.23 ± 1.18), whereas EB-only (2 μg) treated females displayed little to none of these behaviors (ear-wiggling, 0.29 ± 0.16, P < 0.03, two-tailed; hops and darts, 0.00 ± 0.00; P < 0.05, one-tailed). The LI for rats treated with estradiol +oil was considerably lower (1.22 0.28) than that of rats that received EB+P (2.11 ± 0.12, P < 0.01, two-tailed t test). Furthermore, rats treated with EB+oil vehicle displayed an LQ of only 24%, whereas those rats treated with EB+P had an LQ of 75% (P b 0.001, two-tailed t test), which is above the accepted threshold for sexual receptivity of N60–70% (Edwards and Pfeifle, 1983; Bennett et al., 2002). These results demonstrate that under our experimental conditions, these animals were not in estrogen heats, and confirm that proceptive behaviors under these conditions are dependent on progesterone.
Females infused with antisense ODNs to SRC-1 and CBP mRNA into the VMN displayed fewer ear wiggles than did scrambled control-treated rats (Fig. 2A). In addition, antisense to nuclear receptor coactivators infused into the VMN reduced PR-dependent hops and darts (Fig. 2B, P < 0.5, one-tailed t test; a decrease in hops and darts by antisense to coactivator mRNA was predicted). There were no differences in the number of chamber midline crossings between scrambled control (10.00 ± 1.54) and antisense-treated rats (10.25 ± 1.40; P = 0.91), suggesting that a decrease in proceptive behaviors by antisense treatment was not due to differences in locomotor activity. While animals in Exp. 1 (treated with EB only) had a higher number of chamber midline crossings than animals in Exp. 2 (treated with a lower dose of EB and P), these findings are consistent with previous studies revealing that estradiol increases (Rodier and Segal, 1977), and progesterone decreases (Axelson et al., 1981), locomotor activity. Taken together, these findings suggest that SRC-1 and CBP are important for the expression of PR-dependent proceptivity. Interestingly, differences in the lordosis quotient between rats treated with antisense ODN (77 ± 7.0) and scrambled control ODNs (90 ± 5.0, P = 0.14) were not detected. Likewise, no differences in LI were detected between antisense ODN (2.01 ± 0.16) and scrambled control-treated rats (2.14 ± 0.08, P = 0.48). No differences were detected in the incidence of rejections of male rats between antisense (2.54 ± 0.63) and scrambled control-treated females (1.63 ± 0.45, P = 0.24), which is consistent with studies using antisense to PR (Ogawa et al., 1994; Mani et al., 1994), suggesting rejection behaviors are not influenced by PR.
Fig. 2.
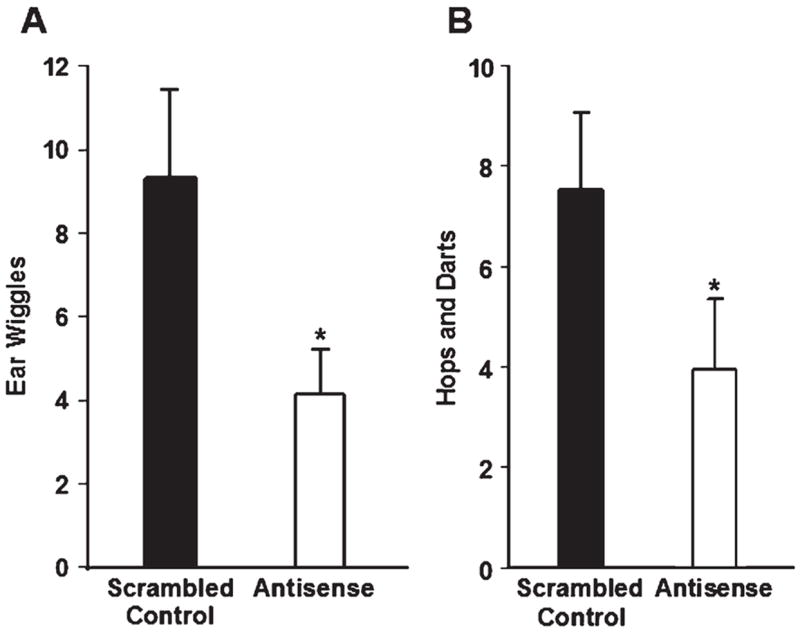
(A) Infusions of antisense ODNs to both SRC-1 and CBP mRNA (n = 23) in the VMN decreased ear wiggling compared to scrambled control ODNs (n = 23). *P < 0.04. (B) Infusion of antisense ODNs to both SRC-1 and CBP mRNA in the VMN had a decrease in hopping and darting compared with scrambled control ODN-treated animals. *P < 0.05; one-tailed t test.
Four antisense- and two control-treated rats were eliminated from data analyses due to cannulae placement that did not meet the criteria described in Methods. As in Exp. 1, none of the behaviors measured differed between antisense-treated rats that did not have appropriately placed cannulae and control-treated rats with cannulae targeting the VMN. However, it should be pointed out that the small number of antisense-treated rats with missed cannula placement makes these results difficult to interpret. Nonetheless, these results suggest that antisense to SRC-1 and CBP mRNA functions in a region-specific manner to affect the expression of PR-dependent proceptive behaviors in Exp. 2.
It was important to determine that antisense treatment, around the time of P, did not disrupt E-induced PR expression in the VMN. There were no differences in the number (Fig. 3A), or mean optical density (Fig. 3B), of PR-immunoreactive cells in the VMN between antisense and control-treated rats. These results suggest that the decrease in PR-dependent proceptive behaviors by antisense treatment in the VMN was not due to a decrease in PR expression.
Fig. 3.
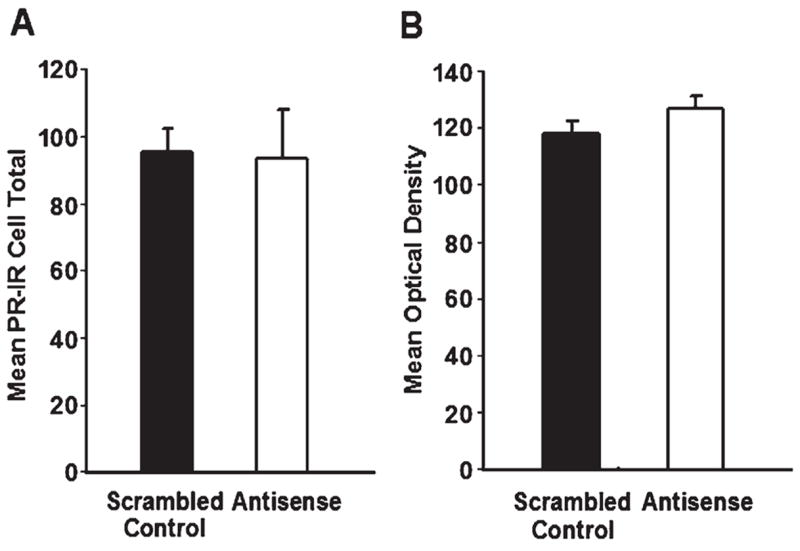
(A) There were no differences in the number of E-induced PR-immunoreactive cells in the VMN between animals treated with bilateral infusion of antisense ODNs to both SRC-1 and CBP mRNA (n = 14) compared with scrambled control ODN-treated animals (n = 13, P = 0.93). (B) The mean optical density of PR-immunoreactive cells did not differ between animals treated with bilateral infusion of antisense ODNs to both SRC-1 and CBP mRNA compared with scrambled control ODN-treated animals (P = 0.18).
It is important to confirm that our antisense technique in fact decreased expression of the target proteins. As shown in Fig. 4A, antisense to SRC-1 and CBP mRNA infused into the VMN around the time of P administration resulted in a decrease in SRC-1 protein, compared to the contralateral scrambled control-treated VMN. Furthermore, treatment of the VMN with antisense to SRC-1 and CBP mRNA decreased CBP protein compared to contralateral VMN treated with the scrambled control ODNs (Fig. 4B). These findings suggest that the antisense infusions were effective in reducing both SRC-1 and CBP proteins in the VMN.
Fig. 4.
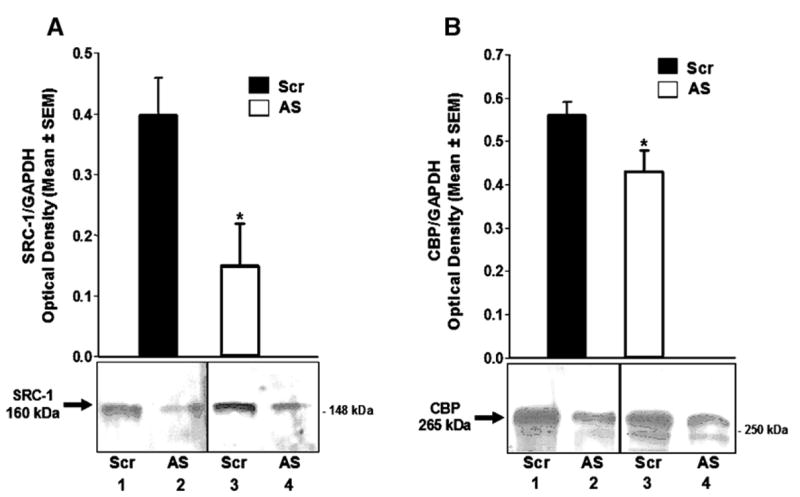
(A) Histogram represents Western blot analysis of the integrated density of SRC-1 immunoreactive bands in the ventromedial hypothalamus of female rats treated on one side of the VMN with antisense ODNs to SRC-1 and CBP mRNA and scrambled control ODNs on the contralateral VMN (n = 6). P < 0.05. Inset is image of SRC-1 immunoreactive bands from 2 representative animals; lanes 1 and 2 are from the hypothalamus of one rat, and lanes 3 and 4 are from the hypothalamus of another rat. (B) Histogram represents Western blot analysis of the integrated density of CBP immunoreactive bands in the ventromedial hypothalamus of female rats treated on one side of the VMN with antisense ODNs to SRC-1 and CBP mRNA and scrambled control ODNs on the contralateral VMN (n = 10). P < 0.01. Inset is image of CBP immunoreactive bands from 2 representative animals; lanes 1 and 2 are from the hypothalamus of one rat, and lanes 3 and 4 are from the hypothalamus of another rat.
Previous studies have demonstrated that SRC-2 is overexpressed in the brain of SRC-1 knockout mice (Xu et al., 1998). Therefore, it was important to determine if the expression of other coactivators, such as SRC-2, was increased in the VMN to compensate for the decrease in SRC-1 and CBP following administration of antisense ODNs to these coactivators. There were no differences in the number SRC-2 immunoreactive cells (Scrambled control: 213 ± 21, n = 6 vs. Antisense: 214 ± 22, n = 9; P = 0.98), total area of SRC-2 immunoreactivity (Scrambled: 6098 ± 811 pixels vs. Antisense: 6181 ± 814; P = 0.95) or mean optical density of SRC-2 immunoreactive cells (Scrambled: 0.022 ±0.002 OD vs. Antisense: 0.024 ± 0.003; P = 0.47) in the VMN of animals treated with antisense to SRC-1 and CBP mRNA compared to animals treated with scrambled control ODNs. These findings suggest that SRC-2 was not upregulated by antisense to SRC-1 and CBP mRNA.
Discussion
We have shown previously that the nuclear receptor coactivators, SRC-1 and CBP, function together to regulate ER-mediated gene expression in the VMN and the expression of hormone-dependent behavior (Molenda et al., 2002). However, this previous study (Molenda et al., 2002) did not distinguish whether nuclear receptor coactivators modulated ER or PR action to influence female sexual behavior. Here, we report that SRC-1 and CBP function in distinct ER- and PR-dependent aspects of female sexual behavior. In Experiment 1, the hypothesis that SRC-1 and CBP function in the VMN to regulate ER-dependent female sexual receptivity was tested. Reduction of SRC-1 and CBP in the VMN by antisense infusion decreased the lordosis quotient in EB-only treated females. Furthermore, in these animals, antisense to SRC-1 and CBP mRNA decreased the intensity of lordosis. These results extend our previous findings that SRC-1 and CBP function in ER-dependent transcription in brain (Molenda et al., 2002) by providing evidence that these nuclear receptor coactivators act in the VMN to modulate specific aspects of ER-dependent sexual receptivity.
In Experiment 2, the hypothesis that SRC-1 and CBP are important for the expression of PR-dependent sexual behaviors was tested. In support of this hypothesis, a variety of in vitro studies reveal that SRC-1 and CBP enhance the transcriptional activity and function of PR (Smith et al., 1996; Tetel et al., 1999; Xu et al., 2000a,b; Liu et al., 2001). In the present studies using E-primed females, infusions of antisense to SRC-1 and CBP mRNA in the VMN around the time of P administration decreased PR-dependent proceptive behaviors. PR-dependent ear wiggling, and hopping and darting, were decreased following antisense to SRC-1 and CBP in the VMN. These findings indicate that SRC-1 and CBP modulate PR action in the VMN and are critical for the full expression PR-dependent proceptive behaviors. One possibility for the reduction of proceptive behaviors by antisense to coactivators is a reduction of E-induced PR in the VMN. However, no differences in E-induced PR expression in the VMN between antisense- and control-treated animals were found, suggesting that the antisense-induced decreases in PR-dependent behaviors were not due to a decrease in E-induced PR. Our findings suggest that SRC-1 and CBP mediate PR action in the VMN to regulate PR-specific aspects of female sexual behaviors.
While it has been shown previously that infusion of antisense to SRC-1 and CBP mRNA into the hypothalamus is effective in reducing coactivator proteins (Auger et al., 2000, 2002; Molenda et al., 2002), it was essential to confirm that SRC-1 and CBP proteins were also reduced in the current paradigm. As determined by Western blot analysis, the antisense infusion paradigm was effective in reducing both SRC-1 and CBP protein expression in the current study.
While administration of antisense to SRC-1 and CBP mRNA decreased ER- and PR-dependent aspects of female sexual behavior, a complete elimination of these behaviors was not expected. Although the majority of PR-containing cells in the VMN express SRC-1 and/or CBP, it is important to note that not all PR cells in this nucleus contain both SRC-1 and CBP (Murphy et al., 2003). It is possible that hormone action in this sub-population of cells was sufficient to elicit low levels of sexual behaviors. Furthermore, it is possible that other nuclear receptor coactivators may be upregulated to compensate for decreases in SRC-1 and CBP levels. For example, in SRC-1 knockout mice, SRC-2 expression is increased in brain (Xu et al., 1998), and has been implicated in the regulation of female sexual receptivity (Apostolakis et al., 2002). However, in the present experiments, we found no differences in the number of SRC-2 immunoreactive cells in the VMN between rats treated with antisense to SRC-1 and CBP mRNA and scrambled control ODNs in Exp. 2. These data suggest that there was no compensation by SRC-2 following the decrease of SRC-1 and CBP by antisense. While other studies have observed effects on hormone action in brain by targeting either SRC-1 or CBP alone (Auger et al., 2000, 2002; Charlier et al., 2005; Apostolakis et al., 2002), we sought to target both SRC-1 and CBP based on the concept that these coactivators function together from in vitro studies (Smith et al., 1996; Tetel et al., 1999; Xu et al., 2000a,b; Liu et al., 2001; Kim et al., 2001) and our previous work in brain (Molenda et al., 2002). However, it should be noted that the present studies are limited in their interpretation given that the effects of one or the other coactivator cannot be distinguished.
In the present experiments, we sought to alter ER and PR action by administering antisense to two nuclear receptor coactivators; SRC-1 and CBP, which are known to function with these receptors in vitro (Oñate et al., 1995; Smith et al., 1996; Xu et al., 2000a,b). In contrast to behavioral experiments that directly target the receptors (e.g., ER and PR) by antisense or knockout approaches (Pollio et al., 1993; Ogawa et al., 1994, 1999; Mani et al., 1994; Lydon et al., 1995; Rissman et al., 1997), we propose that our present studies altered distinct signaling pathway(s) for these steroid receptors by reducing the expression of specific coactivators. For example, in Exp. 2, administration of antisense to SRC-1 and CBP mRNA around the P injection to alter PR function, reduced PR-dependent proceptive behaviors, but no effects were detected on PR-dependent receptivity. One explanation for this outcome is that administration of antisense to SRC-1 and CBP reduced the activity of PR signaling pathway(s) that influence proceptivity, while alternate PR signaling pathways, that regulate PR-dependent receptivity, remained intact and functional. In previous studies (Ogawa et al., 1994; Mani et al., 1994), both sexual receptivity and proceptivity were decreased when rats were infused with antisense to PR into the VMN of E-primed animals. In contrast, our study appears to have dissociated the effects of PR on receptivity and proceptivity.
This dissociation of receptor-dependent behaviors, discussed above, could be due to recruitment of different coactivators by the receptor. In support, in vitro studies indicate that estrogen receptors recruit different p160 family coactivators depending on the estrogen response element (ERE) to which the ER is bound (Hall et al., 2002). For example, estradiol-bound ERα strongly recruits SRC-1 to the Vitellogenin ERE, but recruits SRC-2 much more efficiently when bound to the pS2 ERE. Thus, in different cells, or even within the same cell, eliminating one of the p160 coactivators (e.g., SRC-1) could disrupt one signaling pathway of a receptor; while other pathways of that receptor, that are dependent on different coactivators, would remain intact. Taken together, this molecular dissection of these signaling pathways by decreasing a specific coactivator, could result in one receptor-dependent behavior being disrupted; while another behavior, dependent on that same receptor, would be unaffected. However, we cannot exclude the possibility that ligand-independent activation of PR (Mani and O'Malley, 2002) or brain derived progesterone synthesis (Soma et al., 2005) could contribute to the divergent effects observed on proceptivity and receptivity. In addition, it is possible that these divergent effects could be due to differences in the effectiveness of the antisense treatment between the two experiments.
Our present findings provide further evidence that SRC-1 and CBP function in vivo to modulate ovarian steroid receptor action in brain and hormone-dependent sexual behaviors. These findings demonstrate that nuclear receptor coactivators modulate both ER-dependent sexual receptivity and PR-dependent proceptivity. While further investigation is needed, our approach of targeting nuclear receptor coactivators may allow the molecular dissection of steroid receptor signaling pathways and the corresponding steroid-dependent behaviors.
Acknowledgments
The authors thank Tora Mitra and Rich Rebidue for their expert technical assistance in scoring behavior for the pilot study in Experiment 2. This research was supported in part by National Science Foundation Grant IBN 0080818 and National Institutes of Health DK 61935 (MJT), NS 19327 (JDB) and NIMH Training Grant T32MH47538 (HAMF).
References
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- Apostolakis EM, Ramamurphy M, Zhou D, Onate S, O'Malley BW. Acute disruption of select steroid receptor coactivators prevents reproductive behavior in rats and unmasks genetic adaptation in knockout mice. Mol Endocrinol. 2002;16:1511–1523. doi: 10.1210/mend.16.7.0877. [DOI] [PubMed] [Google Scholar]
- Auger AP, Tetel MJ, McCarthy MM. Steroid receptor coactivator-1 mediates the development of sex specific brain morphology and behavior. Proc Natl Acad Sci. 2000;97:7551–7555. doi: 10.1073/pnas.97.13.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger AP, Perrot-Sinal TS, Auger CJ, Ekas LA, Tetel MJ, McCarthy MM. Expression of the nuclear receptor coactivator, cAMP response element-binding protein, is sexually dimorphic and modulates sexual differentiation of neonatal rat brain. Endocrinology. 2002;143:3009–3016. doi: 10.1210/endo.143.8.8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelson JF, Gerall AA, Albers HE. Effect of progesterone on the estrous activity cycle of the rat. Physiol Behav. 1981;26:631–635. doi: 10.1016/0031-9384(81)90137-2. [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Kouzarides T. The CBP coactivator is a histone acetyltransferase. Nature. 1996;384:641–643. 19–26. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- Barfield RJ, Chen JJ. Activation of estrous behavior in ovariectomized rats by intracerebral implants of estradiol benzoate. Endocrinology. 1977;101:1716–1725. doi: 10.1210/endo-101-6-1716. [DOI] [PubMed] [Google Scholar]
- Beato M, Sánchez-Pacheco A. Interaction of steroid hormone receptors with the transcription initiation complex. Endocr Rev. 1996;17:587–609. doi: 10.1210/edrv-17-6-587. [DOI] [PubMed] [Google Scholar]
- Bennett AL, Blasberg ME, Blaustein JD. Mating stimulation required for mating-induced estrous abbreviation in female rats: effects of repeated testing. Horm Behav. 2002;42:206–211. doi: 10.1006/hbeh.2002.1809. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Erskine MS. Feminine sexual behavior: cellular integration of hormonal and afferent information in the rodent forebrain. In: Pfaff DW, editor. Hormones, Brain and Behavior. Academic Press; New York: 2002. pp. 139–214. [Google Scholar]
- Blaustein JD, Feder HH. Cytoplasmic progestin receptors in guinea pig brain: characteristics and relationship to the induction of sexual behavior. Brain Res. 1979;169:481–497. doi: 10.1016/0006-8993(79)90398-6. [DOI] [PubMed] [Google Scholar]
- Boling JL, Blandau RJ. The estrogen-progesterone induction of mating responses in the spayed female rat. Endocrinology. 1939;25:359–364. [Google Scholar]
- Castro-Vazquez A, Carreno NB. Estrogen but not progesterone facilitates the lordosis reaction to cervicovaginal stimulation of ovariectomized rats. Physiol Behav. 1985;35:21–24. doi: 10.1016/0031-9384(85)90166-0. [DOI] [PubMed] [Google Scholar]
- Charlier TD, Ball GF, Balthazart J. Inhibition of steroid receptor coactivator-1 blocks estrogen and androgen action on male sex behavior and associated brain plasticity. J Neurosci. 2005;25:906–913. doi: 10.1523/JNEUROSCI.3533-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Clemens LG, Weaver DR. The role of gonadal hormones in the activation of feminine sexual behavior. In: Adler NT, Pfaff DW, Goy RW, editors. Handbook of Behavioral Neurobiology. Plenum Press; New York: 1985. pp. 183–227. [Google Scholar]
- Davidson JM, Rodgers CH, Smith ER, Bloch GJ. Stimulation of female sex behavior in adrenalectomized rats with estrogen alone. Endocrinology. 1968;82:193–195. doi: 10.1210/endo-82-1-193. [DOI] [PubMed] [Google Scholar]
- Davis PG, Krieger MS, Barfield RJ, McEwen BS, Pfaff DW. The site of action of intrahypothalamic estrogen implants in feminine sexual behavior: an autoradiographic analysis. Endocrinology. 1982;111:1581–1586. doi: 10.1210/endo-111-5-1581. [DOI] [PubMed] [Google Scholar]
- DeMarzo A, Beck CA, Oñate SA, Edwards DP. Dimerization of mammalian progesterone receptors occurs in the absence of DNA and is related to the release of the 90-kDa heat shock protein. Proc Natl Acad Sci. 1991;88:72–76. doi: 10.1073/pnas.88.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DA, Pfeifle JK. Hormonal control of receptivity, proceptivity and sexual motivation. Physiol Behav. 1983;30:437–443. doi: 10.1016/0031-9384(83)90150-6. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Solicitation behavior in the estrous female rat: a review. Horm Behav. 1989;23:473–502. doi: 10.1016/0018-506x(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Fadem BH, Barfield RJ, Whalen RE. Dose–response and time–response relationships between progesterone and the display of patterns of receptive and proceptive behavior in the female rat. Horm Behav. 1979;13:40–48. doi: 10.1016/0018-506x(79)90033-3. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Vega-Matuszczyk J, Larsson K. Synergistic action of estradiol, progesterone and testosterone on rat proceptive behavior. Physiol Behav. 1991;50:1007–1011. doi: 10.1016/0031-9384(91)90429-r. [DOI] [PubMed] [Google Scholar]
- Franck JA, Ward IL. Intralimbic progesterone and methysergide facilitate lordotic behavior in estrogen-primed female rats. Neuroendocrinology. 1981;32:50–56. doi: 10.1159/000123129. [DOI] [PubMed] [Google Scholar]
- Gilman DP, Hitt JC. Effects of gonadal hormones on pacing of sexual contacts by female rats. Behav Biol. 1978;24:77–87. doi: 10.1016/s0091-6773(78)92926-7. [DOI] [PubMed] [Google Scholar]
- Glass CK, Rose DW, Rosenfeld MG. Nuclear receptor coactivators. Curr Opin Cell Biol. 1997;9:222–232. doi: 10.1016/s0955-0674(97)80066-x. [DOI] [PubMed] [Google Scholar]
- Gorski RA. The possible neural sites of hormonal facilitation of sexual behavior in the female rat. Psychoneuroendocrinology. 1976;1:371–387. [Google Scholar]
- Hall JM, McDonnell DP, Korach KS. Allosteric regulation of estrogen receptor structure, function, and coactivator recruitment by different estrogen response elements. Mol Endocrinol. 2002;16:469–486. doi: 10.1210/mend.16.3.0814. [DOI] [PubMed] [Google Scholar]
- Hardy DF, DeBold JF. The relationship between levels of exogenous hormones and the display of lordosis by the female rat. Horm Behav. 1971;2:287–297. [Google Scholar]
- Hardy DF, DeBold JF. Effects of coital stimulation upon behavior of the female rat. J Comp Physiol Psychol. 1972;78:400–408. doi: 10.1037/h0032536. [DOI] [PubMed] [Google Scholar]
- Hlinak Z, Madlafousek J. Estradiol treatment and precopulatory behavior in ovariectomized female rats. Physiol Behav. 1981;26:171–176. doi: 10.1016/0031-9384(81)90006-8. [DOI] [PubMed] [Google Scholar]
- Hlinak Z, Madlafousek J. Estradiol plus progesterone treatment and precopulatory behavior in ovariectomized female rats. Physiol Behav. 1983;30:221–227. doi: 10.1016/0031-9384(83)90009-4. [DOI] [PubMed] [Google Scholar]
- Hong H, Kohli K, Garabedian MJ, Stallcup MR. GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Mol Cell Biol. 1997;17:2735–2744. doi: 10.1128/mcb.17.5.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Hsiao SJ, Kraus WL. A role for coactivators and histone acetylation in estrogen receptor alpha-mediated transcription initiation. EMBO J. 2001;20:6084–6094. doi: 10.1093/emboj/20.21.6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komisaruk BR, Diakow C. Lordosis, reflex intensity in rats in relation to the estrous cycle, ovariectomy, estrogen administration and mating behavior. Endocrinology. 1973;93:548–557. doi: 10.1210/endo-93-3-548. [DOI] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. Induction of lordosis in female rats: two modes of estrogen action and the effect of adrenalectomy. Horm Behav. 1975;6:259–276. doi: 10.1016/0018-506x(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Kraus WL, Montano MM, Katzenellenbogen BS. Identification of multiple, widely spaced estrogen-responsive regions in the rat progesterone receptor gene. Mol Endocrinol. 1994;8:952–969. doi: 10.1210/mend.8.8.7997237. [DOI] [PubMed] [Google Scholar]
- Kwok RPS, Lundblad JR, Chrivia JC, Richards JP, Bachinger HP, Brennan RG, Roberts SGE, Green MR, Goodman RH. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–229. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- Lauber AH, Romano GJ, Pfaff DW. Sex difference in estradiol regulation of progestin receptor messenger RNA in rat mediobasal hypothalamus as demonstrated by in situ hybridization. Neuroendocrinology. 1991;53:608. doi: 10.1159/000125781. [DOI] [PubMed] [Google Scholar]
- Li H, Gomes PJ, Chen JD. RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc Natl Acad Sci. 1997;94:8479–8484. doi: 10.1073/pnas.94.16.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wong J, Tsai SY, Tsai MJ, O'Malley BW. Steroid receptor coactivator-1 (SRC-1) enhances ligand-dependent and receptor-dependent cell-free transcription of chromatin. Proc Natl Acad Sci. 1999;96:9485–9490. doi: 10.1073/pnas.96.17.9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wong J, Tsai SY, Tsai MJ, O'Malley BW. Sequential recruitment of steroid receptor coactivator-1 (SRC-1) and p300 enhances progesterone receptor-dependent initiation and reinitiation of transcription from chromatin. Proc Natl Acad Sci. 2001;98:12426–12431. doi: 10.1073/pnas.231474798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttge WG, Hughes JR. Intracerebral implantation of progesterone: reexamination of the brain sites responsible for facilitation of sexual receptivity in estrogen- primed ovariectomized rats. Physiol Behav. 1976;17:771–775. doi: 10.1016/0031-9384(76)90038-x. [DOI] [PubMed] [Google Scholar]
- Lydon JP, Demayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, McEwen BS. Oestrogen modulates progestin receptor concentrations in some rat brain regions but not in others. Nature. 1978;274:276–278. doi: 10.1038/274276a0. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SK, O'Malley BW. Mechanism of progesterone receptor action in the brain. In: Pfaff DW, editor. Hormones, Brain and Behavior. Academic Press; New York: 2002. pp. 643–682. [Google Scholar]
- Mani SK, Blaustein JD, Allen JMC, Law SW, O'Malley BW, Clark JH. Inhibition of rat sexual behavior by antisense oligonucleotides to the progesterone receptor. Endocrinology. 1994;136:1409–1414. doi: 10.1210/endo.135.4.7925102. [DOI] [PubMed] [Google Scholar]
- Mani SK, Blaustein JD, O'Malley BW. Progesterone receptor function from a behavioral perspective. Horm Behav. 1997;31:244–255. doi: 10.1006/hbeh.1997.1393. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O'Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- Meijer OC, Steenbergen PJ, de Kloet ER. Differential expression and regional distribution of steroid receptor coactivators SRC-1 and SRC-2 in brain and pituitary. Endocrinology. 2000;141:2192–2199. doi: 10.1210/endo.141.6.7489. [DOI] [PubMed] [Google Scholar]
- Molenda HA, Griffin AL, Auger AP, McCarthy MM, Tetel MJ. Nuclear receptor coactivators modulate hormone-dependent gene expression in brain and female reproductive behavior in rats. Endocrinology. 2002;143:436–444. doi: 10.1210/endo.143.2.8659. [DOI] [PubMed] [Google Scholar]
- Molenda HA, Kilts CP, Allen RL, Tetel MJ. Nuclear receptor coactivator function in reproductive physiology and behavior. Biol Reprod. 2003;69:1449–1457. doi: 10.1095/biolreprod.103.019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SD, Kilts CP, Molenda HA, Tetel MJ. Confocal analysis of neuronal coexpression of nuclear receptor coactivators and steroid receptors in rat hypothalamus. Soc Neurosci Abstr. 2003:726.8. [Google Scholar]
- Ogawa S, Olazabal UE, Parhar IS, Pfaff DW. Effects of intrahypothalamic administration of antisense DNA for progesterone receptor mRNA on reproductive behavior and progesterone receptor immunoreactivity in female rat. J Neurosci. 1994;14:1766–1774. doi: 10.1523/JNEUROSCI.14-03-01766.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor beta gene-deficient (betaERKO) male and female mice. Proc Natl Acad Sci. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oñate SA, Tsai SY, Tsai MJ, O'Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- Parsons B, MacLusky NJ, Krey L, Pfaff DW, McEwen BS. The temporal relationship between estrogen-inducible progestin receptors in the female rat brain and the time course of estrogen activation of mating behavior. Endocrinology. 1980;107:774–779. doi: 10.1210/endo-107-3-774. [DOI] [PubMed] [Google Scholar]
- Parsons B, Rainbow TC, MacLusky NJ, McEwen BS. Progestin receptor levels in rat hypothalamic and limbic nuclei. J Neurosci. 1982;2:1446–1452. doi: 10.1523/JNEUROSCI.02-10-01446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- Pfaff DW. Estrogens and Brain Function. Springer-Verlag; New York: 1980. [Google Scholar]
- Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol. 1979a;288:203–210. [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J Physiol. 1979b;288:189–202. [PMC free article] [PubMed] [Google Scholar]
- Pleim ET, Brown TJ, MacLusky NJ, Etgen AM, Barfield RJ. Dilute estradiol implants and progestin receptor induction in the ventromedial nucleus of the hypothalamus: correlation with receptive behavior in female rats. Endocrinology. 1989;124:1807–1812. doi: 10.1210/endo-124-4-1807. [DOI] [PubMed] [Google Scholar]
- Pleim ET, Baumann J, Barfield RJ. A contributory role for midbrain progesterone in the facilitation of female sexual behavior in rats. Horm Behav. 1991;25:19–28. doi: 10.1016/0018-506x(91)90036-h. [DOI] [PubMed] [Google Scholar]
- Pollio G, Xue P, Zanisi M, Nicolin A, Maggi A. Antisense oligonucleotide blocks progesterone-induced lordosis behavior in ovariectomized rats. Mol Brain Res. 1993;19:135–139. doi: 10.1016/0169-328x(93)90158-l. [DOI] [PubMed] [Google Scholar]
- Powers JB, Valenstein ES. Sexual receptivity: facilitation by medial preoptic lesions in female rats. Science. 1972;175:1003–1005. doi: 10.1126/science.175.4025.1003. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Wersinger SR, Taylor JA, Lubahn DB. Estrogen receptor function as revealed by knockout studies: neuroendocrine and behavioral aspects. Horm Behav. 1997;31:232–243. doi: 10.1006/hbeh.1997.1390. [DOI] [PubMed] [Google Scholar]
- Robyr D, Wolffe AP, Wahli W. Nuclear hormone receptor coregulators in action: diversity for shared tasks. Mol Endocrinol. 2000;14:329–347. doi: 10.1210/mend.14.3.0411. [DOI] [PubMed] [Google Scholar]
- Rodier WI, Segal S. The effect of progesterone on the activity-wheel running of ovariectomized rats. Horm Behav. 1977;9:214–221. doi: 10.1016/0018-506x(77)90057-5. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Barfield RJ. Progesterone in the ventromedial hypothalamus facilitates estrous behavior in ovariectomized estrogen-primed rats. Endocrinology. 1983;113:797–804. doi: 10.1210/endo-113-2-797. [DOI] [PubMed] [Google Scholar]
- Savouret JF, Bailly A, Misrahi M, Rauch C, Redeuilh G, Chauchereau A, Milgrom E. Characterization of the hormone responsive element involved in the regulation of the progesterone receptor gene. EMBO J. 1991;10:1875–1883. doi: 10.1002/j.1460-2075.1991.tb07713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB. Distribution and regulation of steroid hormone receptor gene expression in the central nervous system. In: Seil FJ, editor. Advances in Neurology. Raven Press; New York: 1993. pp. 207–226. [PubMed] [Google Scholar]
- Smith CL, Oñate SA, Tsai MJ, O'Malley BW. CREB binding protein acts synergistically with steroid receptor coactivator-1 to enhance steroid receptor-dependent transcription. Proc Natl Acad Sci. 1996;93:8884–8888. doi: 10.1073/pnas.93.17.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma KK, Sinchak K, Lakhter A, Schlinger BA, Micevych PE. Neurosteroids and female reproduction: estrogen increases 3β-HSD mRNA and activity in rat hypothalamus. Endocrinology. 2005;146:4386–4390. doi: 10.1210/en.2005-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, McKenna NJ, Oñate SA, Tsai SY, Tsai MJ, O'Malley BW. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–197. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- Stromberg H, Svensson SP, Hermanson O. Distribution of CREB-binding protein immunoreactivity in the adult rat brain. Brain Res. 1999;818:510–514. doi: 10.1016/s0006-8993(98)01219-0. [DOI] [PubMed] [Google Scholar]
- Tennent BJ, Smith ER, Davidson JM. The effects of estrogen and progesterone on female rat proceptive behavior. Horm Behav. 1980;14:65–75. doi: 10.1016/0018-506x(80)90016-1. [DOI] [PubMed] [Google Scholar]
- Tennent BJ, Smith ER, Davidson JM. Effects of progesterone implants in the habenula and midbrain on proceptive and receptive behavior in the female rat. Horm Behav. 1982;16:352–363. doi: 10.1016/0018-506x(82)90033-2. [DOI] [PubMed] [Google Scholar]
- Tetel MJ. Nuclear receptor coactivators in neuroendocrine function. J Neuroendocrinol. 2000;12:927–932. doi: 10.1046/j.1365-2826.2000.00557.x. [DOI] [PubMed] [Google Scholar]
- Tetel MJ, Giangrande PH, Leonhardt SA, McDonnell DP, Edwards DP. Hormone-dependent interaction between the amino- and carboxyl-terminal domains of progesterone receptor in vitro and in vivo. Mol Endocrinol. 1999;13:910–924. doi: 10.1210/mend.13.6.0300. [DOI] [PubMed] [Google Scholar]
- Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/ thyroid receptor superfamily members. Ann Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Vathy IU, Etgen AM, Barfield RJ. Actions of RU 38486 on progesterone facilitation and sequential inhibition of rat estrous behavior: correlation with neural progestin receptor levels. Horm Behav. 1989;23:43–56. doi: 10.1016/0018-506x(89)90073-1. [DOI] [PubMed] [Google Scholar]
- Voegel JJ, Heine MJS, Zechel C, Chambon P, Gronemeyer H. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- Whalen RE. Estrogen–progesterone induction of mating in female rats. Horm Behav. 1974;5:157–162. doi: 10.1016/0018-506x(74)90040-3. [DOI] [PubMed] [Google Scholar]
- Xu J, Qiu Y, Demayo FJ, Tsai SY, Tsai MJ, O’Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–1925. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]


