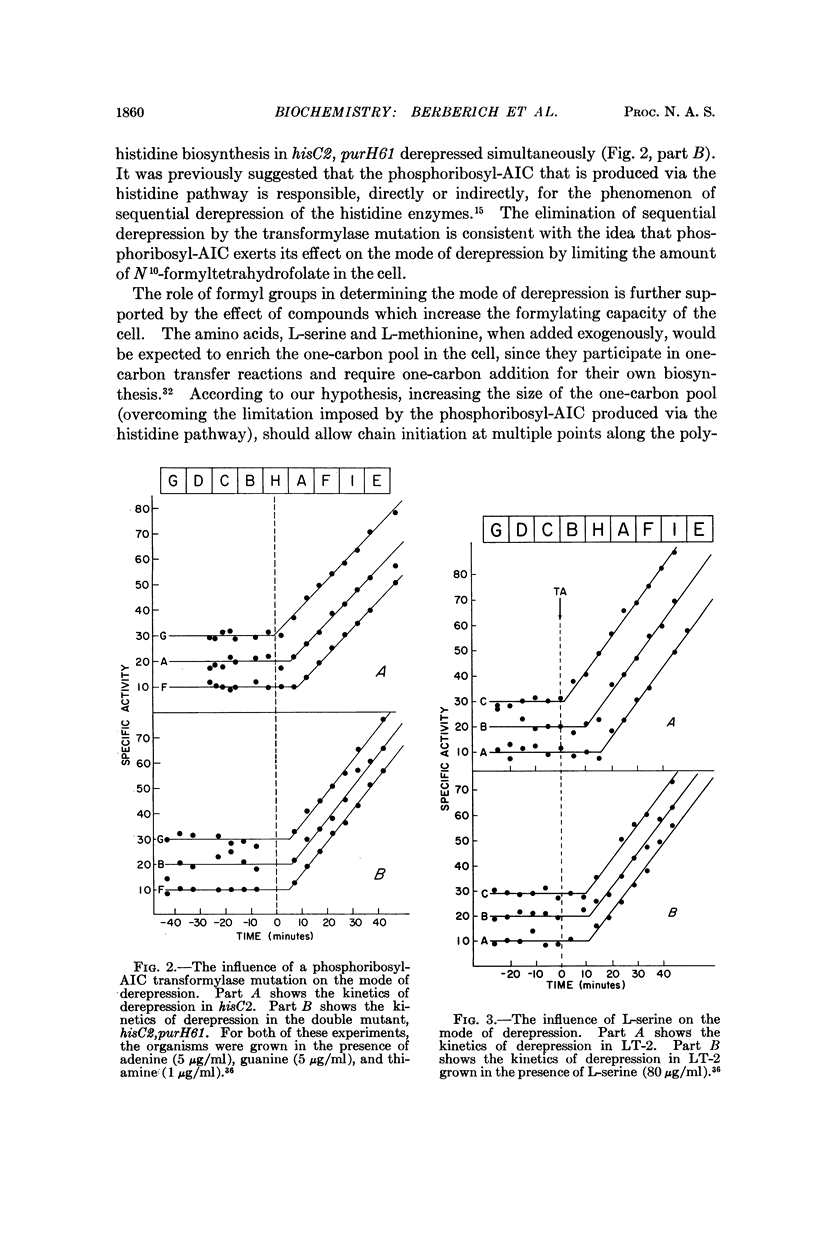
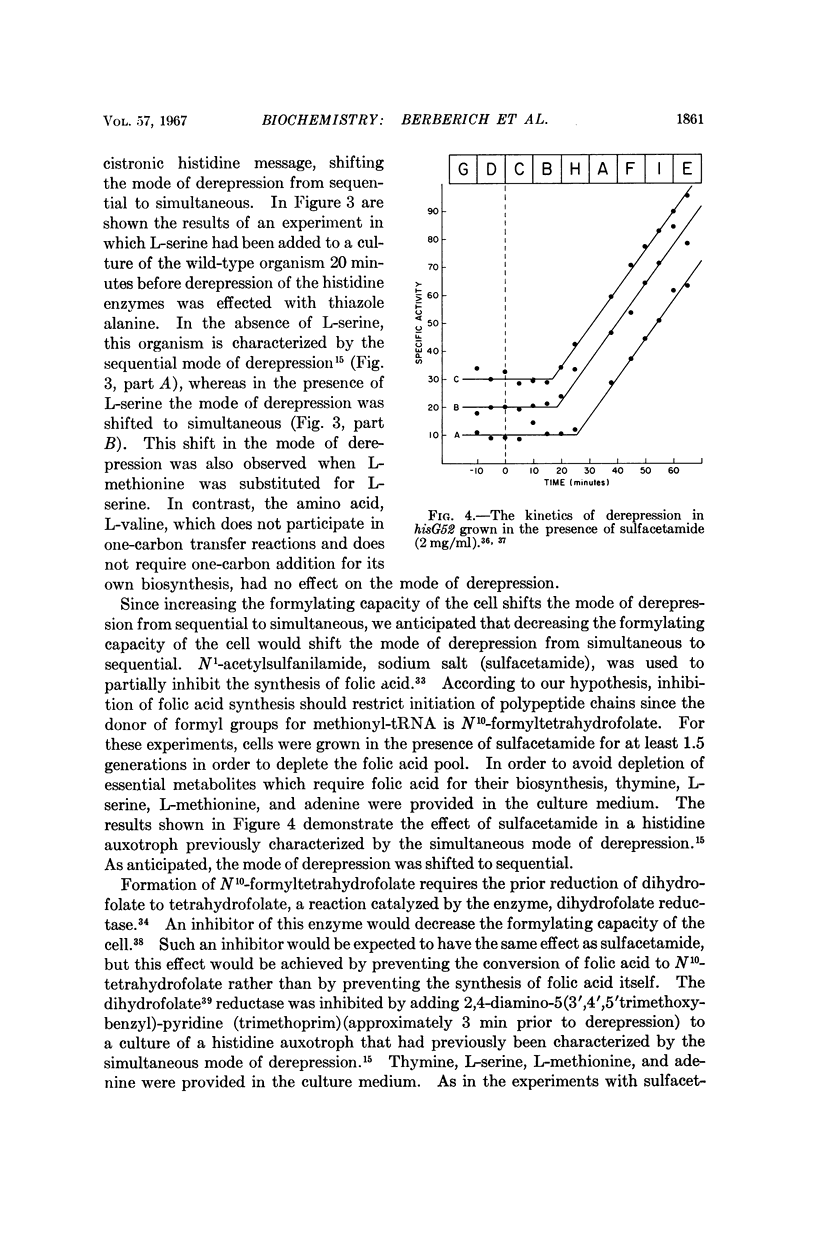
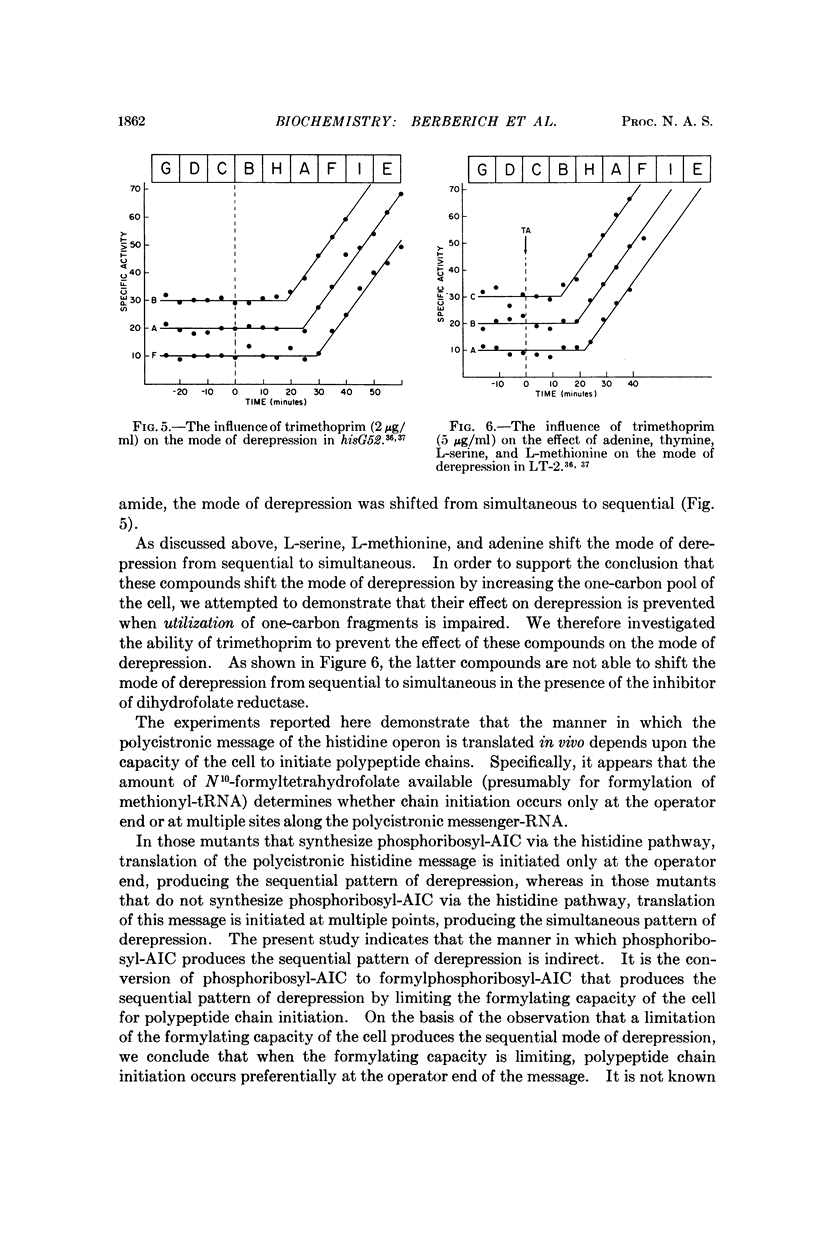
Full text
PDF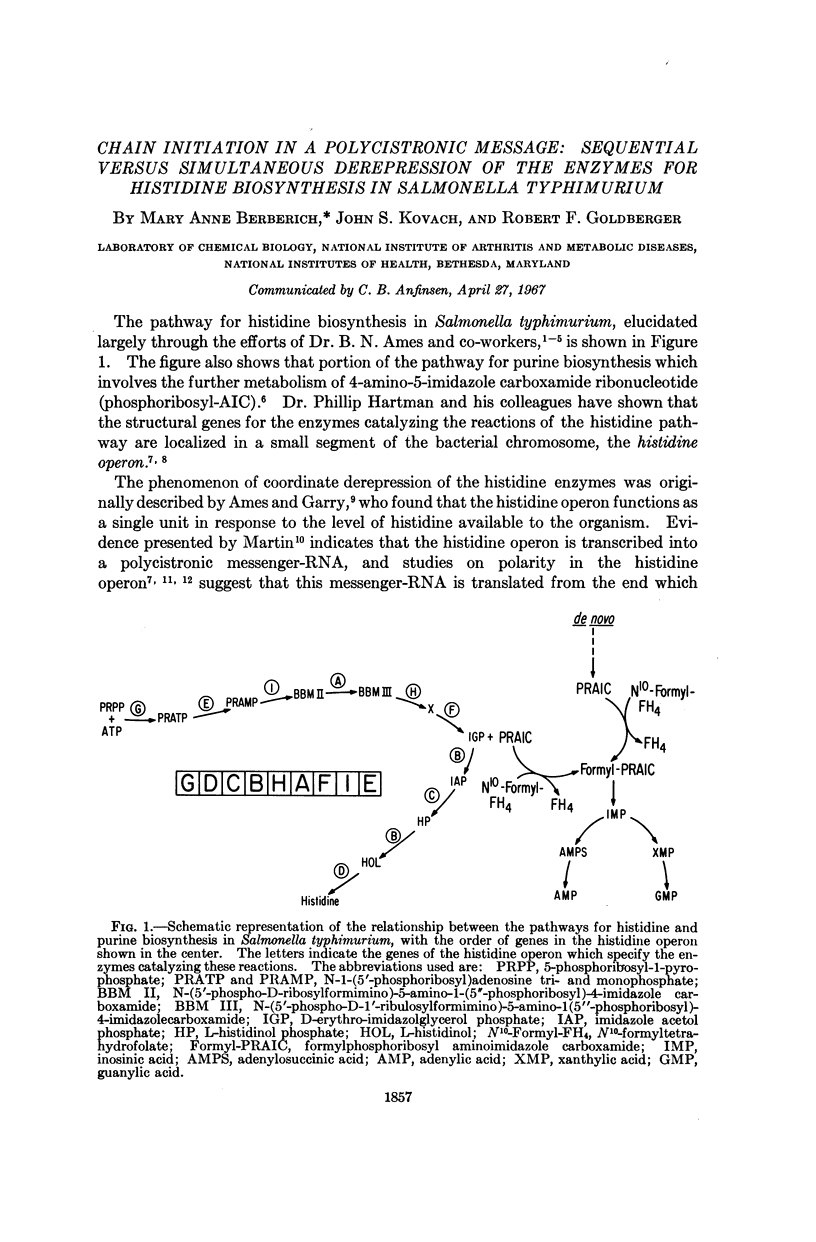




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., GARRY B., HERZENBERG L. A. The genetic control of the enzymes of histidine biosynthesis in Salmonella typhimurium. J Gen Microbiol. 1960 Apr;22:369–378. doi: 10.1099/00221287-22-2-369. [DOI] [PubMed] [Google Scholar]
- AMES B. N., HARTMAN P. E., JACOB F. Chromosomal alterations affecting the regulation of histidine biosynthetic enzymes in Salmonella. J Mol Biol. 1963 Jul;7:23–42. doi: 10.1016/s0022-2836(63)80016-9. [DOI] [PubMed] [Google Scholar]
- AMES B. N., MARTIN R. G., GARRY B. J. The first step of histidine biosynthesis. J Biol Chem. 1961 Jul;236:2019–2026. [PubMed] [Google Scholar]
- Adams J. M., Capecchi M. R. N-formylmethionyl-sRNA as the initiator of protein synthesis. Proc Natl Acad Sci U S A. 1966 Jan;55(1):147–155. doi: 10.1073/pnas.55.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpers D. H., Tomkins G. M. Sequential transcription of the genes of the lactose operon and its regulation by protein synthesis. J Biol Chem. 1966 Oct 10;241(19):4434–4443. [PubMed] [Google Scholar]
- Ames B. N., Garry B. COORDINATE REPRESSION OF THE SYNTHESIS OF FOUR HISTIDINE BIOSYNTHETIC ENZYMES BY HISTIDINE. Proc Natl Acad Sci U S A. 1959 Oct;45(10):1453–1461. doi: 10.1073/pnas.45.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BYRNE R., LEVIN J. G., BLADEN H. A., NIRENBERG M. W. THE IN VITRO FORMATION OF A DNA-RIBOSOME COMPLEX. Proc Natl Acad Sci U S A. 1964 Jul;52:140–148. doi: 10.1073/pnas.52.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberich M. A., Venetianer P., Goldberger R. F. Alternative modes of derepression of the histidine operon observed in Salmonella typhimurium. J Biol Chem. 1966 Oct 10;241(19):4426–4433. [PubMed] [Google Scholar]
- Burchall J. J., Hitchings G. H. Inhibitor binding analysis of dihydrofolate reductases from various species. Mol Pharmacol. 1965 Sep;1(2):126–136. [PubMed] [Google Scholar]
- Eisenstadt J., Lengyel P. Formylmethionyl-tRNA dependence of amino acid incorporation in extracts of trimethoprim-treated Escherichia coli. Science. 1966 Oct 28;154(3748):524–527. [PubMed] [Google Scholar]
- FRIEDKIN M. ENZYMATIC ASPECTS OF FOLIC ACID. Annu Rev Biochem. 1963;32:185–214. doi: 10.1146/annurev.bi.32.070163.001153. [DOI] [PubMed] [Google Scholar]
- Goldberger R. F., Berberich M. A. Sequential repression and derepression of the enzymes for histidine biosynthesis in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1965 Jul;54(1):279–286. doi: 10.1073/pnas.54.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARCKER K., SANGER F. N-FORMYL-METHIONYL-S-RNA. J Mol Biol. 1964 Jun;8:835–840. doi: 10.1016/s0022-2836(64)80164-9. [DOI] [PubMed] [Google Scholar]
- MOAT A. G., FRIEDMAN H. The biosynthesis and interconversion of purines and their derivatives. Bacteriol Rev. 1960 Sep;24(3):309–339. doi: 10.1128/br.24.3.309-339.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOYED H. S., MAGASANIK B. The biosynthesis of the imidazole ring of histidine. J Biol Chem. 1960 Jan;235:149–153. [PubMed] [Google Scholar]
- Margolies M. N., Goldberger R. F. Isolation of the fourth (isomerase) of histidine biosynthesis from Salmonella typhimurium. J Biol Chem. 1966 Jul 25;241(14):3262–3269. [PubMed] [Google Scholar]
- Martin R. G., Silbert D. F., Smith W. E., Whitfield H. J., Jr Polarity in the histidine operon. J Mol Biol. 1966 Nov 14;21(2):357–369. doi: 10.1016/0022-2836(66)90104-5. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Whitfield H. J., Jr, Berkowitz D. B., Voll M. J. A molecular model of the phenomenon of polarity. Cold Spring Harb Symp Quant Biol. 1966;31:215–220. doi: 10.1101/sqb.1966.031.01.029. [DOI] [PubMed] [Google Scholar]
- Roth J. R., Antón D. N., Hartman P. E. Histidine regulatory mutants in Salmonella typhimurium. I. Isolation and general properties. J Mol Biol. 1966 Dec 28;22(2):305–323. doi: 10.1016/0022-2836(66)90134-3. [DOI] [PubMed] [Google Scholar]
- SMITH D. W., AMES B. N. INTERMEDIATES IN THE EARLY STEPS OF HISTIDINE BIOSYNTHESIS. J Biol Chem. 1964 Jun;239:1848–1855. [PubMed] [Google Scholar]
- STENT G. S. THE OPERON: ON ITS THIRD ANNIVERSARY. MODULATION OF TRANSFER RNA SPECIES CAN PROVIDE A WORKABLE MODEL OF AN OPERATOR-LESS OPERON. Science. 1964 May 15;144(3620):816–820. doi: 10.1126/science.144.3620.816. [DOI] [PubMed] [Google Scholar]
- Shih A. Y., Eisenstadt J., Lengyel P. On the relation between ribonucleic acid synthesis and peptide chain initiation in E. coli. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1599–1605. doi: 10.1073/pnas.56.5.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach R. E., Dewey K. F., Brown J. C., Doty P. Formylmethionine codon AUG as an initiator of polypeptide synthesis. Science. 1966 Jul 22;153(3734):416–418. doi: 10.1126/science.153.3734.416. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Viñuela E., Salas M., Ochoa S. Translation of the genetic message, iii. Formylmethionine as initiator of proteins programed by polycistronic messenger RNA. Proc Natl Acad Sci U S A. 1967 Mar;57(3):729–734. doi: 10.1073/pnas.57.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLER J. P. THE NH2-TERMINAL RESIDUES OF THE PROTEINS FROM CELL-FREE EXTRACTS OF E. COLI. J Mol Biol. 1963 Nov;7:483–496. doi: 10.1016/s0022-2836(63)80096-0. [DOI] [PubMed] [Google Scholar]
- Webster R. E., Engelhardt D. L., Zinder N. D. In vitro protein synthesis: chain initiation. Proc Natl Acad Sci U S A. 1966 Jan;55(1):155–161. doi: 10.1073/pnas.55.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]


