Abstract
This review critically reappraises recent scientific evidence concerning central leptin insufficiency versus leptin resistance formulations to explain metabolic and neural disorders resulting from subnormal or defective leptin signaling in various sites in the brain. Research at various fronts to unravel the complexities of the neurobiology of leptin is surveyed to provide a comprehensive account of the neural and metabolic effects of environmentally-imposed fluctuations in leptin availability at brain sites and the outcome of newer technology to restore leptin signaling in a site-specific manner. The cumulative new knowledge favors a unified central leptin insufficiency syndrome over the, in vogue, central resistance hypothesis to explain the global adverse impact of deficient leptin signaling in the brain. Furthermore, the leptin insufficiency syndrome delineates a novel role of leptin in the hypothalamus in restraining rhythmic pancreatic insulin secretion while concomitantly enhancing glucose metabolism and non-shivering thermogenic energy expenditure, sequalae that would otherwise promote fat accrual to store excess energy resulting from consumption of energy-enriched diets. A concerted effort should now focus on development of newer technologies for delivery of leptin or leptin mimetics to specifically target neural pathways for remediation of diverse ailments encompassing the central leptin insufficiency syndrome.
Keywords: leptin insufficiency, brain, obesity, metabolic syndrome, cognition, Alzheimer’s disease
1. INTRODUCTION
Coleman in 1970’s postulated that the absence of a circulating satiety hormone from adipose tissue was responsible for the relentless hyperphagia and morbid obesity in obese ob/ob mice [36, 37]. Isolation of the ob gene from adipose tissue and identification of its protein product, leptin, as a putative “satiety” signal was accomplished in the mid-nineties [143]. On the basis of the long-standing body of clinical and experimental evidence it was generalized that the “satiety” effects of leptin are integrated through the hypothalamus and in the complete absence of leptin, as in ob/ob mice and some humans, hyperphagia and the attendant increased rate of bodyweight gain culminate in morbid obesity, and further, leptin normally exerts a tonic restraint on overeating by modulating the interactive appetite regulating network (ARN) in the hypothalamus [28, 35, 52, 54, 55, 59, 71, 74, 77, 78, 108, 114, 115, 140]. The current worldwide epidemic of obesity attributed to environmental causes and the upsurge in the incidence of a variety of obesity-dependent metabolic afflictions have spurred multidisciplinary research aimed at deciphering the precise nature of the crosstalk between leptin and neurochemical signaling in the hypothalamus [25, 52, 55, 57, 64, 70, 78, 79, 126, 127].
In this context, central leptin resistance has been the cornerstone of the mechanistic insight advocated earlier to explain the environmentally-induced obesity pandemic and related disease cluster of metabolic syndrome [18, 27, 29, 52, 54, 58, 72, 74, 124]. This hypothesis was construed on the conventional perception that loss of central tonic leptin restraint produced by impedance of leptin transport across the blood-brain barrier (BBB) along with disruptions in leptin-induced intracellular signalings in the ARN, favored overeating and accelerated fat accrual. However, subsequent evidence indicated that unregulated eating was neither universal nor significantly concomitant with dietary obesity in animals and humans [5, 20, 28, 44, 54, 55, 97]. Diminution in blood to brain leptin transfer did not compromise intracellular signaling in the ARN because centrally administered leptin remained effective in suppressing weight in obese rodents [28, 44, 59, 76, 110, 135]. Thus, despite the interest generated by this idea, it now appears to be conjectural rather than corroborated by substantial research findings.
An alternate obvious consequence of limiting transport across the BBB is leptin insufficiency at multiple targets in the brain. Indeed, a large body of experimental evidence is consistent with the idea that central leptin insufficiency for extended periods due to dietary and lifestyle changes, orchestrates pathophysiological consequences that include increased rate of fat accrual, decreased energy expenditure and general activity level, hyperinsulinemia, hyperglycemia, neuroendocrine disorders, osteoporosis and impaired learning and memory [7, 9, 12, 13, 20, 22, 31, 41, 42, 45, 49, 50, 67, 80, 88, 95, 107, 123, Fig. 1]. A further crucial issue here, and one that is usually overlooked is the fact that leptin permeability across the BBB, a first step towards development of leptin insufficiency, is itself subject to modulation by a spectrum of endogenous factors. In addition to adiposity, intrinsic circadian rhythms governing ingestive behavior, daily mealtimes, afferent ultradian rhythms in hormonal feedback signaling critical in hypothalamic integration of the daily feeding pattern, wide ranging blood borne neuroactive metabolic variables and aging itself can affect leptin transport from blood to brain [8–14, 29, 73, 82–85, 98, 110–112]. Furthermore, since leptin exerts pleiotropic effects in the brain, BBB-induced suboptimal or defective pattern of leptin signaling at extrahypothalamic targets, either directly or secondarily due to breakdown in hypothalamic integration of energy homeostasis, can potentially contribute to a spectrum of neural disorders.
Fig. 1.
A list of various peripheral and central disorders attributed to leptin insufficiency in the brain.
In light of this new insight into the neurobiology of leptin, this review critically reappraises the body of scientific evidence that argues the merits and demerits of central leptin resistance and leptin insufficiency hypotheses in promoting positive energy balance, the pathophysiological metabolic consequences and CNS disorders. In doing so, an additional goal is to gain a deeper understanding of the dynamic dialogue between leptin and the brain that may be significant for identifying novel loci for designing novel interventional strategies to decelerate the scourge of obesity and related metabolic and neural afflictions.
2. LEPTIN AVAILABILITY IN THE BRAIN
Leptin detected in the two components of the brain, cerebrospinal fluid (CSF) and parenchyma, is derived from the peripheral circulation and synthesis within the brain itself [3, 14, 27, 29, 47, 71, 85, 91, 103, 112, 124, 138, 139, 144]. The major peripheral sources of leptin, adipocytes and stomach, secrete leptin in a pulsatile fashion [6, 109, 110]. The short isoform of the leptin receptor located in the endothelium of the vasculature and epithelium of the choroid plexus of the circumventricular organs most likely transports leptin across the BBB [19, 52, 63, 85, 87, 112]. A variety of endogenous factors that modify the dynamics of leptin entry across BBB are the following:
Leptin entry into the brain is temporally correlated with circadian fluctuations in circulating leptin levels. The lowest amount of entry precedes or is coincident with the nadir in circulating leptin concentrations at the onset of the daily dark phase feeding [111, 141]. The peak level of leptin entry coincides with rising leptin secretion during the post-prandial period and it subsides progressively thereafter along with the gradual fall in blood leptin levels until the next dark phase feeding [111, 141],
Blood-to-brain transport of leptin is reduced in association with decreased circulating leptin levels that follow food deprivation or calorie restriction [6, 52, 72–74, 82],
Leptin entry into the brain is attenuated in ovariectomized rodents contemporaneously with hypoleptinemia [84, 130].
Aging affects leptin availability at CNS sites [9, 10, 12, 13]. In outbred aging mice, despite consuming a relatively stable rodent chow diet, the BBB capacity to transport leptin into the brain gradually declined in obesity-prone (OP), moderately heavier hyperleptinemic mice than in obesity-resistant (OR) lean mice [9, 10, 12, 13].
At the other end of the spectrum, in severely hyperleptinemic young obese OP rodents and human subjects consuming high energy diets, the supply of leptin to various hypothalamic and extrahypothalamic sites was also reduced [11–13, 29, 85, 91, 124].
Blood-borne hormones and circulating metabolic variables also independently modulate the BBB transport system to quantitatively affect leptin availability at CNS sites. For example, α-adrenergic agonist epinephrine, insulin and glucose enhance and triglycerides inhibit the transport of leptin across the BBB [8–14, 47, 83, 85, 91, 98, 138].
Leptin binding proteins in blood may alter levels of leptin availability for transport. Circulating levels of hepatic C-reactive protein (CRP) that binds leptin independently correlate with plasma leptin levels, and fat depletion suppresses CRP levels [32, 53, and unpublished]. Seemingly, increased leptin binding by elevated CRP levels in hyperleptinemic obese subjects quantitatively restricts leptin availability at the level of short isoform of leptin receptors for transport across BBB. Thus, a feedback loop between adipocytes and hepatic cells operates in the periphery to dampen leptin entry into the brain to modulate varied central responses [32, 53].
Leptin entry rates at various locations within and outside the hypothalamus in the brain differ significantly under varied physiological challenges [10, 47, 91]. Hyperleptinemia was reported to be associated with leptin transport to extrahypothalamic sites in the pons, medulla, hippocampus, cerebellum, occipital cortex and midbrain in higher amounts than to the hypothalamic arcuate nucleus (ARC), the site involved in propagation and termination of appetitive drive within the ARN [74, 78, 140]. The functional implication of these variations in leptin levels in extrahypothalamic sites warrants investigation.
Furthermore, brain is another non-adipocyte site of leptin synthesis, where it is likely to modulate neuronal functions by paracrine/autocrine actions. Wilkinson and coworkers [103, 139] detected leptin mRNA expression in specific areas of rat hypothalamus, cortex and cerebellum. We have consistently detected leptin mRNA expression in the rat and mouse hypothalamus [4, 5, 17, 22, 40, 41, 70, 76]. Moreover, fasting decreases leptin mRNA expression in the hypothalamus, as it does in adipose tissue, an observation consistent with the notion that decreased contributions of peripherally and locally derived leptin result in the overall leptin insufficiency at hypothalamic targets in response to fasting-induced depletion of energy stores [103, 139]. Expression of leptin mRNA and leptin protein were also detected in the human hypothalamus [47]. The amount of leptin secreted by the human brain is substantial as leptin outflow was higher in the internal jugular vein when simultaneous arterio-venous blood samples were compared. Interestingly, the spill-over of leptin into the internal jugular vein was greater in obese as compared to lean female subjects [47]. Overall, when one takes into account the pronounced efficacy of enhanced hypothalamic leptin synthesis induced by leptin gene therapy in modulating energy homeostasis [4, 5, 17, 22, 40, 41], the role of leptin produced by the brain is likely to be significant.
In summary, it is clear that reduced availability of leptin in the brain is a consequence of not only the hyperleptinemia in dietary obese young and aging obese rodents and obese human subjects, but also of the hypoleptinemia in the preprandial interval on a daily basis, and fat depletion in response to fasting and caloric restrictions. This analogous pattern of blood to brain entry in response to two opposite patterns of blood leptin concentrations is a provocative new insight that seriously questions the validity, now is vogue, of the leptin resistance hypothesis in explaining the increased fat accrual and obesity due to environmental causes.
3. ENVIRONMENTAL OBESITY, IS IT DUE TO CENTRAL LEPTIN RESISTANCE OR LEPTIN INSUFFICIENCY?
3.1 A. Leptin resistance
The two tenets, impedance to leptin entry imposed at the BBB and extinction of leptin-induced intracellular signaling downstream of leptin binding to the long form of neuronal receptor LRb in the hypothalamus, constitute the core of the leptin resistance hypothesis promulgated several years ago to explain obesity due to environmental causes [9, 12, 13, 15, 18, 27, 29, 52, 54, 55, 58, 59, 65, 104, 105, 108, 120, 121, 140]. According to the conventional underpinning, leptin resistance confers chronic overeating that impels storage of excess energy as fat depots which underlies the accelerated rate of weight gain. However, a careful scrutiny of the results of these investigations reveals little support for the contention that rodents forced to consume high fat diets (HFD) to reproduce clinical obesity, display chronic overeating [5, 12, 20, 28, 44, 59, 76, 92, 97, 110, 115]. Nearly one-half the rodents maintained on HFD are OR and do not exhibit increased food intake. The remaining OP subgroup generally reduce their daily intake when it is assessed in terms of the bulk quantity consumed. In fact, increased food consumed in gram quantity is a rare occurrence in rodents maintained on HFD. The high caloric density, and kcal intake derived from modified macronutrients in the diet and not hyperphagia by OP rodents culminates in increased adiposity.
Whether a persistent increase in appetitive drive is an obligatory universal outcome of central leptin resistance in humans consuming energy-enriched diets remains to be ascertained. To invoke consumatory drive, the distinction between the bulk quantity of daily food intake in grams vs. kcal and macronutrients consumed, has yet to be sorted out prospectively in clinical investigations. Thus, it is obvious that, like in rodents, the decreased leptin availability in the brain that follows consumption of energy-enriched diets most likely accelerates fat accrual by mechanisms other than the perceived overeating response in humans [Fig. 2].
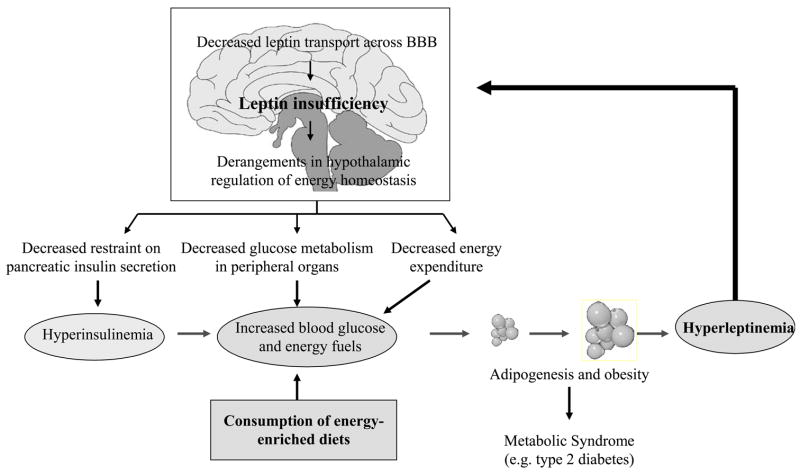
Fig. 2.
A flow chart of sequential metabolic and neural events initiated by consumption of energy-enriched diets leading to leptin insufficiency in the brain and derangements in the hypothalamic regulation of insulin secretion, glucose metabolism and energy expenditure that together promote fat accrual and metabolic disorders including type 2 diabetes. For details see text.
The second tenet of the central leptin resistance hypothesis implies marked disruption in leptin-induced intracellular signaling in the hypothalamus. Initial observations that signal transducer and activator of transcription 3 (STAT-3) is augmented in association with leptin-induced attenuation of food intake and breakdown in this association in HFD-consuming rodents led to the hypothesis that impaired intracellular signaling downstream of leptin-Rb activation in ARN targets is a requisite component of leptin resistance in obese rodents [15, 52, 54, 55, 58, 105, 121]. However, further experimental evidence showed that the restraint on food intake exerted by leptin persisted independent of STAT-3 signaling, and varying degrees of hyperleptinemia was not correlated with altered STAT-3 signaling [15, 105]. Additionally, leptin was recently shown to suppress hypothalamic orexigenic peptidergic signaling in transgenic mice independent of involvement of LRb-STAT-3 signal relay, and that STAT-3 signaling was not required for mediation of leptin-induced tonic restraint on food intake on a daily basis. Thus, the view that suppressed intracellular signaling downstream of leptin-LRb activation in ARN targets is a requisite component of leptin resistance in obese rodents, remains uncertain. A large body of unequivocal evidence demonstrating that leptin, either administered systemically or centrally, continues to inhibit food intake and suppress weight in obese HFD consuming rodents, is inconsistent with the hypothesis [5, 28, 44, 59, 115, 135].
At another level, because systemic administration of leptin enhanced hypothalamic levels of suppressors of cytokine signaling (SOCS-3), it was additionally proposed that this molecule may mediate the suppression of leptin restraint on appetite [65, 104]. Surprisingly, however, SOCS-3-deficient animals continued to display the daily time-related feeding pattern and, although moderately sensitive to exogenous leptin, they maintained a normal response to endogenous circulating leptin because the rate of the age-related increase in fat accrual was undisturbed. Also, partial or complete SOCS-3 deficiency in either the entire brain or solely in the hypothalamus, attenuated, but did not abolish HFD-induced adiposity, and the hypothalamic orexigenic peptidergic signaling, the primary mediators of the inhibitory effects of leptin on food intake and weight, was unaltered in these SOCS-3 deficient animals.
Due to this wide range of ambiguities in the implications that STAT-3 and SOCS-3 intracellular signalings impart central leptin resistance, taken together with several plausible alternate possibilities in these genetically manipulated mice, such as compensatory functional and anatomical reorganizations in the ARN, rearrangements in afferent hormonal feedback signaling involved in weight homeostasis and modifications in leptin transport to the hypothalamus across BBB, the contention that impaired intracellular signaling downstream of leptin entry in the ARN expedites environmentally-induced obesity, remains unsubstantiated.
3.2 B. Leptin insufficiency
A comprehensive assessment of leptin levels in the brain of the non-obese and HFD-induced obese rodents has uncovered an unexpected departure from conventional thinking. As presented earlier, both hypoleptinemia and hyperleptinemia are associated with reduced leptin entry into the brain. It is highly likely that decreased circulating leptin concentrations available for transport across the BBB as seen daily in the preprandial interval and in response to fasting [6, 52, 72–74, 82, 111, 118, 141], are the primary cause of reduced leptin levels in the brain. Though unexpected, in all likelihood, this is a natural defense that initiates and sustains hunger to reinstate energy balance. Indeed, hypoleptinemia promotes the release of orexigenic signals in the ARN for triggering the expression of hunger [74, 75, 77, 78], and as leptin levels rise after commencement of feeding, the release of orexigenic signals and the appetitive drive subside [75, 118, 141].
If this is the underlying physiological consequence of hypoleptinemia, then what is the mechanism and relevance of a similar attenuation of leptin transport to the CNS in the hyperleptinemic obese rodents and human subjects? In accordance with the current evidence, two mechanisms operate simultaneously to impose reduced leptin entry across BBB in response to hyperleptinemia. The first one is the well-established target receptor unresponsiveness rendered by abnormal increases in rhythmic hormonal signaling [6, 73, 80]. Hormonal pulsatility is the norm for optimal target-response and excursions from the normal pattern can extinguish target response due to receptor downregulation [73, 80]. Therefore, as proposed earlier [110, Fig. 2], we postulate that the HFD-induced increased pulsatile leptin secretion is likely to downregulate leptin receptors in the cerebromicrovasculature, especially the short isoform, and thereby markedly attenuate their capacity to transport leptin across the BBB [63, 87]. The possibility that various blood borne metabolic variables may also participate exists. Thus, to prevent undereating and starvation, the expected deleterious health consequences of unrestricted leptin passage to the brain under severe hyperleptinemic conditions, leptin receptor down regulation at the blood-brain interphase is a key defense adjustment to strictly curtail leptin supply to the hypothalamus [Fig. 2].
The second line of defense that aids in diminishing CNS leptin levels is the one precedes the point of leptin passage across the BBB. These include increased binding of leptin to CRP and modulation of blood to brain transport by various metabolic variables in the peripheral circulation [8, 11, 14, 32, 53, 82, 83, 85, 112]. In sum, these two endogenous defensive mechanisms are pressed into high gear to reduce leptin levels in the brain with a common goal of averting undereating and starvation, and initiating those additional hypothalamic mechanisms that promote storage of excess energy as fat, without jeopardizing the daily feeding pattern (see below).
Partial leptin deficiency in humans also is positively correlated with increased fat deposition. Human subjects who inherit one functional copy of ob gene and heterozygous lepob/+ have markedly reduced circulating leptin levels and display increased mass of adipose tissue as compared to controls [48, 108]. Similarly, a minority of Pima Indians with a common form of obesity, not associated with leptin mutations, were hypoleptinemic and exhibited greater fat accrual [119]. Thus, a subthreshold supply of leptin to hypothalamus in these clinical instances is associated with increased adiposity, an inference in line with the bulk of experimental evidence that now endorses central leptin insufficiency in causing dietary obesity.
4. GLOBAL CONSEQUENCES OF CENTRAL LEPTIN INSUFFICIENCY PRODUCED BY ENERGY-RICH DIETS
4.1 OBESITY AND METABOLIC SYNDROME
Compelling evidence links increased visceral adiposity with pathophysiology of the disease cluster of metabolic syndrome. Enhanced rate of fat accumulation is a risk factor for diabetes mellitus, a disease characterized by hyperinsulinemia, insulin resistance, glucose intolerance and hyperglycemia [23, 52, 57, 64, 66, 68–70, 76, 80, 89, 93]. Although the cause and effect relationships between hyperleptinemia, pancreatic insulin secretion and glucose metabolism in the periphery have been explored extensively [68, 69, 89, 93, 127], a crucial role of the leptin-hypothalamic axis in monitoring insulin-glucose homeostasis, independently of the fat depots, has only recently been delineated [5, 17, 20, 22, 23, 42, 44, 76]. Newer experimental approaches undertaken to assess the selective effects of leptin in the hypothalamus on pancreatic insulin secretion and glucose metabolism in the absence of confounding crosstalk in the periphery, have uncovered multiple new ways whereby peripheral hyperleptinemia and central leptin insufficiency concomitantly participate in the etiology of metabolic syndrome [5, 17, 20–23, 132, 133, Fig. 2].
4.1.1 a. Hyperinsulinemia
Although it is generally held that the fat burden of obesity contributes substantially to cause hyperinsulinemia [57, 64, 68, 69, 76, 89, 93], acute inhibition of insulin secretion by central administration of leptin has also been reported [34, 62, 89, 102, 131]. Suppression of ultradian insulin secretion from the pancreas was consistently observed when leptin expression was selectively enhanced by leptin gene transfer in the hypothalamus or in selected hypothalamic sites such as the medial preoptic area (MPOA), paraventricular hypothalamus (PVN), ventromedial hypothalamus (VMH) or ARC [4, 5, 17, 20, 22, 41, 42, 44, 109, 110, 133]. This stably enhanced leptin signaling in the hypothalamus, without leakage to extrahypothalamic sites or to the periphery, also corrected hyperinsulinemia and averted the development of insulin resistance in rodents displaying central leptin deficiency due to aging or consumption of HFD [17, 20, 23, 41, 42, 44]. Seemingly, the combination of restraint on both basal episodic and post-prandial insulin hypersecretion imposed by enhanced leptin signaling in the hypothalamus can sustain circulating insulin concentrations in the normal range [20, 22, 23, 109, 110].
The finding that normally leptin exerts a tonic restraint on pancreatic insulin secretion and HFD-induced central leptin insufficiency in rodents rescinds this restraint to unleash increased insulin secretion, provides a novel etiological insight for the well-documented positive relation in the incidence of obesity and type 2 diabetes. We propose that the positive energy balance generated by consumption of energy-rich diets greatly enhances the rate of storage of excess energy as fat, a process clearly requiring augmented insulin supply [Fig. 2, ]. Therefore, leptin-insufficiency conferred by HFD-induced impaired transport of leptin across BBB, meets that challenge by curtailing the tonic leptin restraint on ultradian insulin efflux from pancreas [20, 22, 23, 109, 110]. The resultant insulin hypersecretion, in turn, promotes adipogenesis and steers excess energy storage in adipocytes [52, 57, 68]. An analysis of the ultradian patterns of insulin secretion in OR and OP rodents affirm this proposal [109, 110]. We observed that OR rats despite consuming HFD do not display hyperleptinemia as do the OP rats. It turns out that unlike OP rats, OR animals develop neither hyperinsulinemia nor the central leptin insufficiency that allows insulin hypersecretion as seen in OP rats [9, 12, 109, 110].
Central leptin-insufficiency is, thus, another intrinsic defense mechanism to monitor the supply of insulin in accordance with the demands for storage of excess energy as fat in the periphery. The predicted untoward consequences of unabated hyperinsulinemia for extended periods are the antecedent pathophysiological sequalae, insulin receptor downregulation, insulin resistance and impaired intracellular signaling in peripheral targets leading to glucose intolerance, hyperglycemia and diabetes mellitus. The evidence that abrogation of leptin insufficiency by central gene therapy reinstated leptin restraint on ultradian insulin secretion, and simultaneously abolished the risk factors of diabetes, is consistent with our proposal that tonic leptin restraint on the basal ultradian and post-prandial pancreatic insulin hypersecretion is an obligatory hypothalamic regulatory component in insulin-glucose homeostasis.
The clinical reports demonstrating that leptin replacement in non-obese severely leptinopenic, lipodystrophic human and rodent models readily abrogated hyperinsulinemia, insulin resistance and diabetes mellitus also corroborate the role of leptin insufficiency in the etiology of diabetes [46, 116, 125]. Since leptin supply selectively in the hypothalamus of ob/ob mice conferred a stable state of normoinsulinemia [20, 132, 133], one can reasonably infer that reinstatement of central leptin restraint on insulin secretion underlies the benefits of systemic leptin administration in these lipodystrophic diabetic models.
The results of several tract tracing studies and those obtained after microinjection of leptin transgene in various hypothalamic and extrahypothalamic sites, imply that the leptin-induced signal relay from various hypothalamic sites traverses along the descending effector neural pathways that terminate in the pancreas [4, 26, 76, 90]. Further, it is evident that propagation of insulin inhibiting signals by leptin are mediated by the hypothalamic NPYergic system and the efferent relay bypasses the dorsal vagal complex en route to the pancreas [21, 78, 79, 96].
4.1.2 b. Hyperglycemia and Glucose Metabolism
The precise nature of leptin in the hypothalamic regulation of glucose homeostasis, independent of insulin involvement, was only recently clarified [20, 132, 133]. Increasing leptin availability selectively in the hypothalamus reduced blood glucose levels and maintained euglycemia in aging rodents as well as in extremely hyperglycemic HFD-consuming rodents and in leptin-deficient ob/ob mice for the lifetime [4, 5, 20, 22, 23, 44, 94, 132, 133]. Remarkably, this perseverance of euglycemia was evident even in the insulin-deficient streptozotocin treated diabetic mice and genetically-induced severely insulinopenic diabetic Akita mice [132, 133, 142, and unpublished]. Evidently, a distinct leptin-responsive hypothalamic mechanism orchestrates euglycemia by accelerating glucose metabolism in brown adipose tissue (BAT), liver, skeletal muscles and adipose tissue independent of insulin involvement [34, 62, 76, 99, 101, 129, 131–134, 136, Fig. 2].
The mechanisms whereby this newly identified distinct regulatory arm of central leptin action interacts with multiple peripheral players already invoked in glucose homeostasis remain to be delineated. Presumably driven by central leptin insufficiency in obese subjects, two events, a diminution in glucose metabolism that raises blood glucose concentrations, and an easing of the restraint that allows the pancreas to hypersecrete insulin, occur in concert to augment fat accrual. The efferent information generated by leptin from various hypothalamic sites, including the VMH [101], to upregulate glucose metabolism is apparently relayed via distinct descending neural pathways to the liver, BAT, white adipose tissue and skeletal muscles [4, 5, 21, 43, 76, 101, 129].
4.2 DECREASED ENERGY EXPENDITURE
Normally, by stimulating non-shivering thermogenesis, leptin also assists in the hypothalamic integration of energy balance in rodents [52, 54, 70, 74, 76, 114]. Distinct efferent pathways relay the leptin-induced messages from the hypothalamic MPOA, PVN and ARC to the BAT to augment energy expenditure [4, 5, 44]. Consequently, a deficit in leptin signaling from the hypothalamus to BAT, just as found in leptin and leptin receptor mutants, HFD consumption also consistently diminished thermogenic energy expenditure [20, 22, 23, 132, 133]. The obvious consequence of reduction in energy expenditure is to conserve energy supply [4, 5, 22, 33, 41, 42, 67, 70, 76]. Thus, as with decreased glucose metabolism, diminished energy expenditure in HFD-consuming rodents also boosts the supply of energy fuels for storage as fat [Fig. 2, ].
4.3 IMPAIRED BRAIN DEVELOPMENT AND COGNITIVE FUNCTION
Reduced brain weight, myelinization and synaptogenesis, the indicators of impaired CNS development and predictors of neurological ailments, have been reported in congenital leptin-deficient humans and rodents [1, 16, 24, 49, 50, 95, 100, 108, 117, 128]. Intriguingly, leptin insufficiency in the brain of obese and diabetic patients, may also adversely impact neural functions [2, 38, 49, 50, 58, 60]. Diabetes mellitus was associated with cognitive deficits, including psychomotor efficiency, attention, learning and memory, intelligence and executive function. Moreover, even non-diabetic individuals suffering either from hyperinsulinemia and insulin resistance or from poor glucose regulation, displayed impaired learning and memory performance, and atrophy of temporal lobe structures, including hippocampus and amygdala [49, 50, 56, 60, 107, 113]. Thus, a chronic low-grade decrease in central leptin levels in humans, a consequence of impaired BBB transport [12, 27, 29, 124], may inflict hippocampal damage, volume loss and atrophy, decrease general cognition performance, and memory impairment.
4.4 OSTEOPOROSIS AND BONE REMODELING
That hypoleptinemia, independent of gonadal hormones, is a common denominator in the pathophysiology of bone remodeling, has only recently been appreciated [6, 7, 67, 130]. Severe loss of fat tissue and hypoleptinemia have long been associated with increased incidence of osteoporosis in patients suffering from anorexia nervosa and in long-distance runners [7]. Leptin deficient ob/ob mice show stunted bone growth and several other abnormal features of bone density, all of which can be corrected by central leptin repletion [7, 67].
5. CAN ONE ALLEVIATE CENTRAL LEPTIN INSUFFICIENCY EXPERIMENTALLY ?
Thus, convergence of evidence concurs with the emerging notion that central leptin insufficiency is a significant contributor to obesity and the disease cluster of metabolic syndrome and certain neural afflictions. Can one alleviate central leptin insufficiency experimentally for extended period to curb obesity and the attending co-morbidities?
Various attempts to increase leptin delivery to the hypothalamus and extrahypothalamic sites with daily injections or continuous infusion systemically and centrally (intracerebroventricular or intrathecal infusion) in pharmacological doses over short periods, have been quite rewarding. Leptin administration to non-obese and HFD-induced obese rodents elicited divergent responses on weight gain and food intake [28, 33, 39, 59, 66, 114, 115, 121]. Whereas suppression of weight gain and fat accrual persisted throughout the entire treatment, daily food intake was inhibited only transiently and it gradually normalized [33, 121]. Presumably, the short-lived decrease in food intake is, as alluded to earlier, an adaptive endogenous response to prevent undereating and starvation without adversely affecting the daily feeding pattern. Maintenance of severe hyperleptinemia by a systemic injection of adenovirus vector encoding the leptin gene, was highly effective in suppressing weight and adiposity. However, these benefits of hyperleptinemia on weight homeostasis, although mediated through the hypothalamus, were questionable due to the immunogenic nature of adenovirus vector [137]. In a limited clinical trial, systemic administration of leptin was less remarkable in reducing weight in obese subjects, possibly due to extreme heterogeneity in the patient population and inadequate leptin dosing [61, 66].
Leptin replacement in various extrahypothalamic sites was also beneficial. Leptin injections reinstated myelinization, synaptogenesis, and promoted overall brain growth in ob/ob mice [1, 16, 23, 24, 60, 100, 117, 128]. Also, the possibility that cognition deficit may be associated with leptin insufficiency has been explored. Leptin-deficient rodents displayed impairment in hippocampal synaptic plasticity and in spatial memory tasks performed in Morris water maze, administration of leptin directly into the hippocampus facilitated hippocampal long-term potentation (LTP) and improved memory performance in young and aged rats and mice [49]. At the cellular level, leptin was able to convert hippocampal short-term potentation (STP) into LTP, via enhancing NMDA receptor function [107]. Furthermore, leptin was shown to modulate amyloid plaque formation in vivo and in vitro [50]. Since aggregation of these proteins promote memory loss in Alzheimer’s disease, these findings reinforced a role of leptin in learning and memory [2, 38]. Consequently, as alluded to earlier, it is highly possible that when leptin transport into the CNS is compromised in obese and aging subjects, and those suffering from metabolic diseases, low levels of leptin in the hippocampus may instill cognitive deterioration [56, 60, 113]. Leptin insufficiency in the brain either alone, or in concert with hyperglycemia and/or hyperinsulinemia is a significant risk factor for impaired cognitive function in the aging population [56, 60, 113].
The second approach recently tested for leptin delivery to the brain is by intranasal administration. Circumvention of the BBB with intranasally applied leptin in rodents rapidly raise brain concentrations to the supraphysiological range with higher levels in the hypothalamus than in extrahypothalamic sites [51, 86, 123]. These high hypothalamic leptin levels, attained independently of circulating concentrations, effectively decreased food intake and weight. The efficacy of intranasal application to deliver leptin directly to various hypothalamic and other brain targets in normal and obese rodents, warrants further investigation.
Finally, central leptin gene therapy, the third modality to deliver leptin is an extremely efficient approach to alleviate leptin insufficiency in anatomically relevant, distinct brain sites [4, 17, 30, 40, 42, 43, 106, 122]. A single central injection of non-replicative, non-immunogenic and non-pathogenic recombinant adeno-associated virus (rAAV) encoding the leptin gene, either intracerebroventricularly or selectively into hypothalamic sites, was highly effective in providing a stable lifetime supply of leptin without leakage to extrahypothalamic sites or to the periphery. The efficacy of central leptin gene therapy in various in vivo paradigms, such as, non-obese prepubertal and adult rodents, dietary obese, leptin-deficient obese and insulinopenic non-obese rodents has been evaluated [4, 5, 17, 20–23, 41, 42, 44, 70, 72, 76, 94, 109, 110, 132, 133]. Central leptin gene therapy conferred the following benefits:
Suppressed the age-related, ovariectomy-induced and dietary obesities and reduced circulating levels of adipokines, such as leptin, tumor necrosis factor α, adiponectin, triglycerides and free fatty acids, with or without a decrease in food intake.
Abolished the gradual age-related rise in circulating insulin in rodents either consuming ad libitum either regular rodent chow or HFD, and conferred normoinsulinemia by restraining episodic basal and postprandial insulin hypersecretion.
Engendered euglycemia, independent of insulin involvement, as evident in two diabetic rodent models: Akita and streptozotocin-treated mice. Further, increased glucose metabolism in BAT, liver and skeletal muscle, along with enhanced insulin sensitivity at these targets was largely responsible for the observed persistent euglycemic response.
Normalized testis weight, abolished the fatty liver response and promoted brain growth in leptin-deficient mice.
Estrous cycles, pregnancy and parturition in female rats were unaffected.
Normalized, through hypothalamic NPY signal relay, skeletal growth as indicated by femoral length, total bone volume and decreased femoral and vertebral cancellous bone volume in leptin-deficient mice.
Ameliorated various life-shortening co-morbidities in leptin deficient ob/ob mice, i.e. visceral fat, hyperglycemia, insulin resistance, hyperinsulinemia and decreased insulin-like growth factor-1 levels. These benefits, along with elevated ghrelin levels, more than doubled the lifespan, extending it to the range found in wild type mice.
In aggregate, the excellent safety profile for treating neurological diseases [23, 81], coupled with our demonstration of the life-long benefits of overcoming leptin insufficiency [23, 81], reinforce the potential of gene transfer technology with rAAV vector in decelerating the incidence of obesity and attendant sequalae of metabolic syndrome and neural disorders of leptin insufficiency [23, 70, 72, 76].
6. CONCLUSION AND THERAPEUTIC IMPLICATIONS
Historically, the term “leptin resistance” was coined to accommodate the presumption that reduced leptin restraint in the hypothalamus imposed by restricted blood to brain transfer of leptin and impaired leptin signal relay within the ARN, elicited varying degrees of unregulated overeating that culminated in overt obesity. The resultant fat burden sustained over extended periods was thought to expedite a spectrum of metabolic afflictions and shorten longevity [23, 52, 57, 64, 68, 76]. A critical survey of evidence assembled from a variety of physiological, molecular and genetic manipulations in support of the leptin resistance hypothesis is equivocal. In fact, the validity of this concept has been challenged by new knowledge of the dynamic fluctuations in brain leptin levels under diverse physiological and nutritional settings and the unequivocal demonstration that central leptin replacement, contrary to previous assumptions [27, 29, 52, 54, 120, 124], can reinstate weight homeostasis in obese rodents consuming energy-enriched diets [5, 28, 33, 44, 58, 59, 115, 135]. On the other hand, as proposed earlier [41, 42, 72, 76], the cumulative body of scientific evidence fits better with the alternate concept of hypothalamic leptin insufficiency in causation of obesity and metabolic syndrome. The major tenet of this postulation is that BBB restricts the blood to brain entry of leptin in response to hyperleptinemia consisting of heightened rhythmic leptin drive induced by energy enriched diets and intrinsic daily secretion pattern, and attendant varied blood-borne factors [6, 8, 11–14, 27, 29, 32, 53, 73, 82–84, 109, 111, 118, 141, Fig. 2]. The after effect of central leptin insufficiency is a two part defense mechanism, one, to prevent undereating and starvation, and retain the daily pattern of appetitive drive, and two, to simultaneously set in motion those varied hypothalamic regulatory processes that promote storage of excess ingested energy as fat. These encompass independent leptin-driven hypothalamic neural relays to provide optimal supply of pancreatic insulin and fuels for storage. What sets this new formulation apart from the leptin resistance hypothesis is the corroborative experimental evidence amassed in the last decade demonstrating that alleviation of leptin insufficiency selectively in the hypothalamus and elsewhere in the brain, can curtail the rate of fat accumulation, avert the risks of incurring the disease cluster of metabolic syndrome and an array of other neural co-morbidities.
Collectively, multidisciplinary strategies undertaken to unravel the intricate neurobiology of leptin signaling clearly documents a plethora of pathophysiological symptoms and ailments whose etiology can be traced to leptin insufficiency in the brain [Fig. 1]. This inference makes a major break with the past where traditionally metabolic afflictions singularly focused on persistent central leptin resistance and not suboptimal availability of leptin in the brain that boosts the risks of incurring obesity, diabetes and other related metabolic diseases. Therefore, we propose that central leptin resistance hypothesis should be replaced by the central leptin insufficiency syndrome to embrace a wide spectrum of interdependent peripheral and central afflictions orchestrated by the common underlying chronic suboptimal leptin signaling in the brain. This recognition can aid in designing newer interventional therapies aimed at normalizing, either individually or in unison, the diverse neural central afflictions inflicted by the imbalance in leptin signaling. These include fat accumulation, pancreatic insulin secretion, peripheral glucose metabolism, energy expenditure, bone remodeling and cognitive function [Fig. 1]. A concerted effort in designing new leptin mimetics, carefully assessing the clinical efficacy and safety of those central leptin delivery approaches that have already been evaluated in rodents, and identifying newer ways to improve leptin delivery to specifically targeted neural pathways, will accelerate development of remedial therapies for varied ailments of the central leptin insufficiency syndrome in domestic animals and humans.
Acknowledgments
The research embodied in this paper was supported by grants from National Institutes of Health (DK 37273 and NS 32727). We acknowledge secretarial assistance of Mr. Nicholas Cross.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahima RS, Bjorbaek C, Osei S, Flier JS. Regulation of neuronal and glial proteins by leptin: implications for brain development. Endocrinology. 1999;140:2755–62. doi: 10.1210/endo.140.6.6774. [DOI] [PubMed] [Google Scholar]
- 2.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–6. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 3.Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, et al. The stomach is a source of leptin. Nature. 1998;394:790–3. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- 4.Bagnasco M, Dube MG, Kalra PS, Kalra SP. Evidence for the existence of distinct central appetite, energy expenditure, and ghrelin stimulation pathways as revealed by hypothalamic site-specific leptin gene therapy. Endocrinology. 2002;143:4409–21. doi: 10.1210/en.2002-220505. [DOI] [PubMed] [Google Scholar]
- 5.Bagnasco M, Dube MG, Katz A, Kalra PS, Kalra SP. Leptin expression in hypothalamic PVN reverses dietary obesity and hyperinsulinemia but stimulates ghrelin. Obes Res. 2003;11:1463–70. doi: 10.1038/oby.2003.196. [DOI] [PubMed] [Google Scholar]
- 6.Bagnasco M, Kalra PS, Kalra SP. Plasma leptin levels are pulsatile in adult rats: effects of gonadectomy. Neuroendocrinology. 2002;75:257–63. doi: 10.1159/000054717. [DOI] [PubMed] [Google Scholar]
- 7.Baldock PA, Sainsbury A, Allison S, Lin EJ, Couzens M, Boey D, et al. Hypothalamic control of bone formation: distinct actions of leptin and y2 receptor pathways. J Bone Miner Res. 2005;20:1851–7. doi: 10.1359/JBMR.050523. [DOI] [PubMed] [Google Scholar]
- 8.Banks WA. Enhanced leptin transport across the blood-brain barrier by alpha 1-adrenergic agents. Brain Res. 2001;899:209–17. doi: 10.1016/s0006-8993(01)02242-9. [DOI] [PubMed] [Google Scholar]
- 9.Banks WA. Is obesity a disease of the blood-brain barrier? Physiological, pathological, and evolutionary considerations. Curr Pharm Des. 2003;9:801–9. doi: 10.2174/1381612033455350. [DOI] [PubMed] [Google Scholar]
- 10.Banks WA, Clever CM, Farrell CL. Partial saturation and regional variation in the blood-to-brain transport of leptin in normal weight mice. Am J Physiol Endocrinol Metab. 2000;278:E1158–65. doi: 10.1152/ajpendo.2000.278.6.E1158. [DOI] [PubMed] [Google Scholar]
- 11.Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, et al. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–60. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- 12.Banks WA, Farr SA, Morley JE. The effects of high fat diets on the blood-brain barrier transport of leptin: failure or adaptation? Physiol Behav. 2006;88:244–8. doi: 10.1016/j.physbeh.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 13.Banks WA, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity is acquired and reversible. Am J Physiol Endocrinol Metab. 2003;285:E10–15. doi: 10.1152/ajpendo.00468.2002. [DOI] [PubMed] [Google Scholar]
- 14.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–11. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 15.Bates SH, Dundon TA, Seifert M, Carlson M, Maratos-Flier E, Myers MG., Jr LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes. 2004;53:3067–73. doi: 10.2337/diabetes.53.12.3067. [DOI] [PubMed] [Google Scholar]
- 16.Bereiter DA, Jeanrenaud B. Altered neuroanatomical organization in the central nervous system of the genetically obese (ob/ob) mouse. Brain Res. 1979;165:249–60. doi: 10.1016/0006-8993(79)90557-2. [DOI] [PubMed] [Google Scholar]
- 17.Beretta E, Dube MG, Kalra PS, Kalra SP. Long-term suppression of weight gain, adiposity, and serum insulin by central leptin gene therapy in prepubertal rats: effects on serum ghrelin and appetite-regulating genes. Pediatr Res. 2002;52:189–98. doi: 10.1203/00006450-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–25. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 19.Bjorbaek C, Elmquist JK, Michl P, Ahima RS, van Bueren A, McCall AL, et al. Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology. 1998;139:3485–3491. doi: 10.1210/endo.139.8.6154. [DOI] [PubMed] [Google Scholar]
- 20.Boghossian S, Dube MG, Torto R, Kalra PS, Kalra SP. Hypothalamic clamp on insulin release by leptin-transgene expression. Peptides. 2006;27:3245–54. doi: 10.1016/j.peptides.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 21.Boghossian S, Lecklin A, Dube MG, Kalra PS, Kalra SP. Increased Leptin Expression in the Dorsal Vagal Complex Suppresses Adiposity without Affecting Energy Intake and Metabolic Hormones. Obesity. 2006;14:1003–9. doi: 10.1038/oby.2006.115. [DOI] [PubMed] [Google Scholar]
- 22.Boghossian S, Lecklin AH, Torto R, Kalra PS, Kalra SP. Suppression of fat deposition for the life time of rodents with gene therapy. Peptides. 2005;26:1512–9. doi: 10.1016/j.peptides.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 23.Boghossian S, Ueno N, Dube MG, Kalra P, Kalra S. Leptin gene transfer in the hypothalamus enhances longevity in adult monogenic mutant mice in the absence of circulating leptin. Neurobiol Aging. 2007;28:1594–1604. doi: 10.1016/j.neurobiolaging.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Bouret SG, Simerly RB. Minireview: Leptin and development of hypothalamic feeding circuits. Endocrinology. 2004;145:2621–6. doi: 10.1210/en.2004-0231. [DOI] [PubMed] [Google Scholar]
- 25.Bray GA, Tartaglia LA. Medicinal strategies in the treatment of obesity. Nature. 2000;404:672–7. doi: 10.1038/35007544. [DOI] [PubMed] [Google Scholar]
- 26.Buijs RM, Chun SJ, Niijima A, Romijn HJ, Nagai K. Parasympathetic and sympathetic control of the pancreas: a role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J Comp Neurol. 2001;431:405–23. doi: 10.1002/1096-9861(20010319)431:4<405::aid-cne1079>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 27.Burguera B, Couce ME, Curran GL, Jensen MD, Lloyd RV, Cleary MP, et al. Obesity is associated with a decreased leptin transport across the blood-brain barrier in rats. Diabetes. 2000;49:1219–23. doi: 10.2337/diabetes.49.7.1219. [DOI] [PubMed] [Google Scholar]
- 28.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–9. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 29.Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–61. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 30.Carter BJ, Burstein H, Templeton-Smith N. Theruapeutics Mechanisms and Strategies. New York, NY: Marcel Dekker; 2004. Adeno-associated virus and AAV vectors for gene delivery; pp. 71–1011. [Google Scholar]
- 31.Chehab FF. Leptin as a regulator of adipose mass and reproduction. Trends Pharmacol Sci. 2000;21:309–14. doi: 10.1016/s0165-6147(00)01514-5. [DOI] [PubMed] [Google Scholar]
- 32.Chen K, Li F, Li J, Cai H, Strom S, Bisello A, et al. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nat Med. 2006;12:425–32. doi: 10.1038/nm1372. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Heiman ML. Chronic leptin administration promotes lipid utilization until fat mass is greatly reduced and preserves lean mass of normal female rats. Regul Pept. 2000;92:113–9. doi: 10.1016/s0167-0115(00)00157-9. [DOI] [PubMed] [Google Scholar]
- 34.Chinookoswong N, Wang JL, Shi ZQ. Leptin restores euglycemia and normalizes glucose turnover in insulin- deficient diabetes in the rat. Diabetes. 1999;48:1487–92. doi: 10.2337/diabetes.48.7.1487. [DOI] [PubMed] [Google Scholar]
- 35.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–9. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 36.Coleman DL. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973;9:294–8. doi: 10.1007/BF01221857. [DOI] [PubMed] [Google Scholar]
- 37.Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–8. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- 38.Convit A. Links between cognitive impairment in insulin resistance: an explanatory model. Neurobiol Aging. 2005;26 (Suppl 1):31–35. doi: 10.1016/j.neurobiolaging.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 39.Della-Fera MA, Choi YH, Hartzell DL, Duan J, Hamrick M, Baile CA. Sensitivity of ob/ob mice to leptin-induced adipose tissue apoptosis. Obes Res. 2005;13:1540–7. doi: 10.1038/oby.2005.189. [DOI] [PubMed] [Google Scholar]
- 40.Dhillon H, Ge Y, Minter RM, Prima V, Moldawer LL, Muzyczka N, et al. Long-term differential modulation of genes encoding orexigenic and anorexigenic peptides by leptin delivered by rAAV vector in ob/ob mice. Relationship with body weight change. Regul Pept. 2000;92:97–105. doi: 10.1016/s0167-0115(00)00155-5. [DOI] [PubMed] [Google Scholar]
- 41.Dhillon H, Kalra SP, Kalra PS. Dose-dependent effects of central leptin gene therapy on genes that regulate body weight and appetite in the hypothalamus. Mol Ther. 2001;4:139–45. doi: 10.1006/mthe.2001.0427. [DOI] [PubMed] [Google Scholar]
- 42.Dhillon H, Kalra SP, Prima V, Zolotukhin S, Scarpace PJ, Moldawer LL, et al. Central Leptin Gene Therapy Suppresses Body Weight Gain, Adiposity and Serum Insulin Without Affecting Food Consumption in Normal Rats: A Long-Term Study. Regul Pept. 2001;99:69–77. doi: 10.1016/s0167-0115(01)00237-3. [DOI] [PubMed] [Google Scholar]
- 43.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 44.Dube MG, Beretta E, Dhillon H, Ueno N, Kalra PS, Kalra SP. Central leptin gene therapy blocks high fat diet-induced weight gain, hyperleptinemia and hyperinsulinemia: effects on serum ghrelin levels. Diabetes. 2002;51:1729–36. doi: 10.2337/diabetes.51.6.1729. [DOI] [PubMed] [Google Scholar]
- 45.Ealey KN, Fonseca D, Archer MC, Ward WE. Bone abnormalities in adolescent leptin-deficient mice. Regul Pept. 2006;136:9–13. doi: 10.1016/j.regpep.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 46.Ebihara K, Kusakabe T, Hirata M, Masuzaki H, Miyanaga F, Kobayashi N, et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab. 2007;92:532–41. doi: 10.1210/jc.2006-1546. [DOI] [PubMed] [Google Scholar]
- 47.Eikelis N, Wiesner G, Lambert G, Esler M. Brain leptin resistance in human obesity revisited. Regul Pept. 2007;139:45–51. doi: 10.1016/j.regpep.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Farooqi IS, Keogh JM, Kamath S, Jones S, Gibson WT, Trussell R, et al. Partial leptin deficiency and human adiposity. Nature. 2001;414:34–5. doi: 10.1038/35102112. [DOI] [PubMed] [Google Scholar]
- 49.Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2006;27:1420–5. doi: 10.1016/j.peptides.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Fewlass DC, Noboa K, Pi-Sunyer FX, Johnston JM, Yan SD, Tezapsidis N. Obesity-related leptin regulates Alzheimer’s Abeta. Faseb J. 2004;18:1870–8. doi: 10.1096/fj.04-2572com. [DOI] [PubMed] [Google Scholar]
- 51.Fliedner S, Schulz C, Lehnert H. Brain uptake of intranasally applied radioiodinated leptin in Wistar rats. Endocrinology. 2006;147:2088–94. doi: 10.1210/en.2005-1016. [DOI] [PubMed] [Google Scholar]
- 52.Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–50. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 53.Florez H, Castillo-Florez S, Mendez A, Casanova-Romero P, Larreal-Urdaneta C, Lee D, et al. C-reactive protein is elevated in obese patients with the metabolic syndrome. Diabetes Res Clin Pract. 2006;71:92–100. doi: 10.1016/j.diabres.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 54.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 55.Friedman JM. Modern science versus the stigma of obesity. Nat Med. 2004;10:563–9. doi: 10.1038/nm0604-563. [DOI] [PubMed] [Google Scholar]
- 56.Gold SM, Dziobek I, Sweat V, Tirsi A, Rogers K, Bruehl H, et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50:711–9. doi: 10.1007/s00125-007-0602-7. [DOI] [PubMed] [Google Scholar]
- 57.Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2595–600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- 58.Hakansson ML, Meister B. Transcription factor STAT3 in leptin target neurons of the rat hypothalamus. Neuroendocrinology. 1998;68:420–7. doi: 10.1159/000054392. [DOI] [PubMed] [Google Scholar]
- 59.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci U S A. 1997;94:8878–83. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harvey J, Solovyova N, Irving A. Leptin and its role in hippocampal synaptic plasticity. Prog Lipid Res. 2006;45:369–78. doi: 10.1016/j.plipres.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. Jama. 1999;282:1568–75. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 62.Hidaka S, Yoshimatsu H, Kondou S, Oka K, Tsuruta Y, Sakino H, et al. Hypoleptinemia, but not hypoinsulinemia, induces hyperphagia in streptozotocin-induced diabetic rats. J Neurochem. 2001;77:993–1000. doi: 10.1046/j.1471-4159.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- 63.Hileman SM, Pierroz DD, Masuzaki H, Bjorbaek C, El-Haschimi K, Banks WA, et al. Characterizaton of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology. 2002;143:775–83. doi: 10.1210/endo.143.3.8669. [DOI] [PubMed] [Google Scholar]
- 64.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299:853–5. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 65.Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjorbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med. 2004;10:734–8. doi: 10.1038/nm1072. [DOI] [PubMed] [Google Scholar]
- 66.Hukshorn CJ, Westerterp-Plantenga MS, Saris WH. Pegylated human recombinant leptin (PEG-OB) causes additional weight loss in severely energy-restricted, overweight men. Am J Clin Nutr. 2003;77:771–6. doi: 10.1093/ajcn/77.4.771. [DOI] [PubMed] [Google Scholar]
- 67.Iwaniec UT, Boghossian S, Lapke PD, Turner RT, Kalra SP. Central leptin gene therapy corrects skeletal abnormalities in leptin-deficient ob/ob mice. Peptides. 2007;28:1012–9. doi: 10.1016/j.peptides.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 69.Kahn SE, Zinman B, Haffner SM, O’Neill MC, Kravitz BG, Yu D, et al. Obesity is a major determinant of the association of C-reactive protein levels and the metabolic syndrome in type 2 diabetes. Diabetes. 2006;55:2357–64. doi: 10.2337/db06-0116. [DOI] [PubMed] [Google Scholar]
- 70.Kalra PS, Kalra SP. Obesity and Metabolic Syndrome: Long-term benefits of central leptin gene therapy. In: Prous JR, editor. Drugs of Today. Barcelona, Spain: Prous Science; 2002. pp. 745–57. [DOI] [PubMed] [Google Scholar]
- 71.Kalra SP. Appetite and body weight regulation: is it all in the brain? Neuron. 1997;19:227–30. doi: 10.1016/s0896-6273(00)80934-4. [DOI] [PubMed] [Google Scholar]
- 72.Kalra SP. Circumventing leptin resistance for weight control. Proc Natl Acad Sci U S A. 2001;98:4279–81. doi: 10.1073/pnas.091101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kalra SP, Bagnasco M, Otukonyong EE, Dube MG, Kalra PS. Rhythmic, reciprocal ghrelin and leptin signaling: new insight in the development of obesity. Regul Pept. 2003;111:1–11. doi: 10.1016/s0167-0115(02)00305-1. [DOI] [PubMed] [Google Scholar]
- 74.Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- 75.Kalra SP, Dube MG, Sahu A, Phelps CP, Kalra PS. Neuropeptide Y secretion increases in the paraventricular nucleus in association with increased appetite for food. Proc Natl Acad Sci U S A. 1991;88:10931–5. doi: 10.1073/pnas.88.23.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kalra SP, Kalra PS. Gene transfer technology: a preventive neurotherapy to curb obesity, ameliorate metabolic syndrome and extend life-expectancy. Trends Pharmacol Sci. 2005;26:488–95. doi: 10.1016/j.tips.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 77.Kalra SP, Kalra PS. Neuropeptide Y: a conductor of the appetite-regulating orchestra in the hypothalamus. In: Kastin AJ, editor. The Handbook of Biologically Active Peptides. Burlington, MA: Academic Press; 2006. pp. 889–94. [Google Scholar]
- 78.Kalra SP, Kalra PS. NPY and Cohorts in Regulating Appetite, Obesity and Metabolic Syndrome: Beneficial Effects of Gene Therapy. Neuropeptides. 2004;38:201–11. doi: 10.1016/j.npep.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 79.Kalra SP, Kalra PS. To subjugate NPY is to improve the quality of life and live longer. Peptides. 2007;28:413–8. doi: 10.1016/j.peptides.2006.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kalra SP, Xu B, Dube MG, Kalra PS. Nutritional infertility, mismanagement in central neuropeptidergic and peripheral insulin - leptin signaling. In: Hansel W, Bray G, Ryan DH, editors. Nutrition and Reproduction. Baton Rouge, LA: State University Press; 1998. pp. 25–70. [Google Scholar]
- 81.Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369:2097–105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 82.Kastin AJ, Akerstrom V. Fasting, but not adrenalectomy, reduces transport of leptin into the brain. Peptides. 2000;21:679–82. doi: 10.1016/s0196-9781(00)00195-9. [DOI] [PubMed] [Google Scholar]
- 83.Kastin AJ, Akerstrom V. Glucose and insulin increase the transport of leptin through the blood-brain barrier in normal mice but not in streptozotocin-diabetic mice. Neuroendocrinology. 2001;73:237–42. doi: 10.1159/000054640. [DOI] [PubMed] [Google Scholar]
- 84.Kastin AJ, Akerstrom V, Maness LM. Chronic loss of ovarian function decreases transport of leptin into mouse brain. Neurosci Lett. 2001;310:69–71. doi: 10.1016/s0304-3940(01)02074-2. [DOI] [PubMed] [Google Scholar]
- 85.Kastin AJ, Pan W. Dynamic regulation of leptin entry into brain by the blood-brain barrier. Regul Pept. 2000;92:37–43. doi: 10.1016/s0167-0115(00)00147-6. [DOI] [PubMed] [Google Scholar]
- 86.Kastin AJ, Pan W. Intranasal leptin: blood-brain barrier bypass (BBBB) for obesity? Endocrinology. 2006;147:2086–7. doi: 10.1210/en.2006-0208. [DOI] [PubMed] [Google Scholar]
- 87.Kastin AJ, Pan W, Maness LM, Koletsky RJ, Ernsberger P. Decreased transport of leptin across the blood-brain barrier in rats lacking the short form of the leptin receptor. Peptides. 1999;20:1449–53. doi: 10.1016/s0196-9781(99)00156-4. [DOI] [PubMed] [Google Scholar]
- 88.Keen-Rhinehart E, Kalra SP, Kalra PS. AAV mediated leptin receptor installation improves energy balance and the reproductive status of obese female Koletsky rats. Peptides. 2005;26:2567–78. doi: 10.1016/j.peptides.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 89.Kieffer TJ, Habener JF. The adipoinsular axis: effects of leptin on pancreatic B-cells. Am J Physiol Endocrinol Metab. 2000;278:E1–E14. doi: 10.1152/ajpendo.2000.278.1.E1. [DOI] [PubMed] [Google Scholar]
- 90.Kreier F, Kap YS, Mettenleiter TC, van Heijningen C, van der Vliet J, Kalsbeek A, et al. Tracing from fat tissue, liver, and pancreas: a neuroanatomical framework for the role of the brain in type 2 diabetes. Endocrinology. 2006;147:1140–7. doi: 10.1210/en.2005-0667. [DOI] [PubMed] [Google Scholar]
- 91.Kurrimbux D, Gaffen Z, Farrell CL, Martin D, Thomas SA. The involvement of the blood-brain and the blood-cerebrospinal fluid barriers in the distribution of leptin into and out of the rat brain. Neuroscience. 2004;123:527–36. doi: 10.1016/j.neuroscience.2003.08.061. [DOI] [PubMed] [Google Scholar]
- 92.Lauterio TJ, Bond JP, Ulman EA. Development and characterization of a purified diet to identify obesity-susceptible and resistant rat populations. J Nutr. 1994;124:2172–8. doi: 10.1093/jn/124.11.2172. [DOI] [PubMed] [Google Scholar]
- 93.Lazar MA. How obesity causes diabetes: not a tall tale. Science. 2005;307:373–5. doi: 10.1126/science.1104342. [DOI] [PubMed] [Google Scholar]
- 94.Lecklin AH, Dube MG, Torto R, Kalra PS, Kalra SP. Perigestational suppression of weight gain with central leptin gene therapy results in lower weight F1 generation. Peptides. 2005;26:1176–87. doi: 10.1016/j.peptides.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 95.Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113:607–15. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 96.Lin EJ, Sainsbury A, Lee NJ, Boey D, Couzens M, Enriquez R, et al. Combined deletion of Y1, Y2, and Y4 receptors prevents hypothalamic neuropeptide Y overexpression-induced hyperinsulinemia despite persistence of hyperphagia and obesity. Endocrinology. 2006;147:5094–101. doi: 10.1210/en.2006-0097. [DOI] [PubMed] [Google Scholar]
- 97.Lindqvist A, de la Cour CD, Stegmark A, Hakanson R, Erlanson-Albertsson C. Overeating of palatable food is associated with blunted leptin and ghrelin responses. Regul Pept. 2005;130:123–32. doi: 10.1016/j.regpep.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 98.Maness LM, Banks WA, Kastin AJ. Persistence of blood-to-brain transport of leptin in obese leptin-deficient and leptin receptor-deficient mice. Brain Res. 2000;873:165–7. doi: 10.1016/s0006-8993(00)02520-8. [DOI] [PubMed] [Google Scholar]
- 99.Masuzaki H, Ogawa Y, Aizawa-Abe M, Hosoda K, Suga J, Ebihara K, et al. Glucose metabolism and insulin sensitivity in transgenic mice overexpressing leptin with lethal yellow agouti mutation: usefulness of leptin for the treatment of obesity-associated diabetes. Diabetes. 1999;48:1615–22. doi: 10.2337/diabetes.48.8.1615. [DOI] [PubMed] [Google Scholar]
- 100.Matochik JA, London ED, Yildiz BO, Ozata M, Caglayan S, DePaoli AM, et al. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. J Clin Endocrinol Metab. 2005;90:2851–4. doi: 10.1210/jc.2004-1979. [DOI] [PubMed] [Google Scholar]
- 101.Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes. 1999;48:287–91. doi: 10.2337/diabetes.48.2.287. [DOI] [PubMed] [Google Scholar]
- 102.Miyanaga F, Ogawa Y, Ebihara K, Hidaka S, Tanaka T, Hayashi S, et al. Leptin as an adjunct of insulin therapy in insulin-deficient diabetes. Diabetologia. 2003;46:1329–37. doi: 10.1007/s00125-003-1193-6. [DOI] [PubMed] [Google Scholar]
- 103.Morash B, Li A, Murphy PR, Wilkinson M, Ur E. Leptin gene expression in the brain and pituitary gland. Endocrinology. 1999;140:5995–8. doi: 10.1210/endo.140.12.7288. [DOI] [PubMed] [Google Scholar]
- 104.Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004;10:739–43. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- 105.Munzberg H, Jobst EE, Bates SH, Jones J, Villanueva E, Leshan R, et al. Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling. J Neurosci. 2007;27:69–74. doi: 10.1523/JNEUROSCI.3168-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Murphy JE, Zhou S, Giese K, Williams LT, Escobedo JA, Dwarki VJ. Long-term correction of obesity and diabetes in genetically obese mice by a single intramuscular injection of recombinant adeno-associated virus encoding mouse leptin. Proc Natl Acad Sci U S A. 1997;94:13921–6. doi: 10.1073/pnas.94.25.13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, et al. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;27:2738–49. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 108.O’Rahilly S, Farooqi IS, Yeo GS, Challis BG. Minireview: human obesity-lessons from monogenic disorders. Endocrinology. 2003;144:3757–64. doi: 10.1210/en.2003-0373. [DOI] [PubMed] [Google Scholar]
- 109.Otukonyong EE, Dube MG, Torto R, Kalra PS, Kalra SP. Central leptin differentially modulates ultradian secretory patterns of insulin, leptin and ghrelin independent of effects on food intake and body weight. Peptides. 2005;26:2559–66. doi: 10.1016/j.peptides.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 110.Otukonyong EE, Dube MG, Torto R, Kalra PS, Kalra SP. High-fat diet-induced ultradian leptin and insulin hypersecretion are absent in obesity-resistant rats. Obes Res. 2005;13:991–9. doi: 10.1038/oby.2005.116. [DOI] [PubMed] [Google Scholar]
- 111.Pan W, Kastin AJ. Diurnal variation of leptin entry from blood to brain involving partial saturation of the transport system. Life Sci. 2001;68:2705–14. doi: 10.1016/s0024-3205(01)01085-2. [DOI] [PubMed] [Google Scholar]
- 112.Pan W, Kastin AJ. Why study transport of peptides and proteins at the neurovascular interface. Brain Res Brain Res Rev. 2004;46:32–43. doi: 10.1016/j.brainresrev.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 113.Paulus K, Schulz C, Lehnert H. Central nervous effects of leptin and insulin on hippocampal leptin and insulin receptor expression following a learning task in Wistar rats. Neuropsychobiology. 2005;51:100–6. doi: 10.1159/000084167. [DOI] [PubMed] [Google Scholar]
- 114.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 115.Pelleymounter MA, Cullen MJ, Healy D, Hecht R, Winters D, McCaleb M. Efficacy of exogenous recombinant murine leptin in lean and obese 10- to 12-mo-old female CD-1 mice. Am J Physiol. 1998;275:R950–9. doi: 10.1152/ajpregu.1998.275.4.R950. [DOI] [PubMed] [Google Scholar]
- 116.Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–50. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–5. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 118.Pu S, Dube MG, Kalra PS, Kalra SP. Regulation of leptin secretion: Effects of aging on daily patterns of serum leptin and food consumption. Regul Pept. 2000;92:107–11. doi: 10.1016/s0167-0115(00)00156-7. [DOI] [PubMed] [Google Scholar]
- 119.Ravussin E, Pratley RE, Maffei M, Wang H, Friedman JM, Bennett PH, et al. Relatively low plasma leptin concentrations precede weight gain in Pima Indians. Nat Med. 1997;3:238–40. doi: 10.1038/nm0297-238. [DOI] [PubMed] [Google Scholar]
- 120.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–53. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sahu A. Resistance to the satiety action of leptin following chronic central leptin infusion is associated with the development of leptin resistance in neuropeptide Y neurones. J Neuroendocrinol. 2002;14:796–804. doi: 10.1046/j.1365-2826.2002.00840.x. [DOI] [PubMed] [Google Scholar]
- 122.Scarpace PJ, Matheny M, Zhang Y, Tumer N, Frase CD, Shek EW, et al. Central leptin gene delivery evokes persistent leptin signal transduction in young and aged-obese rats but physiological responses become attenuated over time in aged-obese rats. Neuropharmacology. 2002;42:548–61. doi: 10.1016/s0028-3908(02)00003-5. [DOI] [PubMed] [Google Scholar]
- 123.Schulz C, Paulus K, Lehnert H. Central nervous and metabolic effects of intranasally applied leptin. Endocrinology. 2004;145:2696–701. doi: 10.1210/en.2003-1431. [DOI] [PubMed] [Google Scholar]
- 124.Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D., Jr Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2:589–93. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- 125.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–6. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 126.Slyper AH. The pediatric obesity epidemic: causes and controversies. J Clin Endocrinol Metab. 2004;89:2540–7. doi: 10.1210/jc.2003-031449. [DOI] [PubMed] [Google Scholar]
- 127.Stein CJ, Colditz GA. The epidemic of obesity. J Clin Endocrinol Metab. 2004;89:2522–5. doi: 10.1210/jc.2004-0288. [DOI] [PubMed] [Google Scholar]
- 128.Steppan CM, Swick AG. A role for leptin in brain development. Biochem Biophys Res Commun. 1999;256:600–2. doi: 10.1006/bbrc.1999.0382. [DOI] [PubMed] [Google Scholar]
- 129.Sved AF, Cano G, Card JP. Neuroanatomical specificity of the circuits controlling sympathetic outflow to different targets. Clin Exp Pharmacol Physiol. 2001;28:115–9. doi: 10.1046/j.1440-1681.2001.03403.x. [DOI] [PubMed] [Google Scholar]
- 130.Torto R, Boghossian S, Dube MG, Kalra PS, Kalra SP. Central leptin gene therapy blocks ovariectomy-induced adiposity. Obesity (Silver Spring) 2006;14:1312–9. doi: 10.1038/oby.2006.149. [DOI] [PubMed] [Google Scholar]
- 131.Toyoshima Y, Gavrilova O, Yakar S, Jou W, Pack S, Asghar Z, et al. Leptin improves insulin resistance and hyperglycemia in a mouse model of type 2 diabetes. Endocrinology. 2005;146:4024–35. doi: 10.1210/en.2005-0087. [DOI] [PubMed] [Google Scholar]
- 132.Ueno N, Dube MG, Inui A, Kalra PS, Kalra SP. Leptin modulates orexigenic effects of ghrelin and attenuates adiponectin and insulin levels and selectively the dark-phase feeding as revealed by central leptin gene therapy. Endocrinology. 2004;145:4176–84. doi: 10.1210/en.2004-0262. [DOI] [PubMed] [Google Scholar]
- 133.Ueno N, Inui A, Kalra PS, Kalra SP. Leptin transgene expression in the hypothalamus enforces euglycemia in diabetic, insulin-deficient nonobese Akita mice and leptin-deficient obese ob/ob mice. Peptides. 2006;27:2332–42. doi: 10.1016/j.peptides.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 134.Uyama N, Geerts A, Reynaert H. Neural connections between the hypothalamus and the liver. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:808–20. doi: 10.1002/ar.a.20086. [DOI] [PubMed] [Google Scholar]
- 135.Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, et al. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest. 1997;99:385–90. doi: 10.1172/JCI119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang JL, Chinookoswong N, Scully S, Qi M, Shi ZQ. Differential effects of leptin in regulation of tissue glucose utilization in vivo. Endocrinology. 1999;140:2117–24. doi: 10.1210/endo.140.5.6681. [DOI] [PubMed] [Google Scholar]
- 137.Wang ZW, Zhou YT, Kakuma T, Lee Y, Higa M, Kalra SP, et al. Comparing the hypothalamic and extrahypothalamic actions of endogenous hyperleptinemia. Proc Natl Acad Sci U S A. 1999;96:10373–8. doi: 10.1073/pnas.96.18.10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wiesner G, Vaz M, Collier G, Seals D, Kaye D, Jennings G, et al. Leptin is released from the human brain: influence of adiposity and gender. J Clin Endocrinol Metab. 1999;84:2270–4. doi: 10.1210/jcem.84.7.5854. [DOI] [PubMed] [Google Scholar]
- 139.Wilkinson M, Morash B, Ur E. The brain is a source of leptin. Front Horm Res. 2000;26:106–25. doi: 10.1159/000061018. [DOI] [PubMed] [Google Scholar]
- 140.Wynne K, Stanley S, McGowan B, Bloom S. Appetite control. J Endocrinol. 2005;184:291–318. doi: 10.1677/joe.1.05866. [DOI] [PubMed] [Google Scholar]
- 141.Xu B, Kalra PS, Farmerie WG, Kalra SP. Daily changes in hypothalamic gene expression of neuropeptide Y, galanin, proopiomelanocortin, and adipocyte leptin gene expression and secretion: effects of food restriction. Endocrinology. 1999;140:2868–75. doi: 10.1210/endo.140.6.6789. [DOI] [PubMed] [Google Scholar]
- 142.Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes. 1997;46:887–94. doi: 10.2337/diab.46.5.887. [DOI] [PubMed] [Google Scholar]
- 143.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 144.Zlokovic BV, Jovanovic S, Miao W, Samara S, Verma S, Farrell CL. Differential regulation of leptin transport by the choroid plexus and blood-brain barrier and high affinity transport systems for entry into hypothalamus and across the blood-cerebrospinal fluid barrier. Endocrinology. 2000;141:1434–41. doi: 10.1210/endo.141.4.7435. [DOI] [PubMed] [Google Scholar]