Abstract
Sox1 null lens fiber cells fail to elongate and have disrupted expression of gamma crystallin. We have evaluated the expression of Sox1 and Pax6 proteins during critical stages of lens morphogenesis, with particular focus on fiber cell differentiation. While Pax6 and Sox1 are co-expressed during early stages of fiber cell differentiation, Sox1 up-regulation coincides temporally with the down-regulation of Pax6, and these proteins therefore display a striking inverse expression pattern in the lens fiber cell compartment. Furthermore, Pax6 is inappropriately expressed in the fiber cells of Sox1 null mice and the Pax6 target, α5 integrin, is simultaneously misexpressed. Finally, we demonstrate a genetic interaction between Sox1 and Pax6, as Sox1 heterozygosity partially rescues the diameter of Pax6Sey lenses by increasing the number of cells in the fiber cell compartment.
Keywords: Sox1, Pax6, α5 integrin, cataract, lens, fiber cell, anterior epithelial layer
1. Results and Discussion
The vertebrate lens is composed of two major cell types, epithelial cells and fiber cells. During development, the lens vesicle is polarized into anterior and posterior compartments. Cells in the posterior lens exit the cell cycle and elongate to form primary fiber cells, while cells in the anterior lens differentiate into epithelium. A subset of anterior epithelial layer (AEL) cells proliferate and their daughters subsequently exit the cell cycle, migrate into the transition zone and differentiate into secondary fiber cells.
Sox1, a Group B1 SRY-like HMG box transcription factor, is required for fiber cell development in mice (Nishiguchi et al., 1998). Mice lacking Sox1 have small, opaque lenses with a persistent hole in the central cavity where fiber cells fail to fully differentiate (Nishiguchi et al., 1998). Sox1 null lenses lose expression of γ-crystallin at E12.5 coincident with the down-regulation of the related gene, Sox2 (Nishiguchi et al., 1998). Although loss of γ-crystallin has been linked to cataract formation (reviewed in Graw, 2004), it is reasonable to hypothesize that other genes downstream of Sox1 also contribute to the fiber cell differentiation defect.
In addition to Sox1, other transcription factors are necessary for proper fiber cell differentiation, including Pax6, c-Maf, Pitx3, Prox1, and FoxE3. Mutation of these factors causes cataracts in both mouse models and humans (Glaser et al., 1994; Hanson et al., 1994; Semina et al., 1998; Wigle et al., 1999; Blixt et al., 2000; Brownell et al., 2000; Duncan et al. 2000; Ring et al., 2000; Semina et al., 2000, 2001; Jamieson et al., 2002; Lyon et al., 2003; Berry et al., 2004; Duncan et al., 2004; Rajaram and Kerpolla, 2004). Most of these transcription factors are downstream of Pax6 in the lens. (Brownell et al., 2000; Dimanlig et al., 2001; Sakai et al., 2001; Chauhan et al., 2002; Reza et al., 2002).
The central role that Pax6 plays in lens development and its relationship with another GroupB1 SRY-like HMG box protein, Sox2, is well documented (Kamachi et al., 2001; Aota et al., 2003; Kondoh et al., 2004; Donner et al., in press). Pax6 is upstream of Sox2 (Furuta and Hogan, 1998), Pax6 and Sox2 cooperatively regulate gene expression (Kamachi et al. 2001, Aota et al., 2003), and Sox2 is required for the maintenance of Pax6 (Donner et al., in press). Appropriate temporal, spatial, and quantitative control of Pax6 expression is required for appropriate lens morphogenesis (Hill et al., 1991; Glaser et al., 1994; Hanson et al., 1994; Grindley et al., 1995; Quinn et al., 1996; Schedl et al., 1996; Brown et al., 1998; Ashery-Padan et al., 2000; Duncan et al., 2000; van Raamsdonk and Tilgham, 2000; Dimanlig et al., 2001; Duncan et al., 2004). PAX6 mutation is causative for several congenital eye defects in humans including aniridia, Peters’ anomaly and cataract (reviewed in Prosser and van Heyningen, 1998). Given the importance of the Pax6 for appropriate lens morphogenesis and the link between Pax6 and Sox2, we sought to evaluate the relationship between Sox1 and Pax6 in the developing lens.
1.1. Fiber cell expression of Sox1 and Pax6 is inversely correlated
We assessed the expression of Sox1 and Pax6 during fiber cell formation and differentiation. At E11.5, Sox1 was first detected in cells in the posterior lens, while Pax6 was expressed in all cells of the lens pit (Fig. 1A–C). By E12.5, primary fiber cells elongated across the lens vesicle and their nuclei were anteriorly translocated. While Sox1 and Pax6 are still expressed in most nuclei at this stage, Sox1 staining increased and the intensity of Pax6 staining diminished in the fiber cell compartment as compared to E11.5 (Fig. 1D–F). At E13.5, Sox1 persisted in fiber cell nuclei, while Pax6 was further down regulated with weakest expression observed in the center of the lens (Fig. 1G–I). By E15.5, Pax6 was no longer detected in fiber cell nuclei while Sox1 expression remained (Fig. 1J–L). From E12.5 to E15.5 Sox1 and Pax6 are co-expressed in the AEL (Fig. 1D, 1F–G, 1I–J, 1L). At the onset of primary fiber cell differentiation, Sox1 and Pax6 are co-expressed, but thereafter Pax6 is down regulated while Sox1 is up regulated and maintained. Thus, while Sox1 and Pax6 are co-expressed in the AEL, they exhibit a transiently overlapping, but subsequently inverse expression pattern in the lens fiber cell compartment.
Fig. 1.

Pax6 and Sox1 expression are inversely correlated in the developing fiber cell compartment. Wild-type eyes at embryonic day (E) 11.5 (A–C), E12.5 (D–F), E13.5 (G–I), and E15.5 (J–L) with immunofluorescence for Pax6 (red) (A, D, G, J), or Sox1 (green) (B, E, H, K) and DAPI nuclear stain (blue) are shown. Additional sections of Sox1βgeo/+ lenses stained for β-galactosidase activity are shown (C, F, I, L) to highlight Sox1 expression in the AEL. The arrows in C highlight the anterior extreme of β-galactosidase activity. The arrows in panels F, I and L indicate β-galactosidase activity in the AEL. The arrowheads in J and K highlight nuclei positive for Sox1 and negative for Pax6. All eyes (in all figures) are oriented anterior to the right and posterior to the left. Abbreviations: ael anterior epithelial layer, fc fiber cell compartment, lv lens vesicle Scale bars in all figures are 100 microns.
1.2. Pax6 expression persists inappropriately in the fiber cells of Sox1−/− lenses
We next assessed Pax6 expression in Sox1−/− eyes. The disruption in fiber cell differentiation in Sox1−/− lenses is evident by E12.5. Unlike wild-type lenses, neither fiber cell elongation nor anterior translocation of nuclei has occurred (Fig. 2A–B). By E13.5, some fiber cell elongation and anterior movement of nuclei was evident in Sox1−/− lenses (Fig. 2C–D). Pax6 expression was inappropriately maintained in posterior cells of the Sox1−/− lens through at least E15.5 (Fig. 2A–F), even after the delayed onset of fiber cell elongation. Sox2 was undetectable in both wild-type and Sox1−/− mice at E12.5 (data not shown), and thus cannot be responsible for the persistence of Pax6. Thus, Sox1 is required for the appropriate repression of Pax6 in differentiating lens fiber cells.
Fig. 2.
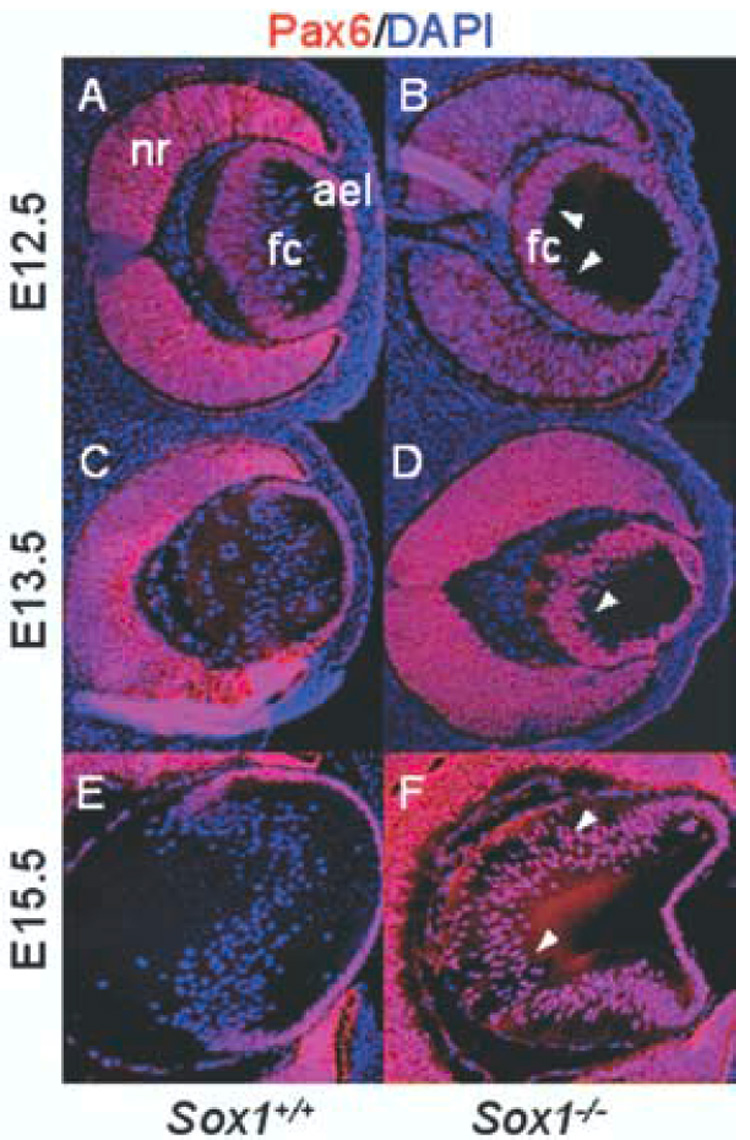
Pax6 expression is inappropriately maintained in the fiber cell compartment of Sox1−/− lenses. Wild-type (A, C, E) and Sox1−/− lenses (B, D, F) are shown with Pax6 immunofluorescence (red) and DAPI nuclear stain (blue) at E12.5 (A–B), E13.5 (C–D), and E15.5 (E–F). Arrowheads indicate fiber cell nuclei positive for Pax6.
1.3. α5 integrin, a Pax6 target, is inappropriately maintained in Sox1 null fiber cells
The persistent and inappropriate expression of the transcription factor Pax6 in the fiber cell compartment of Sox1−/− lenses suggests that factors downstream of Pax6 might also be maintained. The fiber cell differentiation markers α5 and β1 integrin are a co-receptor for fibronectin and are direct downstream targets of Pax6 in the lens (Johansson et al., 1997; Duncan et al., 2000). No major abnormalities were observed in β1 integrin expression in Sox1−/− lenses at E13.5 or 15.5 (data not shown). Thus, misexpression of β1 integrin does not contribute to the Sox1−/− lens phenotype.
The expression of α5 integrin was dynamic during fiber cell differentiation (Fig. 3A–D). At E13.5, α5 integrin was expressed throughout the AEL, the equatorial transition zone, and the fiber cell compartment (Fig. 3A), while α5 integrin expression was reduced in the fiber cell compartment at E15.5 (Fig. 3C). The temporal pattern of α5 integrin expression resembled that of Pax6, which diminished in the fiber cell compartment at E12.5 (Fig. 1D, Fig. 2A) and was absent from the fiber cell compartment by E15.5 (Fig. 1J, Fig. 2E). Down-regulation of α5 integrin began later than that of Pax6, but was complete by E15.5 (Fig. 3C).
Fig. 3.
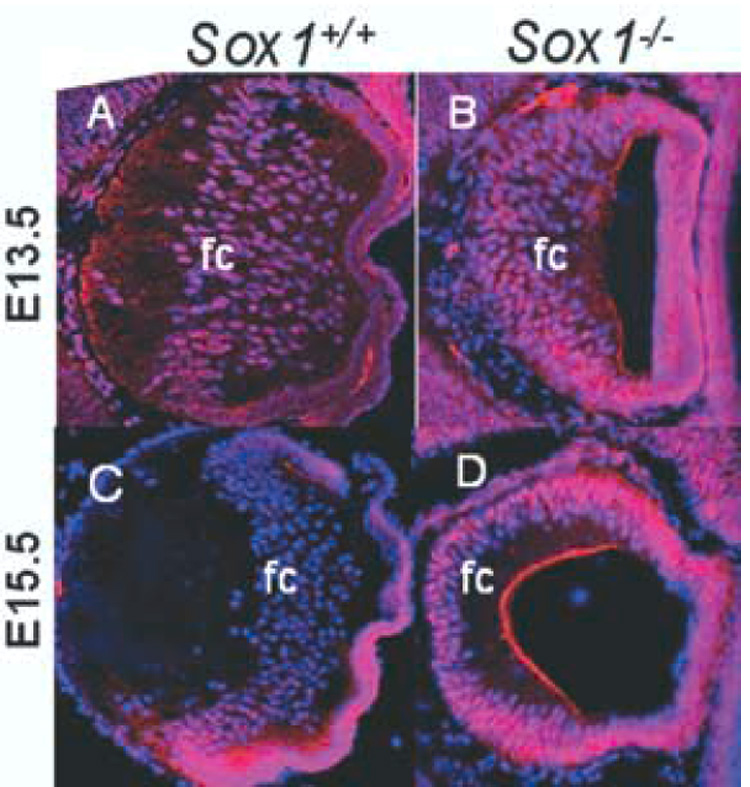
α5 integrin, a Pax6 target, is inappropriately maintained in the fiber cell compartment of Sox1−/− lenses. Wild-type (A, C) and Sox1−/− lenses (B, D) are shown with α5 integrin immunofluorescence (red) and DAPI nuclear stain (blue) at E13.5 (A–B) and E15.5 (C–D). Abbreviations: fc fiber cell compartment
Sox1−/− lenses differed from wild-type lenses in their pattern of α5 integrin expression. At E13.5, as in wild-type lenses, α5 integrin was found in the AEL, the equatorial transition zone, and the fiber cell compartment (Fig. 3B). Unlike wild-type lenses, however, α5 integrin was not down regulated in the fiber cell compartment at E15.5, but persisted (Fig. 3D). Moreover, the persistence of α5 integrin expression followed the pattern of Pax6 expression (compare Figs. 2D, 2F to Figs. 3B, 3D). Thus, in Sox1−/− fiber cells at E15.5, both Pax6 and α5 integrin expression persist inappropriately.
1.4. Loss of Sox1 partially rescues the size of Pax6Sey/+ lenses
To test whether Pax6 and Sox1 interact at the genetic level during fiber cell differentiation, we assessed the histology of lenses with compound Pax6 and Sox1 mutations at E13.5 (Fig. 4A–F), the stage of lens development when Pax6 misexpression was first evident in the Sox1−/− fiber cells. Both the Sox1−/− phenotype and the Pax6Sey/+ phenotype were evident at E13.5 (Fig. 4C–D). The lenses of Pax6Sey/+ mice were misshapen and significantly smaller when compared to those in wild-type mice (maximum lens diameter on dorsal/ventral axis: 307 ± 9.7 microns (mean ± SD, n=5) and 459 ± 21 microns respectively, p < .001) (Fig. 4A, 4D–D’, 4G). The Sox1−/− lenses were also smaller than wild-type lenses and had a large hole between the elongating fiber cells and the AEL resulting from a failure in primary fiber cell differentiation (Fig 4A, 4C). Both Pax6Sey/+ and Sox1+/−; Pax6Sey/+ lenses had variable phenotypes including small lenses and a persistent stalk connecting the lens AEL to the corneal ectoderm (Fig. 4D–E, and data not shown). Lens defects in Sox1+/−; Pax6Sey/+ mice, however, were milder than the Pax6Sey/+ phenotypes. The Sox1+/−; Pax6Sey/+ lenses were larger and less conical in shape than the Pax6Sey/+ lenses (maximum lens diameters 368 ± 28 microns and 307 ± 9.7 microns, respectively, p < .01) (Figure 4D–4D’, 4E–4E’, 4G). Thus, reduction in Sox1 dosage partially rescued the size of Pax6Sey/+ lenses, with the increased lens size resulting from an increase in either fiber cell number or fiber cell elongation. These data demonstrate a genetic interaction between Sox1 and Pax6, and are consistent with Pax6 and Sox1 having opposing affects on fiber cell differentiation.
Fig. 4.
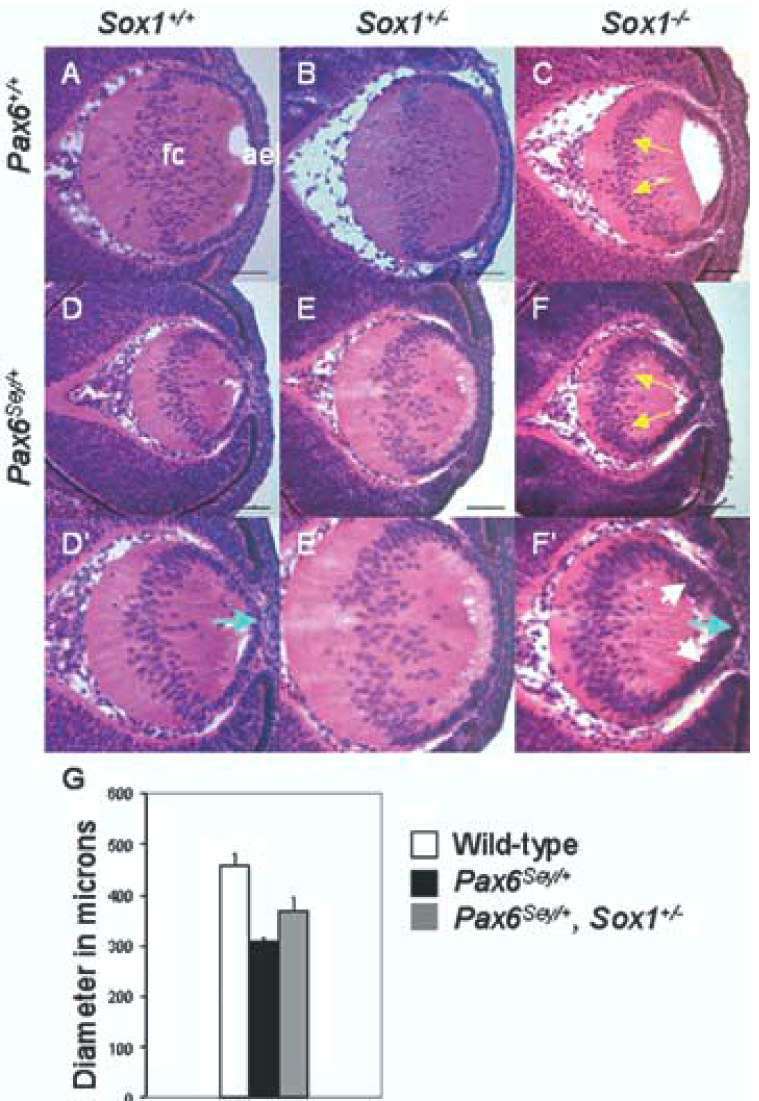
Sox1 heterozygosity partially rescues the size of Pax6Sey/+ lenses. Representative lenses at E13.5 are shown for wild-type (A); Sox1+/− (B); Sox1−/− (C); Pax6Sey/+ (D–D’); Pax6Sey/+, Sox1+/− (E–E’); and Pax6Sey/+, Sox1−/− (F–F’) lenses. High magnification images (D’–F’) show epithelial layer abnormalities. Yellow arrows indicate fiber cell nuclei that have remained in the lens posterior (C, F). White arrows indicate multi-layered AEL (F’) and blue arrows indicate pointed AEL (D’, F’). (G) Maximum lens diameters for wild-type (white box), Pax6Sey/+ (black box), and Pax6Sey/+, Sox1+/− (gray box) lenses are statistically different (see Experimental Procedures).
Pax6Sey/+; Sox1−/− compound mutant lenses were small and grossly misshapen like Pax6Sey/+ lenses (Fig. 4F–F’). In addition, the AEL in the Pax6Sey/+; Sox1−/− lenses was multi-layered and disorganized (Fig. 4F–F’). Thus, unlike the partial rescue observed in lenses with combined heterozygous mutations in Pax6 and Sox1, the combination of Pax6Sey/+ and Sox1−/− had an additive detrimental effect on both lens size and organization of the AEL.
1.5. Pax6Sey/+; Sox+/− lenses have more fiber cells than those of Pax6Sey/+ mice
To determine whether an increase in fiber cell number contributed to the increase in size of Pax6Sey/+; Sox1−/− lenses as compared to Pax6Sey/+ lenses, we counted nuclei in the fiber cell compartments of wild-type, Sox1+/−, Pax6Sey/+ and Sox1+/−; Pax6Sey/+ lenses. To facilitate in the discrimination between fiber cells and epithelial cells, we stained the sections with the epithelial-specific marker, E-cadherin, and the nuclear marker, DAPI. We counted the nuclei of cells that were negative for E-cadherin expression. The fiber cell nuclei number in Sox1+/−; Pax6Sey/+ lenses was statistically significantly higher than in Pax6Sey/+ lenses (fiber cell number 190 ± 31 and 144 ± 13 (mean ± SD), respectively, p< 0.001) (Fig 5E). Thus, the increase in size observed in Sox1+/−; Pax6Sey/+ lenses versus Pax6Sey/+ lenses is due, at least in part, to an increase in fiber cell number.
Fig. 5.
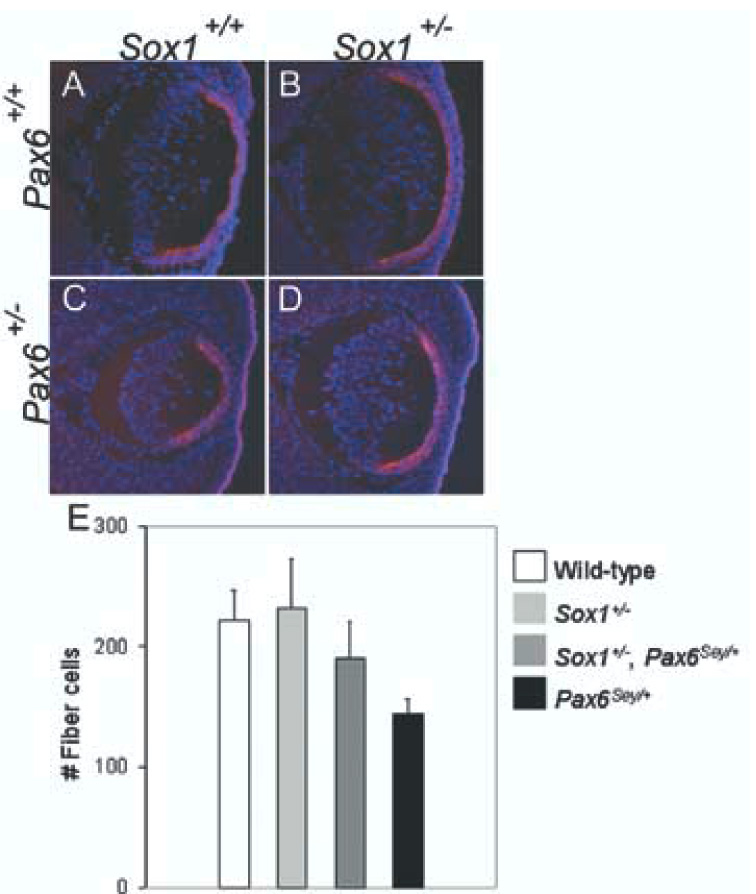
Sox1 heterozygosity increases the number of fiber cells in Pax6Sey/+ lenses. E-cadherin immunofluorescence (red) and DAPI nuclear stain (blue) are shown (A–D) for wild-type (A), Sox1+/− (B), Pax6Sey/+ (C) and Pax6Sey/+, Sox1+/− (D) lenses at E13.5. (E) E-cadherin negative, fiber cell nuclei were counted for wild-type (white box), Sox1+/− (light grey box), Pax6Sey/+ (black box) and Pax6Sey/+, Sox1+/− (dark grey box) lenses and the number of fiber cell nuclei in Pax6Sey/+, Sox1+/− lenses is statistically significantly higher than those in Pax6Sey/+ lenses (see Experimental Procedures).
1.6. Sox1 and Pax6 have both additive and antagonistic activities in the lens
Interestingly Sox1−/−; Pax6Sey/+ lenses are more severely affected than either Sox1−/− or Pax6Sey/+ lenses alone. Thus, combined Sox1 and Pax6 loss-of-function both rescue and disrupt lens development. This result could be reconciled by additive defects in the cells of the AEL in Sox1−/−; Pax6Sey/+ lenses, which normally maintain both Pax6 and Sox1. The AEL of the Sox1−/−; Pax6Sey/+ lens is multi-layered (Fig. 4F–F’). Abnormal morphology of the AEL was also observed in Pax6Sey/+ lenses (Fig. 4D–D’, pointed appearance) although the phenotype was most pronounced in the Sox1−/−; Pax6Sey/+ AEL. This is consistent with the findings of Collinson et al. (2001) that Pax6Sey/+ cells are preferentially lost from the AEL between E12.5 and E16.5 in Pax6Sey/+ ↔ Pax6+/+ chimeric mice. Additional evidence that this additive defect derives from a defect in the AEL comes from lenses evaluated at E15.5. By this stage, secondary fiber cells derived from the AEL are present. At this stage, wild-type, Pax6Sey/+, Sox1+/−; Pax6Sey/+ lenses have phenotypes comparable to those described at E13.5, although the lenses are larger (Fig. 6A–C). In contrast, the Sox1−/−; Pax6Sey/+ lenses are more severely affected at E15.5 (Fig. 6D). The lenses are small and there is no clear delineation between the AEL and the fiber cell compartment. The increase in severity in phenotype between E13.5 and E15.5 indicates that the Sox1−/−; Pax6Sey/+ lens has a progressively degenerative phenotype. Thus, both Sox1 and Pax6 play an important role in organization or maintenance of the AEL and combined loss-of-function of these genes has an additive detrimental effect on the AEL.
Fig. 6.
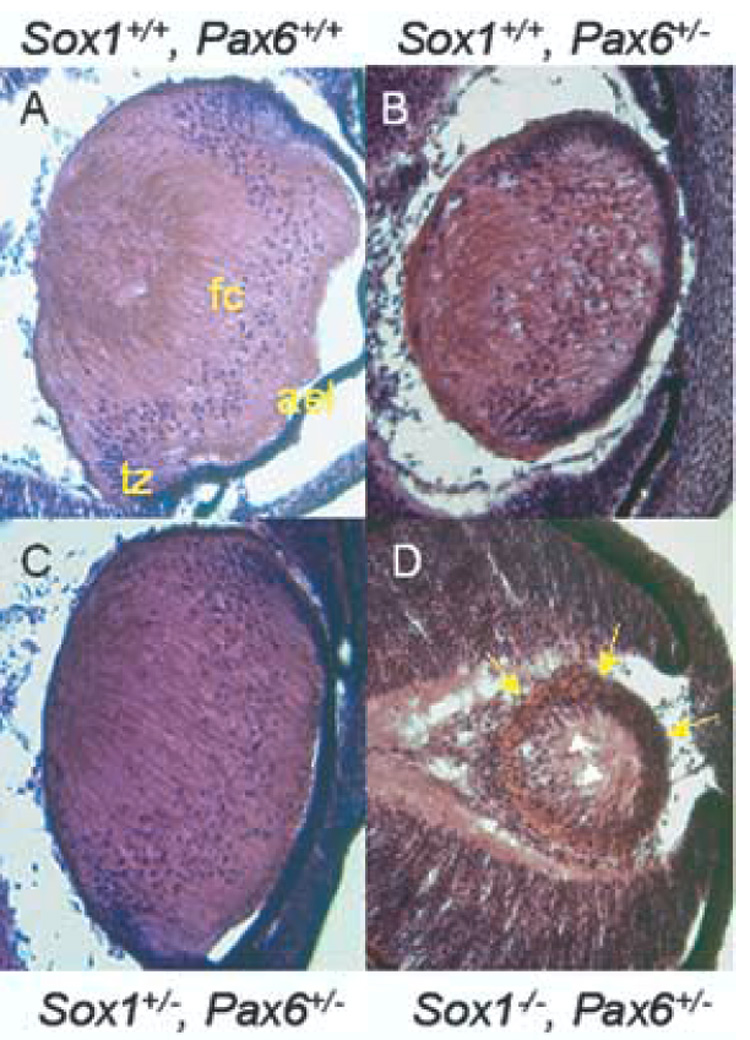
Sox1−/−; Pax6Sey/+ lenses have a progressively degenerative phenotype. H&E stained wild-type (A), Pax6Sey/+ (B), Sox1+/−; Pax6Sey/+ (C), and Sox1−/−; Pax6Sey/+ (D) E15.5 lenses are shown. Distinct compartments for AEL, transition zone (tz), and fiber cells (fc) are evident in panels A–C. In panel D, yellow arrows indicate uniform appearance of cells around the lens perimeter and white arrows indicate the most anterior nuclei of the lens fiber cell compartment.
2. Experimental Procedures
2.1. Mouse work
All mouse work was performed in accordance with protocols approved by the Harvard Animal Care and Usage Committee. Sox1βgeo, Sox1M1, and Pax61-NeuSey/+ (Pax6Sey/+) mice were maintained on a C3H/HeN background (Nishiguchi et al., 1998; Ekonomou et al., 2005). Sox1βgeo and Sox1M1 heterozygotes (Sox1+/−) were crossed to generate Sox1−/− embryos (Ekonomou et al., 2005). Sox1+/− mice were crossed to Pax6Sey/+ mice. Double heterozygotes resulting from these crosses were bred to Sox1+/− mice. Appropriately staged embryos and their extra-embryonic tissues were collected, and genomic DNA from extra-embryonic tissue was screened by PCR (Nishiguchi et al., 1998). The Pax61-NeuSey/+ allele was identified by a PCR fragment polymorphism producing a HincII restriction site.
2.2. Immunofluorescence
Embryos were embedded in paraffin or frozen in OCT (Tissue-Tek). In Fig. 1, stage E11.5 and E13.5 embryos were prepared from frozen sections, while paraffin embedded embryos were utilized to improve histology on eyes at E12.5 and E15.5. In Fig. 2, stage E12.5 and E15.5 sections were prepared from paraffin sections, while frozen embryos were utilized for E13.5. For Fig. 3 and Fig. 5 frozen sections were utilized.
For frozen sections, embryos were fixed in 4% paraformaldehyde (PFA) for 1 hour, equilibrated in 30% sucrose and embedded in OCT. For paraffin sections, embryos were fixed in 4% PFA overnight, dehydrated, and embedded in wax. Sox1 immunofluorescence in paraffin sections required trypsin unmasking (Sigma). Pax6 staining in paraffin (DSHB 1:50, mouse concentrate) was performed as described (Collinson et al., 2003). All frozen sections were unmasked by boiling in Antigen Unmasking Solution (Vector Laboratories) unless otherwise indicated. Sox1 expression in the epithelium was not detectable. The quality of unmasking varied slightly and accounts for the loss of low-level Pax6 staining in some sections. Comparable panels in each figure were always treated together to standardize the quality of unmasking. Staining for β1 integrin (Santa Cruz), α5 integrin (Santa Cruz) and E-cadherin (Zymed® Laboratories) were performed on frozen sections. Detection of α5 and β1 integrin required unmasking in 1mg/mL pepsin; 2.8% glacial acetic acid, pH 3.0 at room temperature.
Indirect visualization was achieved using secondary antibodies with fluorescent conjugates [anti-rabbit Cy3, anti-goat Cy3 (Jackson Immunologicals) and anti-rabbit Alexa-Fluor 488 (Molecular Probes)]. Sections were mounted with Vectashield® plus DAPI (Vector Laboratories), visualized on a Zeiss Axiophot microscope, and photographed with a Leica DFC350 F Digital Camera.
2.3. Histology, maximum diameter calculations, and fiber cell quantification
For maximum diameter calculations, serial transverse paraffin sections from wild-type, compound heterozygous Sox1+/−; Pax6Sey/+ and Pax6Sey/+ mutants were cut through 5 embryos of each genotype. Sections were H&E stained by standard protocols (Nagy et al., 2003). The diameter of each lens was measured from the AEL to the posterior of the fiber cell compartment and the maximum diameters were averaged. A single factor ANOVA and post-hoc Sheffe’s test were utilized to determine the statistical significance of lens maximum diameter differences (wild-type1 459 ± 21 microns, Pax6Sey/+2 307 ± 9.7 microns, and Sox1+/−, Pax6Sey/+3 368 ± 28 microns; mean ± SD). Maximum lens diameters for wild-type, Pax6Sey/+ and Pax6Sey/+; Sox1+/− lenses are statistically different. (n=5 per genotype, p < 0.05, F.05 (2, 12)=3.88; F12=64.4, F13=23.1, F23=10.37).
For fiber cell quantification experiments, serial transverse frozen sections from wild-type, Sox1+/−, Sox1+/−; Pax6Sey/+ and Pax6Sey/+ E13.5 embryos were cut through 5 embryos of each genotype. Immunofluorescence for E-cadherin with DAPI nuclear stain was performed to identify epithelial cells. Cells positive for DAPI, but negative for E-cadherin in sections from the central lens were counted and averaged (1-wild-type 222 ± 24 cells; 2-Sox1+/− 232 ± 40 cells; 3-Sox1+/−; Pax6Sey/+ 190 ± 31 cells, 4-Pax6Sey/+ 144 ± 13 cells). Pair wise t-tests were performed to determine statistical significance of data (p14 < 0.001, p34 < 0.001).
3. Acknowledgements
The authors are grateful to members of the Maas laboratory for helpful discussions and to anonymous reviewers for their helpful comments. The Pax6 antibody developed by A. Kawakami was obtained from the Developmental Studies Hybridoma Bank (NICHD and The University of Iowa). Dr. Robin Lovell-Badge kindly provided the Sox1 antibody. This work was funded by grant R01 EY010123-10 to RLM. ALD was supported by fellowships F32-DE05735, and T32-EY07145 from NEI administered by the Postdoctoral Training Program in the Molecular Bases of Eye Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aota S, Nakajima N, Sakamoto R, Wantanabe S, Ibaraki N, Okazaki K. Pax6 autoregulation mediated by direct interaction of Pax6 protein with the head surface ectoderm-specific enhancer of the mouse Pax6 gene. Dev. Biol. 2003;257:1–13. doi: 10.1016/s0012-1606(03)00058-7. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry V, Yang Z, Addison PK, Francis PJ, Ionides A, Karan G, Jiang L, Lin W, Hu J, Yang R, Moore A, Zhang K, Bhattacharya SS. Recurrent 17 bp duplication in PITX3 is primarily associated with posterior polar cataract (CPP4) J. Med. Genet. 2004;41:e109. doi: 10.1136/jmg.2004.020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blixt A, Mahlapuu M, Aitola M, Pelto-Huikko M, Enerback S, Carlsson P. A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle. Genes Dev. 2000;14:245–254. [PMC free article] [PubMed] [Google Scholar]
- Brown A, McKie M, van Heyningen V, Prosser J. The Human PAX6 Mutation Database. Nucleic Acids Res. 1998;26:259–264. doi: 10.1093/nar/26.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell I, Dirksen M, Jamrich M. Forkhead Foxe3 maps to the dysgenic lens locus and is critical in lens development and differentiation. Genesis. 2000;27:81–93. doi: 10.1002/1526-968x(200006)27:2<81::aid-gene50>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Chauhan BK, Reed NA, Zhang W, Duncan MK, Kilimann MW, Cvekl A. Identification of genes downstream of Pax6 of the mouse lens using cDNA microarrays. J Biol. Chem. 2002;277:11539–11548. doi: 10.1074/jbc.M110531200. [DOI] [PubMed] [Google Scholar]
- Collinson JM, Quinn JC, Buchanan MA, Kaufman MH, Wedden SE, West JD, Hill RE. Primary defects in the lens underlie complex anterior segment abnormalities of the Pax6 heterozygous eye. Proc. Natl. Acad. Sci. 2001;98:9688–9693. doi: 10.1073/pnas.161144098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson JM, Quinn JC, Hill RE, West JD. The roles of Pax6 in the cornea, retina, and olfactory epithelium of the developing mouse embryo. Dev. Biol. 2003;255:303–312. doi: 10.1016/s0012-1606(02)00095-7. [DOI] [PubMed] [Google Scholar]
- Dimanlig PV, Faber SC, Auerbach W, Markarenkova HP, Lang RA. The upstream ectoderm enhancer in Pax6 has an important role in lens induction. Development. 2001;128:4415–4424. doi: 10.1242/dev.128.22.4415. [DOI] [PubMed] [Google Scholar]
- Donner AL, Episkopou V, Maas RL. Sox2 and Pou2f1 interact to control lens and olfactory placode development. Dev. Biol. doi: 10.1016/j.ydbio.2006.10.047. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MK, Kozmik Z, Cveklova K, Piatigorsky J, Cvelk A. Overexpression of PAX6(5a) in lens fiber cells results in cataract and upregulation of (alpha)5(beta)1 integrin expression. J. Cell Sci. 2000;113:3173–3185. doi: 10.1242/jcs.113.18.3173. [DOI] [PubMed] [Google Scholar]
- Duncan MK, Xie L, David LL, Robinson ML, Taube JR, Cui W, Reneker LW. Ectopic Pax6 expression disturbs lens fiber cell differentiation. Invest. Ophthalmol. Vis. Sci. 2004;45:3589–3598. doi: 10.1167/iovs.04-0151. [DOI] [PubMed] [Google Scholar]
- Ekonomou A, Kazanis I, Malas S, Wood H, Alifragis P, Denaxa M, Karagogeos D, Constanti A, Lovell-Badge R, Episkopou V. Neuronal migration and ventral subtype identity in the telencephalon depend on SOX1. PLoS Biol. 2005;3:e186. doi: 10.1371/journal.pbio.0030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Hogan BL. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser T, Jepeal L, Edwards JG, Young SR, Favor J, Maas RL. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat. Genet. 1994;7:463–471. doi: 10.1038/ng0894-463. [DOI] [PubMed] [Google Scholar]
- Graw J. Congenital hereditary cataracts. Int. J. Dev. Biol. 2004;48:1031–1044. doi: 10.1387/ijdb.041854jg. [DOI] [PubMed] [Google Scholar]
- Grindley JC, Davidson DR, Hill RE. The role of Pax-6 in eye and nasal development. Development. 1995;121:1433–1442. doi: 10.1242/dev.121.5.1433. [DOI] [PubMed] [Google Scholar]
- Hanson IM, Fletcher JM, Jordan T, Brown A, Taylor D, Adams RJ, Punnett HH, van Heyningen V. Mutations at the PAX6 locus are found in heterogeneous anterior segment malformations including Peters’ anomaly. Nat. Genet. 1994;6:168–173. doi: 10.1038/ng0294-168. [DOI] [PubMed] [Google Scholar]
- Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- Jamieson RV, Perveen R, Kerr B, Carette M, Yardley J, Heon E, Wirth MG, van Heyningen V, Donnai D, Munier F, Black GC. Domain disruption and mutation of the bZIP transcription factor, MAF, associated with cataract, ocular anterior segment dysgenesis and coloboma. Hum. Mol. Genet. 2002;11:33–42. doi: 10.1093/hmg/11.1.33. [DOI] [PubMed] [Google Scholar]
- Johansson S, Svineng G, Wennerberg K, Armulik A, Lohikangas L. Fibronectin-integrin interactions. Front. Biosci. 1997;2:d126–d146. doi: 10.2741/a178. [DOI] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Tanouchi A, Sekido R, Kondoh H. Pax6 and SOX2 form a co-DNA-binding partner complex regulates initiation of lens development. Genes Dev. 2001;15:1272–1286. doi: 10.1101/gad.887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh H, Uchikawa M, Kamachi Y. Interplay of Pax6 and SOX2 in lens development as a paradigm of genetic switch mechanisms for cell differentiation. Int. J. Dev. Biol. 2004;48:819–827. doi: 10.1387/ijdb.041868hk. [DOI] [PubMed] [Google Scholar]
- Lyon MF, Jamieson RV, Perveen R, Glenister PH, Griffiths R, Boyd y, Glimcher LH, Favor J, Munier FL, Black GC. A dominant mutation with the DNA-binding domain of the bZIP transcription factor Maf causes murine cataract and results in selective alteration of DNA binding. Hum. Mol. Genet. 2003;12:585–594. [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the mouse embryo: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2003. [Google Scholar]
- Nishiguchi S, Wood H, Kondoh H, Lovell-Badge R, Episkopou V. Sox1 directly regulates the gamma-crystallin genes and is essential for lens development in mice. Genes Dev. 1998;12:776–781. doi: 10.1101/gad.12.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser J, van Heyningen V. PAX6 mutations reviewed. Hum. Mutat. 1998;11:93–108. doi: 10.1002/(SICI)1098-1004(1998)11:2<93::AID-HUMU1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Quinn JC, West JD, Hill RE. Multiple functions for Pax6 in mouse eye and nasal development. Genes Dev. 1996;10:435–436. doi: 10.1101/gad.10.4.435. [DOI] [PubMed] [Google Scholar]
- Rajaram N, Kerpolla TK. Synergistic transcription activation by Maf and Sox and their subnuclear localization are disrupted by a mutation in Maf that causes cataract. Mol. Cell Biol. 2004;24:5694–5709. doi: 10.1128/MCB.24.13.5694-5709.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reza HM, Ogino H, Yasuda K. L-Maf, a downstream target of Pax6, is essential for chick lens development. Mech. Dev. 2002;116:61–73. doi: 10.1016/s0925-4773(02)00137-5. [DOI] [PubMed] [Google Scholar]
- Ring BZ, Cordes SP, Overbeek PA, Barsh GS. Regulation of mouse lens fiber cell development and differentiation by the Maf gene. Development. 2000;127:307–317. doi: 10.1242/dev.127.2.307. [DOI] [PubMed] [Google Scholar]
- Sakai M, Serria MS, Ikeda H, Yoshida K, Imaki J, Nishi S. Regulation of c-maf gene expression by Pax6 in cultured cells. Nucleic Acids Res. 2001;29:1228–1237. doi: 10.1093/nar/29.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl A, Ross A, Lee M, Engelkamp D, Rashbass P, van Heyningen V, Hastie ND. Influence of PAX6 gene dosage on development: overexpression causes severe eye abnormalities. Cell. 1996;86:71–82. doi: 10.1016/s0092-8674(00)80078-1. [DOI] [PubMed] [Google Scholar]
- Semina EV, Ferrell RE, Mintz-Hittner HA, Bitoun P, Alward WL, Reiter RS, Funkauser C, Daack-Hirsch S, Murray JC. A novel homeobox gene PITX3 is mutated in families with autosomal-dominant cataracts and ASMD. Nat. Genet. 1998;19:167–170. doi: 10.1038/527. [DOI] [PubMed] [Google Scholar]
- Semina EV, Murray JC, Reiter R, Hrstka RF, Graw J. Deletion in the promoter region and altered expression of Pitx3 homeobox gene in aphakia mice. Hum. Mol. Genet. 2000;9:1575–1585. doi: 10.1093/hmg/9.11.1575. [DOI] [PubMed] [Google Scholar]
- Semina EV, Brownell I, Mintz-Hittner HA, Murray JC, Jamrich M. Mutations in the human forkhead transcription factor FOXE3 associated with anterior segment ocular dysgenesis and cataracts. Hum. Mol. Genet. 2001;10:231–236. doi: 10.1093/hmg/10.3.231. [DOI] [PubMed] [Google Scholar]
- van Raamsdonk CD, Tilghman SM. Dosage requirement and allelic expression of PAX6 during lens placode formation. Development. 2000;127:5439–5448. doi: 10.1242/dev.127.24.5439. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999;21:318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]


