Abstract
Alterations in genes involved in nucleotide excision repair (NER) are associated with three genetic disorders, xeroderma pigmentosum (XP), Cockayne syndrome (CS) and trichothiodystrophy (TTD). The transcription and repair factor TFIIH is a central component of NER and mutations of its subunits are associated with all three diseases. A recent report provides a molecular basis for how mutations in the NER endonuclease XPG that affect the interaction of TFIIH might give rise to CS features. In cells of XP-G patients with a combined XP and CS phenotype, XPG fails to associate with TFIIH and as a consequence the CAK subunit dissociates from core TFIIH. A simplified, but general model of how various assembly and disassembly states of TFIIH can be invoked to explain different disease states is discussed. Accordingly, defects in specific enzymatic functions typically result in XP, dissociation of the CAK subunit from TFIIH is associated with XP/CS and a more generalized destabilization of TFIIH gives rise to TTD. While this classification provides a useful framework to understand how alterations in TFIIH correlate with disease states, it does not universally apply and relevant exception and alternative explanations are discussed.
Keywords: nucleotide excision repair, transcription, xeroderma pigmentosum (XP), Cocakyne syndrome (CS), trichothiodistrophy (TTD), TFIIH
Introduction
Nucleotide excision repair (NER) removes a large variety of lesions from DNA and can be subdivided into two subpathways - global genome repair (GG-NER), which removes lesions from all region of the genome, and transcription-coupled repair (TC-NER), which refers to the accelerated removal of damage from the transcribed strands of active genes [1]. The two pathways employ a common set of proteins (including TFIIH, XPG, XPA, RPA and ERCC1-XPF), but differ in their mode of damage recognition. GG-NER relies on the XPC/HR23B and likely XPE (DDB1/2) genes to find damaged sites in DNA, while TC-NER is initiated by a stalled RNA polymerase and additionally involves the CSA, CSB and XAB2 genes [2,3]. Defects in genes involved in nucleotide excision repair (NER) are associated with three disorders, xeroderma pigmentosum (XP), Cockayne syndrome (CS) and trichodiodistrophy (TTD) [4]. Of these, XP represents a prototypical DNA repair disorder and is characterized by extreme sensitivity to UV light and a 2000-fold increased incidence in skin cancer. Severely affected XP patients additionally suffer from late-onset neurological defects. This skin-cancer prone phenotype is readily explained by the inability of XP patients to repair UV-induced DNA lesions in skin tissues exposed to sunlight. By contrast, CS and TTD patients are not overtly cancer prone, but they experience additional symptoms. CS patients suffer from severe neurological abnormalities, short stature, lack of subcutaneous fat, hypogonadism, bird-like faces, tooth decay and cataracts and have a short life span averaging 12.5 years. TTD patients share some similarities with CS patients and suffer from photosensitivity, mental retardation, small stature, ichthyotic skin and unusual facial features. The defining characteristic of TTD patients is the presence of sulfur-deficient brittle hair and nails caused by reduced levels of cysteine-rich matrix proteins.
CS and TTD are linked to transcription defects
How may we account for the complex phenotypes of CS and TTD? CS is caused by mutations in either the CSA or CSB genes and was initially characterized as a defect in TC-NER. This defect does not account for the complex CS phenotype however, since most of the XP patients (except those of the XP-C and XP-E groups) are also TC-NER deficient, but do not suffer from the typical CS symptoms. Based on the association of CSB with RNA polymerase II and some impairment of transcription in CS cells in particular after DNA damage, a general (mild) defect in transcription has been invoked to explain the CS symptoms [5-8]. Surprisingly, null mutations in CSB result in a disease termed UV-sensitive syndrome (UVSS), which displays a milder phenotype than hypomorphic CSB mutations associated with CS [9]. It remains to be established, how the dominant-negative alleles characteristic of CS-B give rise to the severe CS phenotype. Interestingly, a subset of XP patients with defects in the XPB, XPD and XPG genes suffer from a combined XP/CS phenotype [10,11]. In line with the suspected connection of CS with transcription, the two hecliases XPB and XPD are subunits of the transcription/NER factor TFIIH [12-15]. An association of XPG with transcription has been suggested by studies of its yeast homolog Rad2 [16], although an equivalent role has not yet been demonstrated for the human protein. A link between TTD and transcription is provided by the observation that cells expressing TTD-causing alleles of XPB, XPD or TTD-A result in the destabilization of TFIIH [17,18]. It has been suggested that genes that are expressed at very high levels critically depend on stable TFIIH in terminally differentiated cells where TFIIH de novo synthesis is reduced [19,20]. The brittle hair in TTD patients for example may thus be explained by the reduced amount of TFIIH in keratinocytes in the hairshaft, leading to reduced expression of the abundant cysteine-rich matrix proteins that crosslink the hair.
Different states of TFIIH result in different phenotypes
While it is has become apparent that an altered state of TFIIH is responsible for the XP/CS and TTD phenotypes, it has not been obvious how mutations in XPG might give rise to XP/CS. A recent study by Tanaka, Egly and coworkers sheds new light on the connection between XPG and TFIIH, demonstrating that mutations in XPG that are associated with an XP/CS phenotype affect the assembly state of TFIIH [21]. Here this finding is placed in context of the recent literature by correlating the XP, XP/CS and TTD phenotypes with distinct (dis)assembly states of TFIIH with specific biochemical and physiological properties.
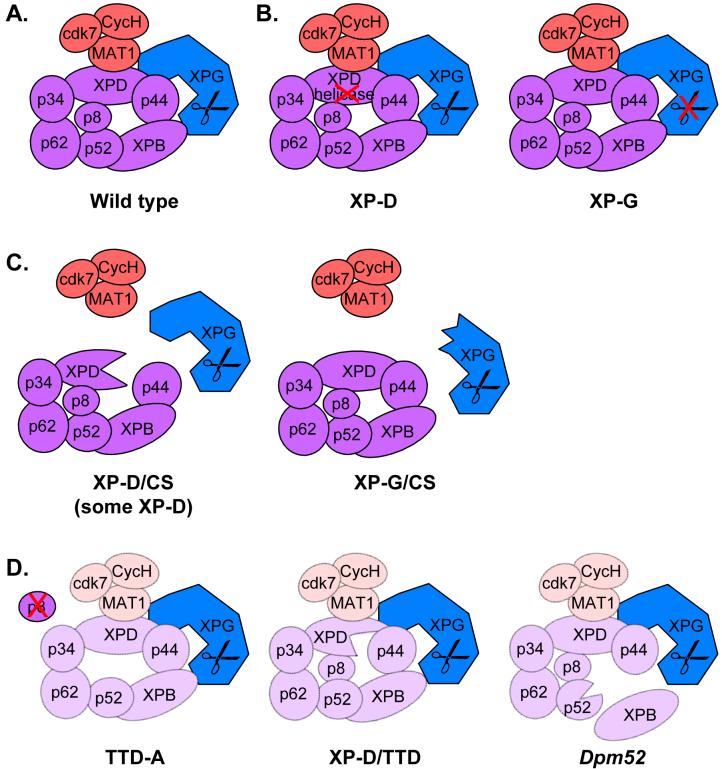
TFIIH contains ten subunits and can be divided up into the core complex (XPB, XPD, p62, p52, p44, p32 and p8/TTDA) and the cdk-activating complex (CAK, consisting of cdk7, MAT1 and cyclin H), which is linked to the core complex via XPD (Figure 1A). The core complex is sufficient to support the NER function of TFIIH [22,23], while the CAK complex is required for the phosphorylation of RNA polymerase II and numerous nuclear receptors (NR) leading to transactivation of NR genes [24,25]. The integrity of the TFIIH core complex therefore appears to be specifically important for NER and basal transcription, while the association of the CAK complex is specifically needed for NR transactivation in addition to basal transcription. Although this classification is a simplified view of the complex functional network orbiting TFIIH, it provides a framework for understanding how distinct molecular defects can be correlated with disease phenotypes. For the purposes of considering disease states in this review, XPG will be considered as a part of TFIIH, although this association is not constitutive in nature for the purposes of NER.
Figure 1. Correlation of assembly and disease states in TFIIH.
A. In wild-type cells TFIIH consists of 10 subunits; XPB, XPD, p62, p52, p44, p34 and p8 form the core (purple), the CAK subunit (orange) consisting of MAT1, cdk7 and cyclinH is linked through XPD. A fraction of XPG (blue) is constitutively associated with TFIIH. B. Mutations in XPD that affect its helicase activity and in XPG that affect its endonuclease activity, but not protein-protein interactions within TFIIH give rise to a XP phenotype. C. Mutations in XPD that disturb the interaction with p44 and in XPG that affect the interaction with the TFIIH core lead to the dissociation of the CAK subunit and combined XP/CS features. Certain XP-causing C-terminal mutations in XP-D also cause dissociation of the CAK subunit, but no CS symptoms. D. Mutations in TTD-A/p8 and XPD lead to an overall destabilization of TFIIH and result in a TTD phenotype. Similar destabilization of TFIIH and TTD-like phenotype is caused by a mutation in the Drosophila homolog of p52, Dpm52.
Specific defects in enzymatic activities of XPG and TFIIH are associated with XP
The basis of the defects in XPG or XPD leading to a classical XP phenotype is the most straightforward to understand at the molecular level. The handful of XPG alleles that are associated with an XP phenotype (some with late onset CS symptoms) display a defect in the NER reaction [26]. The best characterized of these alleles, XP125LO, harbors the mutation A792V. This mutation is located immediately adjacent to the catalytically important E791 and leads to a loss of the endonculease activity that mediates the 3′ incision in NER [27]. This mutation does however not interfere with the association of XPG with TFIIH [21]. A similar trend is observed for XPD alleles that cause exclusively an XP phenotype. Typical XP-causing alleles of XPD are characterized by a mutation in one of the motifs required for helicase activity (such as XP43KO2-D234N) or alternatively weaken the association with p44 (for example XP6BE-R683W). Both properties lead to a defect in helicase and NER activity, but they interfere less with basal transcription activity [25]. Even in the case of XP6BE, the weakened association with p44 does not lead to overall decreased cellular levels of TFIIH or dissociation of the CAK subunit. Typically, mutations in XPG and XPD that are associated with an XP phenotype therefore affect only the NER activity, but do not compromise the association of the CAK subunit with the core TFIIH and retain normal overall cellular levels of TFIIH (Figure 1B). Consequently, basal and activated transcription are not significantly affected. There are important exceptions to this rule however, as C-terminal mutations in XP-D that are associated with an XP phenotype have been found to lead to dissociation of the CAK subunit of TFIIH and to defective nuclear receptor transactivation [24,25].
Mutations in XPG and XPD can cause dissociation of the CAK-complex and CS
Mutations in XPD, in particular those leading to XP/CS, can weaken the interaction between XPD and p44, leading to the dissociation of the CAK subunit from TFIIH resulting in a defect in activated transcription [24,25]. How certain mutations in XPG would fit within that paradigm has not been obvious. Roles for XPG in transcription [16] and the repair of oxidative DNA damage have been suggested to contribute to the CS phenotype, but the importance of the latter connection in particular is still uncertain [26]. The recent study from the Tanaka and Egly laboratories has provided a possible answer to this question and might provide an explanation for the XP/CS phenotype in more general terms [21]. These investigators studied what properties of certain XPG alleles might cause the CS symptoms of the XP-G/CS patients . A native purification scheme of a tagged version of XPG from cell extracts revealed that XPG associates tightly with TFIIH. The high affinity of these two NER components had been noted earlier, but a functional importance of this interaction beyond NER reaction had not yet been established [28,29]. XPG alleles that lack the C-terminus and cause an XP-G/CS phenotype [11] (for example XPCS1RO, which lacks the C-terminal 260 amino acids) failed to stably associate with TFIIH, while those that cause a classical XP phenotype (for example XP125LO see above) still bound TFIIH efficiently. In cells in which XPG failed to associate with TFIIH, the CAK subunit was found to dissociate from the core TFIIH complex, similar to what was observed in cells harboring XP-D/CS alleles (Figure 1C). The defect could be fully restored by expression of wild-type XPG, and conversely, could be induced by knock-down of XPG by siRNA. The authors suggest that certain CS features in XP-G/CS patients, such as loss of subcutaneous fat tissue and hypogonadism are at least partially due to defective transactivation of NRs, similar to what had been observed in XP-D/CS patients [24,30,31]. While this model provides a reasonable explanation based on the characterization of the XP-G and XP-G/CS cells, it leaves some questions unanswered for XP-D. Mutations in the C-terminus of XPD can lead to the dissociation of the CAK subunit and nuclear receptor transactivation, regardless of whether they are associated with XP or XP/CS phenotypes. Clearly, further studies are required to resolve this issues. Furthermore, the CAK subunit remains stably associated with TFIIH and NR transactivation is normal in cells from other CS complementation groups, namely CSA, CSB and XP-B/CS, suggesting that defective NR transactivation does not provide a universal explanation for the CS phenotype. An important question for the future is therefore how various transcriptional deficiencies are responsible for some common features of CS.
TTD cells have reduced levels of TFIIH
As mentioned above a decreased stability of TFIIH has been found in the cell lines of TTD patients [17,18,32] and it has been suggested that the correspondingly lower levels of TFIIH provide a problem for proteins that are expressed at high levels in terminally differentiated cells. For the purposes of discussing assembly states of TFIIH, the p8 subunit of TFIIH, which is mutated in TTD-A cells, provides an instructive example. p8 had remained elusive because of its small size until it was discovered with the help of a proteomic approach and identified as the gene mutated in the TTD-A complementation group [33,34]. The lack of p8 is responsible for the reduced levels of TFIIH in TTD-A cells, and transfection of p8 into TTD-A restored the stability of TFIIH to wild-type levels in those cells [34]. Similarly, TTD-causing mutations in XPD were found to give rise to reduced levels of TFIIH in living cells [25]. The TTD disease state can therefore be described as one of overall decreased levels of TFIIH (Figure 1D). In addition to contributing to overall TFIIH stability, p8 has been found to specifically stimulate NER activity of TFIIH [23,35]. By contrast, p8 does not appear to have a specific role in transcription other than stabilizing TFIIH. Indeed, null mutations of p8, in contrast to XPB and XPD mutations, are compatible with cell viability indicating that p8 is not essential for transcription. The TTD phenotype can therefore be summarized as one with decreased TFIIH activity and a specifically reduced NER activity. The TFIIH present in TTD cells however remains associated with the CAK subunit and is functional in activated and basal transcription.
Could other subunits of TFIIH be associated with XP, CS or TTD?
The observation that many of the p8, XPB and XPD alleles found in XP, CS and TTD patients lead to a destabilization of the TFIIH complex raises the question why no mutations in the p44 or p52 subunits that can also lead to destabilization of TFIIH have been associated with human disease. The p44 gene is duplicated in humans, making it unlikely that mutations would be found in individuals in both alleles of the two genes [36]. Studies in Drosophila melanogaster revealed that mutations in other TFIIH subunits could at least in principle be associated with disease states. Analysis of the marionette gene, which encodes the Drosophila p52 subunit of TFIIH, provided evidence that deletion of p52 can cause TTD-like phenotypes [37]. A null mutant of Dmp52 was shown to be lethal in flies, while hypomorphic mutations were associated with TTD-like symptoms including cuticle defects, brittle bristles and sterility, similar to what had previously been observed in hay (DmXPB) mutants. Similarly to cells from human TTD patients, cells from D. melanogaster marionette mutants had reduced levels of cellular TFIIH (Figure 1D). Introduction of mutations analogous to ones found in marionette into the human p52 furthermore established an importance of p52 in anchoring XPB in TFIIH and found a stimulatory role of the XPB ATPase activity by p52 [37,38]. An analogous role had previously been established for p44 interacting with XPD [25,36]. It appears therefore that based on their molecular and cellular properties, mutations in the human p44 and p52 genes could in principle also yield TTD and CS-like phenotypes.
Unresolved questions
The observations discussed above provide a framework for understanding how mutations in XPB, XPD, TTD-A/p8 and XPG can give rise to different disease states. The model depicted in Figure 1 is however an oversimplification of the complex genotype/phenotype relationships. For example, certain mutation in the C-terminus of XP-D that are associated with an XP or TTD phenotype also result in a weakened association of the CAK subunit with TFIIH and defective nuclear receptor transactivation [25]. Why do patients with these alleles not display any CS symptoms? Is the association of the CAK subunit with TFIIH in their cells only partially defective or is there another fundamental cellular defect in CS cells that we do not understand yet? Conversely, the CAK subunit remains associated with TFIIH in XP-B/CS, CS-A and CS-B cells [21]. While certain deficiencies in transcription in particular after DNA damage in CS-A and CS-B cells have been described, they are clearly different in nature from the defects in activated transcription observed in XP-D/CS and XP-G/CS cells. There are even examples in the literature where an identical mutation in an XPB disease-causing allele (containing the mutation F99S) gives rise to XP in one and XP/CS in another patient [39]. Whether these differences are due to a cryptic activity of the second presumed inactive allele (that is different in the two patients) or can be explained by unidentified difference in the genetic background remains to be determined. A full understanding of the complex genotype/phenotype relationships clearly requires further studies.
Unrelated observation by Lehmann and coworkers of uncontrolled DNA breakage in XP-D/CS (but not XP-D) cell lines in response to UV damage provided another potential distinguish feature of the XP/CS phenotype [40,41]. In response to DNA damage, even if introduced on a plasmid rather than global treatment of cells, breaks were introduced in the cellular DNA in XP-D/CS cells in a transcription- and NER-dependent manner, but these breaks were not localized at sites of DNA damage. It has been proposed that they might occur at sites of transcription initiation. Although the significance of these breaks and the mechanisms by which they are introduced remains to be established, they are a distinctive feature of XP-D/CS cells and might contribute to the unique XP/CS phenotype.
The observation of the different phenotypic manifestations should also provide an impetus for the further study of the activities of TFIIH and XPG in NER and transcription. The interaction between TFIIH and XPG has been well documented and involves several subnits of TFIIH and at least two regions in XPG [42-46]. Available evidence does not implicate a constitutive interaction between TFIIH and XPG in NER [47] and the majority of the XPG in the nucleus is apparently not associated with a large molecular weight complex [48]. It remains to be established why, in the light of the data of Ito et al. [21], not even a subpopulation of XPG appears to be stably associated with TFIIH in live cell imaging studies. In the context of NER, TFIIH recruits XPG to sites of DNA damage in an ATP-dependent fashion, where XPG is then involved in preincision complex formation and carries out the incision 3′ to the lesion. By contrast the work of Ito et al. suggest that for its role in activated transcription, XPG needs to be associated with TFIIH in a constitutive manner to ensure association of the CAK subunit. Whether XPG has a structural or enzymatic role in this context in addition to stabilizing the TFIIH complex and how the formation of two apparently distinct TFIIH complexes is regulated remains to be established.
Acknowledgements
Research on NER in the author’s laboratory is supported by the New York State Office of Science and Technology and Academic Research (NYSTAR) Grant No. C040069, the Human Frontier Science Organization Grant RGP7/2004 and the Structural Cell Biology of DNA Repair Program Grant 2 P01 CA92584-06.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. ASM Press; Washington DC: 2005. [Google Scholar]
- [2].Gillet LC, Schärer OD. Molecular mechanisms of Mammalian global genome nucleotide excision repair. Chem. Rev. 2006;106:253–276. doi: 10.1021/cr040483f. [DOI] [PubMed] [Google Scholar]
- [3].Svejstrup JQ. Mechanisms of transcription-coupled DNA repair. Nat. Rev. Mol. Cell. Biol. 2002;3:21–29. doi: 10.1038/nrm703. [DOI] [PubMed] [Google Scholar]
- [4].Lehmann AR. DNA repair-deficient diseases, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie. 2003;85:1101–1111. doi: 10.1016/j.biochi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- [5].van Gool AJ, Citterio E, Rademakers S, van Os R, Vermeulen W, Constantinou A, Egly JM, Bootsma D, Hoeijmakers JH. The Cockayne syndrome B protein, involved in transcription-coupled DNA repair, resides in an RNA polymerase II-containing complex. Embo J. 1997;16:5955–5965. doi: 10.1093/emboj/16.19.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tantin D, Kansal A, Carey M. Recruitment of the putative transcription-repair coupling factor CSB/ERCC6 to RNA polymerase II elongation complexes. Mol. Cell. Biol. 1997;17:6803–6814. doi: 10.1128/mcb.17.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Balajee AS, May A, Dianov GL, Friedberg EC, Bohr VA. Reduced RNA polymerase II transcription in intact and permeabilized Cockayne syndrome group B cells. Proc. Natl. Acad. Sci. U S A. 1997;94:4306–4311. doi: 10.1073/pnas.94.9.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rockx DA, Mason R, van Hoffen A, Barton MC, Citterio E, Bregman DB, van Zeeland AA, Vrieling H, Mullenders LH. UV-induced inhibition of transcription involves repression of transcription initiation and phosphorylation of RNA polymerase II. Proc. Natl. Acad. Sci. U S A. 2000;97:10503–10508. doi: 10.1073/pnas.180169797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Horibata K, Iwamoto Y, Kuraoka I, Jaspers NG, Kurimasa A, Oshimura M, Ichihashi M, Tanaka K. Complete absence of Cockayne syndrome group B gene product gives rise to UV-sensitive syndrome but not Cockayne syndrome. Proc. Natl. Acad. Sci. U S A. 2004;101:15410–15415. doi: 10.1073/pnas.0404587101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Taylor EM, Broughton BC, Botta E, Stefanini M, Sarasin A, Jaspers NG, Fawcett H, Harcourt SA, Arlett CF, Lehmann AR. Xeroderma pigmentosum and trichothiodystrophy are associated with different mutations in the XPD (ERCC2) repair/transcription gene. Proc. Natl. Acad. Sci. U S A. 1997;94:8658–8663. doi: 10.1073/pnas.94.16.8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nouspikel T, Lalle P, Leadon SA, Cooper PK, Clarkson SG. A common mutational pattern in Cockayne syndrome patients from xeroderma pigmentosum group G: implications for a second XPG function. Proc. Natl. Acad. Sci. U S A. 1997;94:3116–3121. doi: 10.1073/pnas.94.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [12].Schaeffer L, Roy R, Humbert S, Moncollin V, Vermeulen W, Hoeijmakers JH, Chambon P, Egly JM. DNA repair helicase: a component of BTF2 (TFIIH) basic transcription factor. Science. 1993;260:58–63. doi: 10.1126/science.8465201. [DOI] [PubMed] [Google Scholar]
- [13].Drapkin R, Reardon JT, Ansari A, Huang JC, Zawel L, Ahn K, Sancar A, Reinberg D. Dual role of TFIIH in DNA excision repair and in transcription by RNA polymerase II. Nature. 1994;368:769–72. doi: 10.1038/368769a0. [DOI] [PubMed] [Google Scholar]
- [14].Guzder SN, Sung P, Bailly V, Prakash L, Prakash S. RAD25 is a DNA helicase required for DNA repair and RNA polymerase II transcription. Nature. 1994;369:578–581. doi: 10.1038/369578a0. [DOI] [PubMed] [Google Scholar]
- [15].Schaeffer L, Moncollin V, Roy R, Staub A, Mezzina M, Sarasin A, Weeda G, Hoeijmakers JH, Egly JM. The ERCC2/DNA repair protein is associated with the class II BTF2/TFIIH transcription factor. Embo J. 1994;13:2388–2392. doi: 10.1002/j.1460-2075.1994.tb06522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee SK, Yu SL, Prakash L, Prakash S. Requirement of yeast RAD2, a homolog of human XPG gene, for efficient RNA polymerase II transcription. implications for Cockayne syndrome. Cell. 2002;109:823–834. doi: 10.1016/s0092-8674(02)00795-x. [DOI] [PubMed] [Google Scholar]
- [17].Vermeulen W, Bergmann E, Auriol J, Rademakers S, Frit P, Appeldoorn E, Hoeijmakers JH, Egly JM. Sublimiting concentration of TFIIH transcription/DNA repair factor causes TTD-A trichothiodystrophy disorder. Nat. Genet. 2000;26:307–313. doi: 10.1038/81603. [DOI] [PubMed] [Google Scholar]
- [18].Botta E, Nardo T, Lehmann AR, Egly JM, Pedrini AM, Stefanini M. Reduced level of the repair/transcription factor TFIIH in trichothiodystrophy. Hum. Mol. Genet. 2002;11:2919–2928. doi: 10.1093/hmg/11.23.2919. [DOI] [PubMed] [Google Scholar]
- [19].de Boer J, de Wit J, van Steeg H, Berg RJ, Morreau H, Visser P, Lehmann AR, Duran M, Hoeijmakers JH, Weeda G. A mouse model for the basal transcription/DNA repair syndrome trichothiodystrophy. Mol. Cell. 1998;1:981–990. doi: 10.1016/s1097-2765(00)80098-2. [DOI] [PubMed] [Google Scholar]
- [20].Viprakasit V, Gibbons RJ, Broughton BC, Tolmie JL, Brown D, Lunt P, Winter RM, Marinoni S, Stefanini M, Brueton L, Lehmann AR, Higgs DR. Mutations in the general transcription factor TFIIH result in beta-thalassaemia in individuals with trichothiodystrophy. Hum. Mol. Genet. 2001;10:2797–2802. doi: 10.1093/hmg/10.24.2797. [DOI] [PubMed] [Google Scholar]
- [21].Ito S, Kuraoka I, Chymkowitch P, Compe E, Takedachi A, Ishigami C, Coin F, Egly JM, Tanaka K. XPG Stabilizes TFIIH, Allowing Transactivation of Nuclear Receptors: Implications for Cockayne Syndrome in XP-G/CS Patients. Mol. Cell. 2007;26:231–243. doi: 10.1016/j.molcel.2007.03.013. [DOI] [PubMed] [Google Scholar]
- [22].Araujo SJ, Tirode F, Coin F, Pospiech H, Syvaoja JE, Stucki M, Hubscher U, Egly JM, Wood RD. Nucleotide excision repair of DNA with recombinant human proteins: definition of the minimal set of factors, active forms of TFIIH, and modulation by CAK. Genes Dev. 2000;14:349–359. [PMC free article] [PubMed] [Google Scholar]
- [23].Coin F, Proietti De Santis L, Nardo T, Zlobinskaya O, Stefanini M, Egly JM. p8/TTD-A as a repair-specific TFIIH subunit. Mol. Cell. 2006;21:215–226. doi: 10.1016/j.molcel.2005.10.024. [DOI] [PubMed] [Google Scholar]
- [24].Keriel A, Stary A, Sarasin A, Rochette-Egly C, Egly JM. XPD mutations prevent TFIIH-dependent transactivation by nuclear receptors and phosphorylation of RARalpha. Cell. 2002;109:125–135. doi: 10.1016/s0092-8674(02)00692-x. [DOI] [PubMed] [Google Scholar]
- [25].Dubaele S, Proietti De Santis L, Bienstock RJ, Keriel A, Stefanini M, Van Houten B, Egly JM. Basal transcription defect discriminates between xeroderma pigmentosum and trichothiodystrophy in XPD patients. Mol. Cell. 2003;11:1635–1646. doi: 10.1016/s1097-2765(03)00182-5. [DOI] [PubMed] [Google Scholar]
- [26].Clarkson SG. The XPG story. Biochimie. 2003;85:1113–1121. doi: 10.1016/j.biochi.2003.10.014. [DOI] [PubMed] [Google Scholar]
- [27].Constantinou A, Gunz D, Evans E, Lalle P, Bates PA, Wood RD, Clarkson SG. Conserved residues of human XPG protein important for nuclease activity and function in nucleotide excision repair. J. Biol. Chem. 1999;274:5637–5648. doi: 10.1074/jbc.274.9.5637. [DOI] [PubMed] [Google Scholar]
- [28].Mu D, Park CH, Matsunaga T, Hsu DS, Reardon JT, Sancar A. Reconstitution of human DNA repair excision nuclease in a highly defined system. J. Biol. Chem. 1995;270:2415–2418. doi: 10.1074/jbc.270.6.2415. [DOI] [PubMed] [Google Scholar]
- [29].Araujo SJ, Nigg EA, Wood RD. Strong functional interactions of TFIIH with XPC and XPG in human DNA nucleotide excision repair, without a preassembled repairosome. Mol. Cell. Biol. 2001;21:2281–2291. doi: 10.1128/MCB.21.7.2281-2291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Compe E, Drane P, Laurent C, Diderich K, Braun C, Hoeijmakers JH, Egly JM. Dysregulation of the peroxisome proliferator-activated receptor target genes by XPD mutations. Mol. Cell. Biol. 2005;25:6065–6076. doi: 10.1128/MCB.25.14.6065-6076.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Drane P, Compe E, Catez P, Chymkowitch P, Egly JM. Selective regulation of vitamin D receptor-responsive genes by TFIIH. Mol. Cell. 2004;16:187–197. doi: 10.1016/j.molcel.2004.10.007. [DOI] [PubMed] [Google Scholar]
- [32].Vermeulen W, Rademakers S, Jaspers NG, Appeldoorn E, Raams A, Klein B, Kleijer WJ, Hansen LK, Hoeijmakers JH. A temperature-sensitive disorder in basal transcription and DNA repair in humans. Nat. Genet. 2001;27:299–303. doi: 10.1038/85864. [DOI] [PubMed] [Google Scholar]
- [33].Ranish JA, Hahn S, Lu Y, Yi EC, Li XJ, Eng J, Aebersold R. Identification of TFB5, a new component of general transcription and DNA repair factor IIH. Nat. Genet. 2004;36:707–713. doi: 10.1038/ng1385. [DOI] [PubMed] [Google Scholar]
- [34].Giglia-Mari G, Coin F, Ranish JA, Hoogstraten D, Theil A, Wijgers N, Jaspers NG, Raams A, Argentini M, van der Spek PJ, Botta E, Stefanini M, Egly JM, Aebersold R, Hoeijmakers JH, Vermeulen W. A new, tenth subunit of TFIIH is responsible for the DNA repair syndrome trichothiodystrophy group A. Nat. Genet. 2004;36:714–719. doi: 10.1038/ng1387. [DOI] [PubMed] [Google Scholar]
- [35].Giglia-Mari G, Miquel C, Theil AF, Mari PO, Hoogstraten D, Ng JM, Dinant C, Hoeijmakers JH, Vermeulen W. Dynamic interaction of TTDA with TFIIH is stabilized by nucleotide excision repair in living cells. PLoS Biol. 2006;4:e156. doi: 10.1371/journal.pbio.0040156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Coin F, Marinoni JC, Rodolfo C, Fribourg S, Pedrini AM, Egly JM. Mutations in the XPD helicase gene result in XP and TTD phenotypes, preventing interaction between XPD and the p44 subunit of TFIIH. Nat. Genet. 1998;20:184–188. doi: 10.1038/2491. [DOI] [PubMed] [Google Scholar]
- [37].Fregoso M, Laine JP, Aguilar-Fuentes J, Mocquet V, Reynaud E, Coin F, Egly JM, Zurita M. DNA repair and transcriptional deficiencies caused by mutations in the Drosophila p52 subunit of TFIIH generate developmental defects and chromosome fragility. Mol. Cell. Biol. 2007;27:3640–3650. doi: 10.1128/MCB.00030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Coin F, Oksenych V, Egly JM. Distinct Roles for the XPB/p52 and XPD/p44 Subcomplexes of TFIIH in Damaged DNA Opening during Nucleotide Excision Repair. Mol. Cell. 2007;26:245–256. doi: 10.1016/j.molcel.2007.03.009. [DOI] [PubMed] [Google Scholar]
- [39].Oh KS, Khan SG, Jaspers NG, Raams A, Ueda T, Lehmann A, Friedmann PS, Emmert S, Gratchev A, Lachlan K, Lucassan A, Baker CC, Kraemer KH. Phenotypic heterogeneity in the XPB DNA helicase gene (ERCC3): xeroderma pigmentosum without and with Cockayne syndrome. Hum. Mutat. 2006;27:1092–1103. doi: 10.1002/humu.20392. [DOI] [PubMed] [Google Scholar]
- [40].Berneburg M, Lowe JE, Nardo T, Araujo S, Fousteri MI, Green MH, Krutmann J, Wood RD, Stefanini M, Lehmann AR. UV damage causes uncontrolled DNA breakage in cells from patients with combined features of XP-D and Cockayne syndrome. Embo J. 2000;19:1157–1166. doi: 10.1093/emboj/19.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Theron T, Fousteri MI, Volker M, Harries LW, Botta E, Stefanini M, Fujimoto M, Andressoo JO, Mitchell J, Jaspers NG, McDaniel LD, Mullenders LH, Lehmann AR. Transcription-associated breaks in xeroderma pigmentosum group D cells from patients with combined features of xeroderma pigmentosum and Cockayne syndrome. Mol. Cell. Biol. 2005;25:8368–8378. doi: 10.1128/MCB.25.18.8368-8378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Iyer N, Reagan MS, Wu KJ, Canagarajah B, Friedberg EC. Interactions involving the human RNA polymerase II transcription/nucleotide excision repair complex TFIIH, the nucleotide excision repair protein XPG, and Cockayne syndrome group B (CSB) protein. Biochemistry. 1996;35:2157–2167. doi: 10.1021/bi9524124. [DOI] [PubMed] [Google Scholar]
- [43].Gervais V, Lamour V, Jawhari A, Frindel F, Wasielewski E, Dubaele S, Egly JM, Thierry JC, Kieffer B, Poterszman A. TFIIH contains a PH domain involved in DNA nucleotide excision repair. Nat. Struct. Mol. Biol. 2004;11:616–622. doi: 10.1038/nsmb782. [DOI] [PubMed] [Google Scholar]
- [44].Thorel F, Constantinou A, Dunand-Sauthier I, Nouspikel T, Lalle P, Raams A, Jaspers NG, Vermeulen W, Shivji MK, Wood RD, Clarkson SG. Definition of a Short Region of XPG Necessary for TFIIH Interaction and Stable Recruitment to Sites of UV Damage. Mol. Cell. Biol. 2004;24:10670–10680. doi: 10.1128/MCB.24.24.10670-10680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dunand-Sauthier I, Hohl M, Thorel F, Jaquier-Gubler P, Clarkson SG, Schärer OD. The spacer region of XPG mediates recruitment to nucleotide excision repair complexes and determines substrate specificity. J. Biol. Chem. 2005;280:7030–7037. doi: 10.1074/jbc.M412228200. [DOI] [PubMed] [Google Scholar]
- [46].Hohl M, Dunand-Sauthier I, Staresincic L, Jaquier-Gubler P, Thorel F, Modesti M, Clarkson SG, Schärer OD. Domain swapping between FEN-1 and XPG defines regions in XPG that mediate nucleotide excision repair activity and substrate specificity. Nucleic Acids Res. 2007;35:3053–3063. doi: 10.1093/nar/gkm092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Riedl T, Hanaoka F, Egly JM. The comings and goings of nucleotide excision repair factors on damaged DNA. Embo J. 2003;22:5293–5303. doi: 10.1093/emboj/cdg489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zotter A, Luijsterburg MS, Warmerdam DO, Ibrahim S, Nigg A, van Cappellen WA, Hoeijmakers JH, van Driel R, Vermeulen W, Houtsmuller AB. Recruitment of the nucleotide excision repair endonuclease XPG to sites of UV-induced DNA damage depends on functional TFIIH. Mol. Cell. Biol. 2006;26:8868–8879. doi: 10.1128/MCB.00695-06. [DOI] [PMC free article] [PubMed] [Google Scholar]