Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) is linked to the development of Kaposi's sarcoma (KS), a vascular spindle cell tumor primarily consisting of proliferating endothelial cells. Although KSHV has been shown to infect primary human endothelial cells and convert them into spindle shapes, KSHV infection is largely latent, and efforts to establish a highly efficient and sustainable infection system have been unsuccessful. A recombinant KSHV, BAC36, that has high primary-infection efficiency in 293 cells has been obtained (F. C. Zhou, Y. J. Zhang, J. H. Deng, X. P. Wang, H. Y. Pan, E. Hettler, and S. J. Gao, J. Virol. 76:6185-6196, 2002). BAC36 contains a green fluorescent protein cassette which can be used to conveniently monitor viral infection. Here, we describe the establishment of a KSHV lytic-replication-permissive infection cell model using BAC36 virions to infect primary human umbilical vein endothelial cell (HUVEC) cultures. BAC36 infection of HUVEC cultures has as high as 90% primary-infection efficiency and consists of two phases: a permissive phase, in which the cultures undergo active viral lytic replication, producing a large number of virions and concomitantly resulting in large-scale cell death, and a latent phase, in which the surviving cells from the permissive phase switch into latent infection, with a small number of cells undergoing spontaneous viral lytic replication, and proliferate into bundles of spindle cells with KS slit-like spaces. An assay for determining the KSHV titer in a virus preparation has also been developed. The cell model should be useful for examining KSHV infection and replication, as well as for understanding the development of KS.
Kaposi's sarcoma (KS) is a vascular tumor, primarily consisting of proliferating spindle-shaped endothelial cells and infiltrates of inflammatory cells (53). A human gammaherpesvirus, KS-associated herpesvirus (KSHV), initially identified in a KS lesion from an AIDS patient (16), has been convincingly linked to the development of all four clinical forms of KS, including classical KS, AIDS-related KS, African endemic KS, and immunosuppressed posttransplant KS (6). KSHV genomes and encoded gene products are detected in the majority of spindle cells and sporadically in monocytes, macrophages, keratinocytes, and lymphocytes in KS tumors, but not in adjacent tissues or control tissues from patients without KS (5, 19, 22, 26, 34, 49, 60). KSHV has also been associated with other lymphoproliferative diseases, including primary effusion lymphoma (PEL) and multicentric Castleman's disease (12).
Like those of other herpesviruses, the life cycle of KSHV consists of latent and lytic phases (43). Following primary infection, KSHV establishes latent infection in the host. During latent replication, KSHV expresses only a restricted number of genes, including latent nuclear antigen (LNA or LANA), v-cyclin, v-FLIP, and kaposin. The latent virus can be reactivated into lytic replication by host or environmental factors, such as immunosuppression associated with iatrogenic organ transplantation, during which lytic-cycle genes are expressed, usually in a coordinated fashion. These include genes encoding structural proteins, such as viral capsid and tegument proteins; proteins that are essential for viral replication; and regulatory proteins. In KS lesions, the majority of spindle cells are latently infected by KSHV and expressed viral latent genes, suggesting an essential role of latent infection for the maintenance of persistent viral infection and the development of KS; however, a small number of spindle cells in KS lesions also undergo spontaneous lytic replication, expressing viral lytic proteins (4, 19, 22, 34, 47, 60, 61). Many of these lytic proteins have regulatory functions which can modulate different cellular pathways and therefore have been proposed to have important roles in the initiation, promotion, and sustaining of KS tumors through an autocrine and paracrine mechanism (13, 15, 40, 42).
Characterizations of KSHV have relied heavily on the growth of the virus in cell culture systems (43). KSHV has been cultured through the isolation of cell lines from PEL, in which the virus is primarily maintained in latent replication but can be reactivated into lytic replication through induction with various chemicals, such as phorbol ester, 12-O-tetradecanoyl phorbol-13-acetate (TPA) (2, 8-10, 14, 21, 29, 30, 32, 37, 51, 62). The ability to grow KSHV in PEL cell lines has been instrumental in the development of specific serologic assays (28, 29, 33, 59) and molecular characterizations of the virus (43). Nevertheless, further understanding of the delicate viral and cellular interplay, particularly during primary viral infection, requires the establishment of a reliable primary infection system. Virus preparations from PEL cell lines and occasionally from KS tumors are infectious to several cell types, including monocytes, fibroblasts, lymphocytes, and keratinocytes (11, 27, 35, 41, 44, 48, 50, 63). Nevertheless, primary-infection efficiencies in these cell types are usually low, and the cultures cannot sustain long-term virus growth. Several reports have documented KSHV infection of human primary endothelial cells (18, 25, 38, 45). In the initial report, KSHV was shown to infect only a small number of cells in primary human bone marrow microvascular endothelial cell cultures and primary human umbilical vein endothelial cell (HUVEC) cultures, but the cells in infected cultures acquired a spindle shape and were maintained for >12 months while the control cultures underwent senescence within 3 weeks of culture (25). It was proposed that the small number of infected cells provided a paracrine effect to sustain the cultures. Subsequent study showed that KSHV could infect primary human dermal microvascular endothelial cell (DMVEC) cultures and form colonies or plaques of spindle-shaped cells (18). Again, the primary-infection efficiency in this system was low, even though the virus eventually infected the entire cultures after 2 to 3 weeks. Paradoxically, to sustain long-term KSHV infection, it was necessary to periodically replenish the cultures with uninfected endothelial cells at a ratio of 10 portions of normal cells to 1 portion of infected cells. To facilitate the manipulation of primary endothelial cells, HPV E6- and E7-immortalized DMVEC cultures were used as targets for KSHV infection (45). In this system, the primary-infection efficiency remained low even though the virus also eventually spread to the entire cultures, which could now be stably maintained. More recently, KSHV infection of telomerase-immortalized microvascular endothelial (TIME) cells was shown to be extremely efficient, reaching the entire cultures within 2 to 3 days of infection; however, the infected cells were unable to sustain persistent KSHV infection, and the cultures quickly lost the virus after several passages (38). The limitations, such as low primary-infection efficiency and/or failure of long-term sustainability for virus growth, of the above-mentioned systems have restricted their use for KSHV characterization, especially virus-cell interactions at the initial stage of infection. Furthermore, even in systems that can sustain persistent KSHV infection, the cultures are predominantly in the viral latent phase, with a small number of cells (1 to 10%) undergoing lytic replication (18, 45). No active viral lytic replication has been observed in any stages of infection in these systems. Consequently, the lack of a productive permissive infection system has hampered the understanding of KSHV infection and replication. Recently, a recombinant KSHV, BAC36, has been obtained by cloning the full-length viral genome into a bacterial artificial chromosome (BAC) and recovering it in 293 cells (65). Recombinant virions generated from BAC36 are highly infectious to 293 cells, with primary-infection efficiency close to 50%, which can be further enhanced using concentrated virus preparations. Although BAC36 can be stably maintained in 293 cells for several months, KSHV replication in 293 cells resembles that in other infection systems, in which it quickly establishes latent infection following primary infection without active productive lytic replication (65). Here, we describe the establishment of a cell model using BAC36 to infect primary HUVEC cultures. We have observed ∼90% primary-infection efficiency using concentrated virus preparations. We have found that, unlike other infection systems, efficient infection of HUVEC cultures by BAC36 is for permissive lytic replication at the early stage of infection, producing large amounts of infectious virions. Infected cultures form bundles of spindle-shaped cells, which are reminiscent of KS vascular structures, and establish latency at a late stage of infection.
MATERIALS AND METHODS
Cell culture and virus preparation.
BCBL-1 and 293 cells harboring a recombinant KSHV, BAC36, have been described previously (65). Primary HUVECs were obtained from Clonetics (Walkersville, Md.). BCBL-1 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (Sigma, St. Louis, Mo.), 50 μg of gentamicin/ml, and 2 mM glutamine. 293 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 50 μg of gentamicin/ml, and 2 mM glutamine. HUVECs were cultured in endothelial cell growth medium (BulletKit; Clonetics) containing human endothelial growth factor, human fibroblast growth factor B, vascular endothelial growth factor, ascorbic acid, hydrocortisone, long R3-IGF-1, and heparin as instructed by the manufacturer. To induce KSHV lytic replication, the cultured cells were treated with 25 ng of TPA (Sigma)/ml for 2 to 6 days as previously described (65). To concentrate the virus, supernatant from TPA-induced culture was centrifuged two times at 5,000 × g for 10 min each time to eliminate cell debris, filtered through a 0.45-μm-pore-size filter, and centrifuged again at 100,000 × g for 1 h, using 20% sucrose as cushion. The final pellet was dissolved in culture medium overnight and adjusted to a desired volume. Undissolved debris was eliminated by centrifugation at 5,000 × g for 10 min. All the procedures for virus concentration were handled at 4°C. Virus preparations used for this study were concentrated 10-fold and had titers of 8 × 105 to 10 × 105 green fluorescent protein (GFP)-expressing cells/ml (see below for virus titration). The concentrated virus preparation was aliquoted and stored at −80°C before use.
Virus infection.
Primary HUVECs or 293 cells were seeded in either flasks (75 or 25 cm2) or plates (6, 12, 24, or 96 well) 1 day before infection and infected with virus preparations containing BAC36 virions at 70 to 80% confluency as described previously (65). Flasks (75 and 25 cm2) containing ∼3 × 106 and 1 × 106 cells/flask were infected with 3 and 1 ml of virus preparation/flask, while 6-, 12-, 24-, and 96-well plates containing ∼4 × 105, 1.6 × 105, 8 × 104, and 2 × 104 cells/well were infected with 400, 150, 80, and 20 μl of virus preparation/well, respectively. Since BAC36 contains a GFP cassette, GFP expression was used to monitor infection. For long-term cultures, the medium was changed every 2 days. For split cultures, the cells were trypsinized and passaged at a ratio of 1:3 every 3 to 4 days.
Virus titration.
Tested samples were subjected to twofold serial dilution. The diluted virus inocula (three to six repeats for each supernatant) were used to infect 293 cells at 4 × 104/well in a 96-well plate at 20 μl/well as previously described (65). The plates were examined with an inverted fluorescence microscope 30 h postinfection for the numbers of cells expressing GFP in each well. Wells inoculated with the last samples that had GFP-positive cells were used to calculate the virus titer of the undiluted original sample.
Analysis of KSHV gene expression.
KSHV protein expression was detected by immunofluorescence assay (IFA) (29). Cells were seeded in and allowed to adhere to four-chamber slides. The slides were fixed and permeabilized in 2% paraformaldehyde with 0.25% Triton X-100 in PBS for 5 min and then in 2% paraformaldehyde in PBS for 5 min. LNA encoded by ORF73 was detected with a rat anti-LNA monoclonal antibody (ABI, Columbia, Md.) and revealed with a rabbit anti-rat immunoglobulin tetramethyl rhodamine isothiocyanate conjugate (Sigma). Minor capsid protein (mCP) encoded by ORF65 was detected with a mouse monoclonal antibody and revealed with a rabbit anti-mouse immunoglobulin G tetramethyl rhodamine isothiocyanate conjugate (DAKO, Carpinteria, Calif.).
RESULTS
BAC36 infects primary HUVEC cultures at high primary-infection efficiency.
The full-length KSHV genome was previously cloned into a BAC (65). Virus generated from the recombinant KSHV BAC36 can infect 293 cells at high efficiency. BAC36 has a GFP cassette, inserted at the PmeI site of the noncoding region between ORF18 and ORF19 of the viral genome, that can be conveniently used to monitor infection of cells by the virus. Since the spindle cells in KS tumors have been identified as endothelial cells (23, 64), we wanted to determine whether BAC36 virus could efficiently infect primary human endothelial cells. BAC36 virus concentrated 10-fold by differential centrifugation was used to infect low-passage (<10) primary HUVECs. As shown in Fig. 1A, GFP expression was visible in some cells by 16 h postinfection. By day 2 postinfection, >90% of the cells expressed GFP, indicating infection of the cells by the virus. The percentage of cells expressing GFP continued to increase after day 2 postinfection and eventually reached the entire cultures in most cases. However, in some infected cultures, we observed a small number of GFP-negative cells (<10%) for the entire culture period, suggesting that they either were never infected by the virus or, for unknown reasons, had lost GFP expression despite viral infection.
FIG. 1.
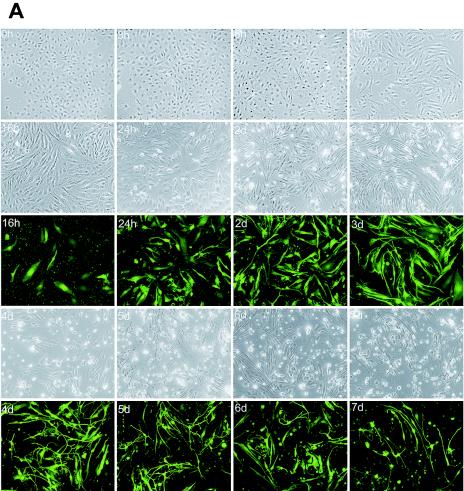
Infection, spindle conversion, and formation of bundle structures in primary HUVEC cultures infected by recombinant KSHV BAC36. (A) Primary HUVEC cultures were infected with BAC36 virus and observed at different time points. On day 2 postinfection, close to 90% of the cells expressed GFP, an indication of BAC36 infection. Spindle conversion was observed as early as 6 h postinfection and maintained throughout the infection period. A large amount of cell death occurred 1 week after infection, but a small number of cells survived and continued to proliferate, forming large bundle structures with KS slit-like spaces. (B) BAC36-infected cultures passaged by cell splitting at an early stage of infection were spared cell death crisis, quickly forming large bundle structures of spindle cells. (C) Mock-infected control primary HUVEC cultures maintained cobblestone shapes throughout the culture period. d, days.
BAC36 infection of HUVEC cultures induces spindle conversion, morphology of neuronal cells, and bundles of spindle cells resembling KS vascular structures.
Other investigators have reported spindle conversion of endothelial cells by KSHV infection (18, 25, 38, 45). BAC36 infection of HUVECs also resulted in drastic morphological changes. Spindle conversion was observed as early as 3 h postinfection and became obvious after 6 h postinfection (Fig. 1A, 3h and 6h). The alterations in cell morphology progressed rapidly. By 10 h, the majority of the infected cells had fully acquired spindle shapes (Fig. 1A, 10h). The significant increase in cell length during spindle conversion was not at the expense of cell width; on the contrary, most cells had increases in width in addition to length. Thus, spindle conversion simultaneously resulted in an increase in overall cell size. By 16 h postinfection, the widths and lengths of the infected cells were about one to two and four to six times those of the cobblestone-shaped control cells (Fig. 1A, 16h). In addition, some infected cells aligned into small groups (three to six cells), or bundles, that often overlapped with each other during the first 2 to 3 days of infection (Fig. 1A, 2d and 3d). In contrast to the infected cultures, no obvious change in cell morphology and/or growth was observed for control cells, as expected, since the cultures were close to confluency at the time of mock infection (Fig. 1C).
FIG. 3.
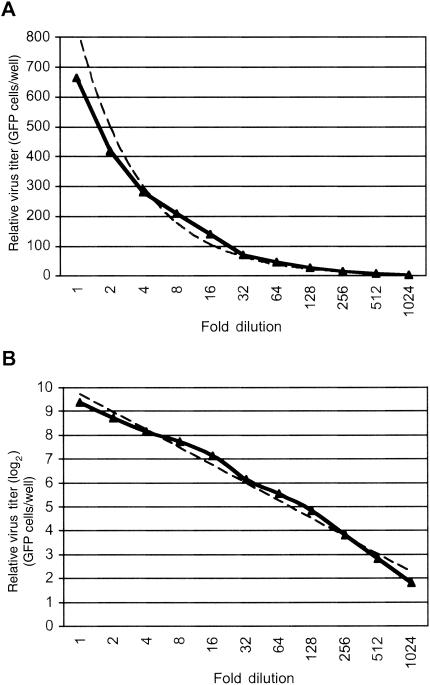
Determination of virus titer. (A) Representative experiment to determine the titer of a virus preparation by measuring the number of cells expressing GFP. The virus sample was subjected to twofold serial dilution and examined in 293 cells in a 96-well plate; the number of cells expressing GFP/well was determined 30 h postinfection. The experiment was repeated three times; however, only the average number of GFP-expressing cells/well was plotted because the standard deviations were too small (range, 0 to 3.5 GFP-expressing cells/well) to be reflected on the scale. (B) The number of GFP-expressing cells/well shown in panel A was converted to a log2 scale. The solid lines represent the values from the experiments, while the dashed lines represent the trend lines of the data.
FIG. 2.
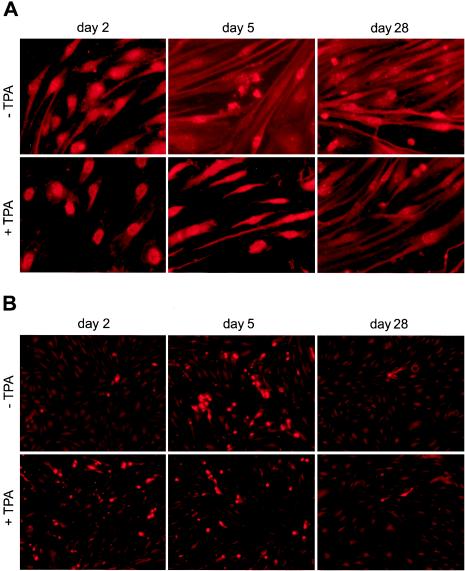
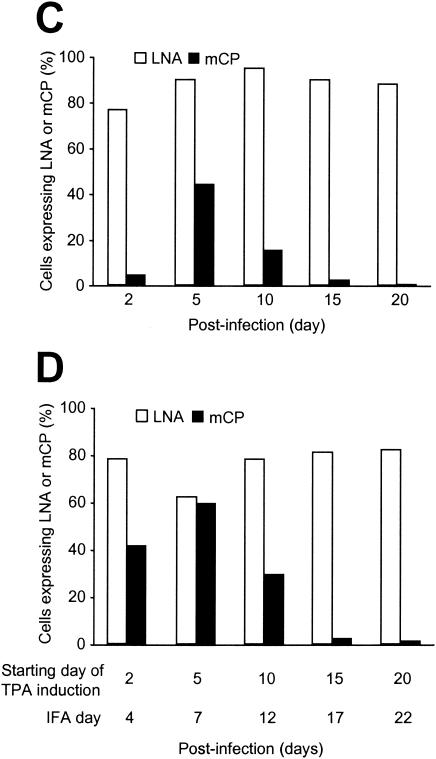
Expression of KSHV latent and lytic proteins in BAC36-infected HUVEC cultures at different time points postinfection. (A) Expression of KSHV latent protein LNA in uninduced cells and cells induced with TPA for 2 days. Over 90% of the cells in uninduced cultures and over 80% of the cells in TPA-induced cultures were positive for LNA. (B) Expression of KSHV lytic protein mCP in uninduced cells (−TPA) and cells induced with TPA for 2 days (+TPA). Less than 5% of the cells were positive for mCP on day 2 postinfection, which increased to 45% on day 5 postinfection. TPA induction on day 2 postinfection increased mCP-positive cells to 45% (examined on day 4 postinfection), and TPA induction on day 5 postinfection increased mCP-positive cells to 60% (examined on day 7 postinfection). Less than 3% of the cells were positive for mCP after day 28 postinfection with or without TPA induction. (C) Kinetics of LNA and mCP expression in BAC36-infected cultures. (D) Kinetics of LNA and mCP expression in BAC36-infected cultures induced with TPA for 2 days.
BAC36-infected HUVEC cultures continued to show morphological changes several days postinfection. The cell shapes became more longitudinal as infection progressed. By day 3 postinfection, some cells reached a length/width ratio of up to 30. Multiple branches in a single cell were also visible by day 3 and became more obvious by days 4 and 5 postinfection, forming network structures in the cultures. These cells had morphologies resembling those of differentiated neuronal cells. In some cases, cells from the entire culture or a large proportion of the culture appeared to be connected, forming a large network (Fig. 1A, 4d).
FIG. 4.
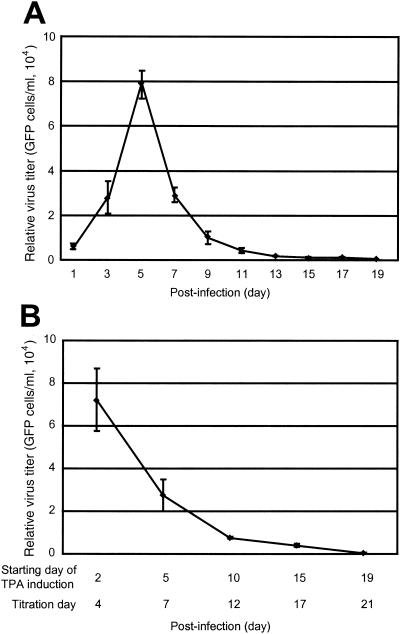
Virus production in BAC36-infected primary HUVEC cultures at different time points postinfection. (A) Virus production in uninduced BAC36-infected cultures. The infected cultures produced large amounts of virus at the early stage of infection and peaked on day 5 postinfection. However, virus production quickly decreased after the peak and reached zero after day 19 postinfection. (B) Virus production in TPA-induced BAC36-infected cultures. TPA-induced cultures produced large amounts of virus at the early stage of infection (day 4 postinfection) but remained at the same level as uninduced cultures (A). As in uninduced cultures, virus production also quickly decreased after the peak of virus production and reached zero after day 21 postinfection. Virus titers are given as 104 GFP-expressing cells per milliliter.
We have also observed cell death by day 4 postinfection, which became severe by days 5 and 6 postinfection (Fig. 1A, 4d to 6d). A large number of the cells were dead by days 7 and 8 postinfection; however, a small number of the infected cells survived and continued to proliferate, resulting in the formation of foci or colonies in the culture (Fig. 1A, 7d and 8d). Small foci of several spindle cells (5 to 20) were visible after 2 weeks postinfection (Fig. 1A, 15d to 23d) and continued to grow, forming larger foci (Fig. 1A, 28d and 35d). In some cases, the foci occupied the entire cultures 1 month postinfection. Infected cells in the foci also maintained the spindle shape. At the late stage of infection (>23 days postinfection), the cells tended to grow into bundles, each containing a large number of spindle cells, which was reminiscent of the vascular structures in KS tumors (Fig. 1A, 28d to 35d). The spaces between the spindle bundles were also reminiscent of the slit-like spaces in KS tumors.
Ciufo and colleagues have reported the observation of colonies or plaques consisting of KSHV-infected spindle cells in DMVEC cultures (18). In their system, the primary-infection efficiency was low, and the cultures did not go through the cell death crisis. The morphology of the surrounding cells of the colonies also remained healthy, with cobblestone shapes. The foci that we have observed usually did not have surrounding cobblestone-shaped cells, since almost all cells were infected.
BAC36-infected HUVEC cultures were stably maintained for 4 to 5 months, a life span which was substantially longer than that of the mock-infected cultures, which went into senescence after 6 to 7 weeks of culture. Late-stage infected cultures continued to express GFP and were positive for LNA (see below), indicating that they were able to maintain KSHV infection. Nevertheless, infected cultures eventually went into senescence. Attempts to isolate immortalized clones have not been successful.
BAC36-infected HUVEC cultures maintained viral infection after consecutive passages by cell splitting.
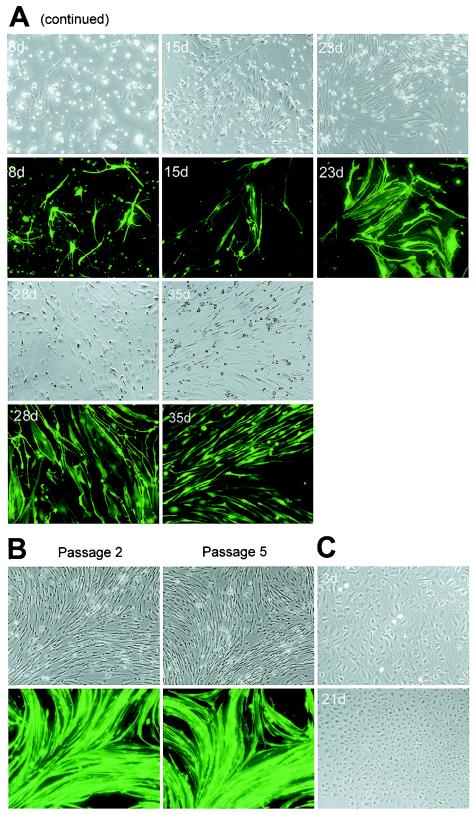
Previous studies have shown that KSHV can infect TIME cells at high primary-infection efficiency; however, infected cells quickly lost the virus after several passages (38). To determine whether BAC36-infected HUVECs can still maintain stable viral infection after passage, infected cultures were split every 3 to 4 days over a period of 35 days (10 passages). During this period, BAC36-infected HUVEC cultures maintained infection without losing the virus, as shown by the presence of GFP expression (Fig. 1B and below). Interestingly, if the cultures were split at an early stage of infection (within 2 to 3 days), they were spared a cell death crisis. All the split cultures formed bundles consisting of large numbers of orderly aligned spindle-shaped cells within 2 to 3 days after passage (Fig. 1B). Similar to unsplit cultures, the split infected cultures were maintained for 4 to 5 months before going into senescence. The split infected cultures also continued to express GFP and were positive for LNA at a late stage of culture.
Expression of KSHV latent- and lytic-cycle proteins in BAC36-infected HUVEC cultures.
To determine KSHV latent and lytic replication in BAC36-infected HUVEC cultures, we examined the expression of the KSHV latent-cycle protein LNA and lytic-cycle protein mCP by IFA with the respective specific monoclonal antibodies. On day 2 postinfection, the majority of the cells (∼80%) already expressed LNA (Fig. 2A), while only 3 to 5% of the cells expressed mCP (Fig. 2B). However, continuous examination of the cultures revealed that the number of cells expressing mCP quickly increased, reaching 45% by day 5 postinfection, while the number of cells expressing LNA remained high (80 to 95%) (Fig. 2A to C). Since mCP is a viral capsid protein that is expressed at a late stage of virus lytic replication, these results indicated that close to half of the infected cells were in active lytic replication. It is worth noting that active lytic replication coincided with or preceded the period of cell death crisis. It is unknown whether the observed high number of cells expressing LNA and low number of cells expressing mCP on day 2 postinfection reflected a status of KSHV transient latency or simply rapid LNA expression during primary infection.
Further examination of the infected cultures revealed that the percentage of BAC36-infected HUVECs expressing LNA remained high after day 5 postinfection, while the percentage of mCP-positive cells started to decrease, reaching <3% after day 15 postinfection and remaining at this low level for the rest of the culture period. These results indicated that the majority of the infected cells were in latent replication after day 15 postinfection. It remains to be determined whether the cells that survived the cell death crisis were in viral latent-replication status at the time of the crisis or were at viral lytic replication but had by then switched into latent replication. The detection of the majority of the cells in latent replication and a small percentage of cells undergoing spontaneous lytic replication at a late stage of infection is reminiscent of KS tumors and is concordant with other culture systems (18, 25, 38, 45, 65).
To test the inducibility of BAC36-infected HUVEC cultures for lytic replication, we treated the cells with TPA at 25 ng/ml for 2 days and examined the expression of LNA and mCP. Induction of the infected cells on day 2 postinfection increased the percentage of cells expressing mCP from <5 to 45% (Fig. 2B and D). However, since the percentage of mCP-positive cells had already reached 45% in parallel uninduced cultures (Fig. 2B and C), it appeared that TPA treatment did not further enhance viral lytic replication at this specific time. Nevertheless, induction with TPA on day 5 (examined on day 7) postinfection increased the percentage of mCP-positive cells to 60%, and the percentage remained higher than that in the uninduced cells (29 versus 18%) until day 10 postinfection, although the overall percentage of mCP-positive cells also decreased quickly after day 5 postinfection, similar to uninduced cultures (Fig. 2C and D). These results suggest that TPA treatment indeed induced lytic replication in a subset of the cells from days 5 to 10 postinfection. The percentage of mCP-positive cells in TPA-induced cultures decreased to 3% by day 15 postinfection, which was the same level as in uninduced cultures, and remained at this level at subsequent time points (Fig. 2C and D).
Virus production in BAC36-infected HUVEC cultures.
Because we have observed high levels of KSHV lytic protein expression in BAC36-infected HUVEC cultures at the early stage of infection, we further determined whether the lytic replication was productive by measuring infectious virus in the culture supernatants. We took advantage of the GFP cassette in the BAC36 genome and developed an assay to quantitatively determine the numbers of infectious BAC36 virions in the culture supernatants. We calculated the average number of 293 cells expressing GFP in one well (GFP-expressing cells/well) in a 96-well plate inoculated with the tested supernatant and converted it to GFP-expressing cells/ml. Figure 3 is an example of the use of this assay to determine the titer of a supernatant sample from a BAC36-infected HUVEC culture. The supernatant was subjected to twofold serial dilution, and each of the diluted samples was tested in a 96-well plate previously seeded with 293 cells. Although an almost linear curve was obtained when the number of GFP-expressing cells/well was converted to a log2 scale (Fig. 3B), the actual number of GFP-expressing cells/well did not have a linear correlation with the dilution (Fig. 3A). At high virus titer (when the supernatant was diluted <32-fold), the number of GFP-expressing cells/well decreased less rapidly than the actual dilution, suggesting that infection might have occurred at >2 multiplicities of infection; however, at low virus titer (when the supernatant was diluted >64-fold), the number of GFP-expressing cells/well correlated almost linearly with the actual dilution (Fig. 3A). Therefore, samples at higher dilution but still able to infect 293 cells are likely more reliable for calculating the virus titer of the undiluted original sample. Using this assay, we determined the titers of the virus preparations used in this study to be ∼8 × 105 to 10 × 105 GFP-expressing cells/ml. We then determined the virus titers in the BAC36-infected HUVEC cultures. On day 1 postinfection, a low level of virus at an average of 5 × 103 GFP-expressing cells/ml was observed in the supernatants of the infected cultures (Fig. 4A). Since there was minimal expression of viral lytic protein at this time (Fig. 2B and C), the low virus titers picked up by the assay were unlikely to come from viral lytic replication; instead, they were the virions from the inocula attached to the cells, though we washed the plates three times after the initial inoculation period. The amount of virus produced was increased to an average of 3 × 104 GFP-expressing cells/ml on day 3 postinfection and reached a peak on day 5 postinfection (average, 8 × 104 GFP-expressing cells/ml) (Fig. 4A). These results were in concordance with the expression of viral lytic protein (Fig. 2B and C) and reflected active viral lytic replication at the initial stage of infection. After day 5 postinfection, the production of virus started to decrease, and it approached zero after day 15 postinfection (Fig. 4A).
The results of virus production in BAC36-infected HUVEC cultures induced with TPA were also in concordance with the expression of viral proteins. Maximum virus production was observed on day 4 postinfection (average, 7.2 × 104 GFP-expressing cells/ml) but did not surpass that of parallel uninduced cultures (Fig. 4). These results suggest that the uninduced cultures were already under full lytic replication at the peak of viral lytic replication, and TPA treatment did not further increase viral lytic replication. Similar to uninduced cultures, virus production quickly decreased after the peak, but it appeared that slightly more virus was produced than in uninduced cultures, particularly from days 12 to 17 postinfection (average, 9 × 103 GFP-expressing cells/ml on day 12 and 5 × 103 GFP-expressing cells/ml on day 17 versus 5 × 103 GFP-expressing cells/ml on day 12 and 1 × 103 GFP-expressing cells/ml on day 17) (Fig. 4). Almost no virus production was observed with TPA treatment after day 21 postinfection (Fig. 4B). These results again suggested that KSHV had established latency after day 15 postinfection and that the latently infected cultures were not responsive to TPA induction for lytic replication.
Viral replication in split BAC36-infected HUVEC cultures resembled that in late-stage unsplit cultures.
Since early split BAC36-infected cultures did not go through cell death crisis and quickly formed bundles of spindle cells, we were interested in determining the status of viral replication in these cultures. IFA showed that almost all cells expressed LNA, while a small percentage of the cells (3 to 5%) also expressed mCP (data not shown). Virus titration showed production of minimal virus in the supernatants of these cultures (data not shown). These results indicated that BAC36-infected HUVEC cultures passaged by cell splitting behaved similarly to the late stage of unsplit cultures.
Passage of BAC36 virus to new cultures by de novo infection.
To determine whether virions produced in BAC36-infected HUVEC cultures during active viral lytic replication can be continuously passaged in cell cultures by de novo infection, we used supernatants collected at the peak of lytic replication (day 5 postinfection) to infect new HUVEC cultures after concentrating them fivefold. By day 2 postinfection, >50% of the cells in the newly infected HUVEC culture expressed GFP. Supernatants from the second-generation cultures were again collected on day 5 postinfection and used to infect new HUVEC cultures. A total of five such serial de novo infection passages were carried out. In each case, we were able to observe ∼40 to 50% primary-infection efficiency in HUVEC cultures on day 2 postinfection, indicating that the virus can be efficiently passaged in HUVEC cultures.
DISCUSSION
We have established a KSHV infection cell model by using a recombinant KSHV, BAC36, to infect primary HUVEC cultures. KSHV replication in this system can be divided into two phases: a permissive phase, which is at the early stage of infection with close to half of the cells in active viral lytic replication, and a latent phase, which is at the late stage of infection with most of the infected cells in viral latent replication. This system has the following unique features: (i) high primary-infection efficiency; (ii) active productive viral lytic replication at the early stage of infection; (iii) induction of a large number of cell deaths in the permissive phase with a small number of cells surviving the crisis, continuing to proliferate, and eventually establishing viral latent infection; (iv) infected cells sustaining KSHV infection after long-term culture and/or cell passages by cell splitting; and (v) BAC36 infection converting primary HUVECs into KS-like spindle cells, which form bundle structures at both early and late stages of infection.
A critical stage in viral infection is the primary infection period, during which the fates of the virus and the infected cells are determined: on one hand, the cells mount antiviral defense mechanisms in an attempt to clear the invader; on the other hand, the virus employs specific strategies to evade cellular antiviral defenses or alter cellular environments to favor its own replication. In order to examine the early events of viral infection, it is essential to establish an infection cell model that has high primary-infection efficiency. Although KSHV has previously been shown to infect primary human endothelial cells, the primary-infection efficiencies were low (18, 25, 45). KSHV primary infection of TIME cells was very efficient, but the infected cells lost the virus after several passages (38). Because of the immortalized nature of the TIME cell line, its use for investigating cellular immortalization and transformation is limited. In our system, primary infection of HUVECs is highly efficient, reaching 90% of the cells in the cultures (Fig. 1). BAC36 infection prolongs the life span of the primary HUVEC cultures to 4 to 5 months, during which the cultures maintain stable BAC36 infection, compared to a life span of 6 to 7 weeks for the uninfected cultures. BAC36 infection in the infected cultures is also stable after cell passages by cell splitting. This efficient infection cell model should allow the analysis of the sophisticated interplay between KSHV and cells, such as viral manipulation of cell cycle progression and the expression kinetics of viral and cellular genes, which can provide insight into viral replication and altered cellular pathways during primary infection.
All previously reported KSHV infection systems do not support permissive KSHV lytic replication (11, 18, 25, 27, 35, 38, 41, 44, 45, 48, 50, 63, 65). In these systems, KSHV establishes latent infection without going through active lytic replication following primary infection. In contrast to these reported systems, highly efficient infection of HUVECs by BAC36 is lytic replication permissive at an early stage of infection. This conclusion is supported by the following evidence: (i) close to half of the cells in BAC36-infected HUVEC cultures expressed the KSHV lytic-cycle mCP gene (Fig. 2B and C), (ii) infected cultures produced large amounts of infectious virus at the peak of active lytic replication (Fig. 4A), and (iii) TPA induction can hardly enhance viral lytic replication (Fig. 2B to D and 4). The establishment of this lytic-replication-permissive infection system should be useful for examining KSHV lytic replication and the functions of KSHV genes.
Several reports have described spindle conversion of human primary endothelial cells by KSHV (18, 25). We have observed spindle conversion of BAC36-infected HUVEC cultures as early as 6 h postinfection and continuing throughout the infection period (Fig. 1A). Early spindle conversion was also associated with significant increase in cell size, suggesting active protein and DNA synthesis, activities seen at S phase of the cell cycle, in the infected cells. In fact, KSHV infection of primary HUVEC cultures indeed promotes cell entry from G0-G1 into S phase (unpublished data). Following rapid spindle conversion and cell size increase at the early stage of infection, we have observed cell death in a large number of cells at and after the peak of KSHV active lytic replication (Fig. 1A, 6d to 8d). Thus, KSHV infection appears to drive primary HUVECs into S phase and to induce cell death. A number of other herpesviruses have been shown to regulate the cell cycle to favor their replication (20, 36, 58). KSHV appears to exploit a similar strategy.
A small number of cells survived the cell death crisis occurring during active viral replication (Fig. 1A, 15d). The survival of these cells is particularly important because they are the cells that continue to proliferate and eventually establish latent infection in the cultures. At this point, it is unknown whether the surviving cells remain latent or undergo lytic replication during the permissive phase. Understanding the mechanisms preventing the death of these cells, and those controlling the switch from lytic to latent infection in the cultures, might provide insight into the development of KS tumors.
It is intriguing that BAC36-infected HUVECs also developed morphology similar to that of neuronal cells, with multiple branches at the early stage of infection (Fig. 1A, 4d and 5d). It remains to be determined whether infected cells at this stage indeed express markers of neuronal cells and respond to extracellular neuronal signaling. Specific signaling of neuronal cells has been demonstrated in other types of cancers and implicated in their development (17, 31, 39, 52).
How does KSHV modulate cellular pathways to alter cell morphology and regulate cell cycle progression? The cell receptors of KSHV have been identified to be α3β1 integrins and possibly some other types of integrins (1). Binding of KSHV to its receptors can activate the MEK/ERK pathway (46) and possibly other MAPK pathways, which could be the molecular basis by which infected cells undergo alterations of morphology and cell cycle progression at the early stage of viral infection. Activation of MAPK pathways has been shown to accelerate cell entry into S phase of the cell cycle and cell polarization (7, 24, 56, 57). Nevertheless, the initial binding of KSHV virions to the receptors is unlikely to be a factor for long-term maintenance of the spindle shapes of the infected HUVEC cultures, particularly at the late stage of infection, which manifests minimal production of infectious virus (Fig. 4A). In KS tumors, the majority of the tumor cells are in latent replication, with a small number of them undergoing spontaneous lytic replication (4, 19, 22, 34, 47, 60, 61). It has been proposed that these lytically infected cells produce regulatory products, such as viral interleukin-6, that could have autocrine-paracrine signaling effects on the cells. In our cell model, almost all cells were latently infected by KSHV at the late stage of infection, with minimal cells undergoing lytic replication, which is reminiscent of KS tumors. Similar observations were reported in other culture systems (18, 25, 45, 65). Therefore, inflammatory cytokines produced through autocrine-paracrine loops of KSHV lytic-replication products are possible factors for maintaining the spindle shape of the infected cells. Nevertheless, conditioned media from BAC36-infected HUVEC cultures at the late stage of infection do not induce spindle conversion (unpublished data). Furthermore, BAC36-infected HUVEC cultures quickly regained spindle shapes after cell splitting, a procedure that gives the cells round shapes and at the same time eliminates any virus-induced autocrine and paracrine factors from the cultures. In Ciufo's system, although a small number of the infected cells also undergo spontaneous lytic replication, uninfected cells surrounding the spindle cell colonies remain in the cobblestone shape, arguing against the paracrine theory (18). For the above reasons, we propose that a KSHV latent gene(s), which we named the spindle gene(s), was the determinant for maintaining the spindle morphology of KSHV-infected endothelial cells. The expression kinetics of this gene(s) is also likely to influence the switch from viral lytic to latent replication in the infected cultures. A final answer to the question of whether viral lytic replication is necessary for maintaining the spindle morphology and sustaining KSHV-infected cultures could only be determined by generating a KSHV mutant with the deletion of a viral gene, such as that for RTA, that is essential for the activation of viral lytic replication and examining it in an infection system of primary endothelial cell cultures.
KS tumors have distinct vascular structures of spindle cells. We have observed bundle structures of BAC36-infected HUVEC cultures which are reminiscent of KS vascular structures at both early and late stages of infection (Fig. 1A). Large bundle structures are particularly prominent at the late stage of infection and consist of KS slit-like spaces (Fig. 1A, 28d and 35d). It will be important to further identify the cell adhesion molecules that are involved in the formation of bundle structures after viral infection. It is interesting that BAC36-infected cultures split at an early stage of infection behaved like unsplit cultures, which were spared a cell death crisis and quickly acquired large bundle structures, at a late stage of infection. The alterations of cell adhesion molecules and related signaling pathways after cell splitting could contribute to the rapid morphology transition of the split cultures in this case. It is worth noting that viral replication in the split cultures was also similar to that of unsplit cultures at a late stage of infection, which was predominantly in the latent phase. It appears that large bundle structures are associated with viral latent infection, which could also be regulated by signaling pathways of cell adhesion molecules.
Why is our infection system lytic replication permissive at an early stage of infection, which has never been described before? Obviously, virus sources and procedures for preparing virus stocks and cell cultures could all contribute to the differences between our system and those of others. First, we have shown that cell splitting at an early stage of infection can affect virus replication. We cannot determine whether the cultures are split at an early stage of infection in other systems. Second, the primary-infection efficiency in our system is high, while those of most other systems are low. It is possible that lytic replication also occurs at the early stage of infection in other systems. Indeed, cell death and/or cytopathic effect, indications of likely viral lytic replication, have been observed at early stages of infection in some of these systems (25, 27). However, since the primary-infection efficiency is low, virus production in the cultures could also be low and thus be ignored. In addition, the level of viral lytic replication and infectious-virus production has not always been examined in other systems. KSHV infection of TIME cells has a high primary-infection rate; however, infected cells appear to quickly establish latent infection without active viral lytic replication (38). Third, genetic variations of the virus sources could also account for the differences. Defective viruses, in some cases named defective interfering virus because of their ability to interfere with the replication of wild-type virus, have been well documented for other herpesviruses (3, 54). Both genomic rearrangements and deletions can result in the generation of defective viruses. For example, the KSHV genome in the BC-1 cell line is rearranged and contains a viral genome of 250 kb instead of the predicted 180 kb. The BC-1 genomic sequences are duplicated both in the long unique coding region and the terminal repeat region (55). During viral lytic replication, these viral genomes probably have to be rearranged in order to be effectively packaged to generate infectious virions. Lytic-replication-defective viruses have been found in cultures of three other PEL cell lines and isolated cell cultures that exclusively harbor the defective viruses (J. H. Deng, Y. J. Zhang, and S.-J. Gao, presented at the 3rd International Workshop on Kaposi's Sarcoma-Associated Herpesvirus and Related Agents, 6 to 10 July, 2000, and unpublished data). One of the defective viruses has a deletion of 87 kb in the genome and is defective in viral lytic replication (Deng et al., 3rd International Workshop on Kaposi's Sarcoma-Associated Herpesvirus and Related Agents). If high numbers of defective viruses are present in the cultures (even infectious virions can still be obtained after complementation by helper viruses) it is likely that the virus preparations are of low quality. Even though high primary-infection efficiencies could be achieved with these virus preparations, the majority of the de novo-infected cells might still have received the defective genomes, which would result in defective viral lytic replication. The use of BAC36 can certainly avoid these potential problems because it contains the full-length KSHV genome without genetic rearrangements and deletions (65).
Finally, what are the meanings of the results observed so far from our cell model for KSHV infection in vivo and the development of KS? First, active lytic replication at the early stage of infection can certainly generate large numbers of virions and facilitate the spread of virus to other cells. Second, spindle conversion and the acquisition of neuronal cell morphology with multiple branches at the early stage of infection can also facilitate the spread of virus, as well as molecular signaling to other cells to favor viral infection. Third, for long-term infection, latent infection is probably the best way for the virus to establish persistent infection in the host and also likely the best way to avoid host immune systems. Fourth, in concordance with the observation that KS is a highly angiogenic tumor, KSHV-induced large bundle structures at the late stage of infection can not only continue to facilitate virus spread but, more importantly, secure connections to other cells or sources of nutrition and sustain the growth of cells that in return nurture the latent viruses. A somewhat disappointing but no less interesting result is the failure to obtain permanent KSHV-immortalized primary HUVEC cultures. However, this outcome might reflect the true scenario in vivo, in which dominant KSHV latent infection, possibly in combination with sporadic lytic replication, is the driving force for the growth of the angiogenic KS tumors, at least at the early stage of KS development.
Acknowledgments
This work was supported in part by the Association for International Cancer Research, the Charlotte Geyer Foundation, and NIH grant RO1CA096512 to S.-J. Gao.
REFERENCES
- 1.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin α3β1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. [DOI] [PubMed] [Google Scholar]
- 2.Arvanitakis, L., E. A. Mesri, R. G. Nador, J. W. Said, A. S. Asch, D. M. Knowles, and E. Cesarman. 1996. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood 88:2648-2654. [PubMed] [Google Scholar]
- 3.Barrett, A. D., and N. J. Dimmock. 1986. Defective interfering viruses and infections of animals. Curr. Top. Microbiol. Immunol. 128:55-84. [DOI] [PubMed] [Google Scholar]
- 4.Blasig, C., C. Zietz, B. Haar, F. Neipel, S. Esser, N. H. Brockmeyer, E. Tschachler, S. Colombini, B. Ensoli, and M. Sturzl. 1997. Monocytes in Kaposi's sarcoma lesions are productively infected by human herpesvirus 8. J. Virol. 71:7963-7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boshoff, C., T. F. Schulz, M. M. Kennedy, A. K. Graham, C. Fisher, A. Thomas, J. O. D. McGee, R. A. Weiss, and J. J. O'Leary. 1995. Kaposi's sarcoma-associated herpes virus (KSHV) infects endothelial and spindle cells. Nat. Med. 1:1274-1278. [DOI] [PubMed] [Google Scholar]
- 6.Boshoff, C., and R. A. Weiss. 2001. Epidemiology and pathogenesis of Kaposi's sarcoma-associated herpesvirus. Phil. Trans. R. Soc. Lond. B 356:517-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buck, M., and M. Chojkier. 2003. Signal transduction in the liver: C/EBPβ modulates cell proliferation and survival. Hepatology 37:731-738. [DOI] [PubMed] [Google Scholar]
- 8.Cannon, J. S., D. Ciufo, A. L. Hawkins, C. A. Griffin, M. J. Borowitz, G. S. Hayward, and R. F. Ambinder. 2000. A new primary effusion lymphoma-derived cell line yields a highly infectious Kaposi's sarcoma herpesvirus-containing supernatant. J. Virol. 74:10187-10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carbone, A., A. M. Cilia, A. Gloghini, D. Capello, T. Perin, D. Bontempo, V. Canzonieri, U. Tirelli, R. Volpe, and G. Gaidano. 2000. Primary effusion lymphoma cell lines harbouring human herpesvirus type-8. Leuk. Lymphoma 36:447-456. [DOI] [PubMed] [Google Scholar]
- 10.Carbone, A., A. M. Cilia, A. Gloghini, D. Capello, M. Todesco, S. Quattrone, R. Volpe, and G. Gaidano. 1998. Establishment and characterization of EBV-positive and EBV-negative primary effusion lymphoma cell lines harbouring human herpesvirus type-8. Br. J. Haematol. 102:1081-1089. [DOI] [PubMed] [Google Scholar]
- 11.Cerimele, F., F. Curreli, S. Ely, A. E. Friedman-Kien, E. Cesarman, and O. Flore. 2001. Kaposi's sarcoma-associated herpesvirus can productively infect primary human keratinocytes and alter their growth properties. J. Virol. 75:2435-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cesarman, E. 2002. The role of Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in lymphoproliferative diseases. Recent Results Cancer Res. 159:27-37. [DOI] [PubMed] [Google Scholar]
- 13.Cesarman, E., E. A. Mesri, and M. C. Gershengorn. 2000. Viral G protein-coupled receptor and Kaposi's sarcoma: a model of paracrine neoplasia? J. Exp. Med. 191:417-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cesarman, E., P. S. Moore, P. H. Rao, G. Inghirami, D. M. Knowles, and Y. Chang. 1995. In vitro establishment and characterization of two AIDS-related lymphoma cell lines containing Kaposi's sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood 86:2708-2714. [PubMed] [Google Scholar]
- 15.Chang, J., R. Renne, D. Dittmer, and D. Ganem. 2000. Inflammatory cytokines and the reactivation of Kaposi's sarcoma-associated herpesvirus lytic replication. Virology 266:17-25. [DOI] [PubMed] [Google Scholar]
- 16.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 17.Chave, H. S., A. C. Gough, K. Palmer, S. R. Preston, and J. N. Primrose. 2000. Bombesin family receptor and ligand gene expression in human colorectal cancer and normal mucosa. Br. J. Cancer 82:124-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciufo, D. M., J. S. Cannon, L. J. Poole, F. Y. Wu, P. Murray, R. F. Ambinder, and G. S. Hayward. 2001. Spindle cell conversion by Kaposi's sarcoma-associated herpesvirus: formation of colonies and plaques with mixed lytic and latent gene expression in infected primary dermal microvascular endothelial cell cultures. J. Virol. 75:5614-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis, M. A., M. Sturzl, C. Blasig, A. Schreier, H.-G. Guo, M. Reitz, S. R. Opalenik, and P. J. Browning. 1997. Expression of human herpesvirus 8-encoded cyclin D in Kaposi's sarcoma spindle cells. J. Natl. Cancer Inst. 89:1868-1874. [DOI] [PubMed] [Google Scholar]
- 20.Derfuss, T., and E. Meinl. 2002. Herpesviral proteins regulating apoptosis. Curr. Top. Microbiol. Immunol. 269:257-272. [DOI] [PubMed] [Google Scholar]
- 21.Drexler, H. G., C. C. Uphoff, G. Gaidano, and A. Carbone. 1998. Lymphoma cell lines: in vitro models for the study of HHV-8+ primary effusion lymphomas (body cavity-based lymphomas). Leukemia 12:1507-1517. [DOI] [PubMed] [Google Scholar]
- 22.Dupin, N., C. Fisher, P. Kellam, S. Ariad, M. Tulliez, N. Franck, E. van Marck, D. Salmon, I. Gorin, J. P. Escande, R. A. Weiss, K. Alitalo, and C. Boshoff. 1999. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc. Natl. Acad. Sci. USA 96:4546-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ensoli, B., G. Barillari, L. Buonaguro, and R. C. Gallo. 1991. Molecular mechanisms in the pathogenesis of AIDS-associated Kaposi's sarcoma. Adv. Exp. Med. Biol. 303:27-38. [DOI] [PubMed] [Google Scholar]
- 24.Fingar, D. C., S. Salama, C. Tsou, E. Harlow, and J. Blenis. 2002. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 16:1472-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flore, O., S. Rafii, S. Ely, J. J. O'Leary, E. M. Hyjek, and E. Cesarman. 1998. Transformation of primary human endothelial cells by Kaposi's sarcoma-associated herpesvirus. Nature 394:588-592. [DOI] [PubMed] [Google Scholar]
- 26.Foreman, K. E., P. E. Bacon, E. D. Hsi, and B. J. Nickoloff. 1997. In situ polymerase chain reaction-based localization studies support role of human herpesvirus-8 as the cause of two AIDS-related neoplasms: Kaposi's sarcoma and body cavity lymphoma. J. Clin. Investig. 99:2971-2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foreman, K. E., J. Friborg, W. Kong, C. Woffendin, P. J. Polverini, B. J. Nickoloff, and G. J. Nabel. 1997. Propagation of a human herpesvirus from AIDS-associated Kaposi's sarcoma. N. Engl. J. Med. 336:163-171. [DOI] [PubMed] [Google Scholar]
- 28.Gao, S.-J., L. Kingsley, D. R. Hoover, T. J. Spira, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, P. Parry, Y. Chang, and P. S. Moore. 1996. Seroconversion of antibodies against Kaposi's sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi's sarcoma. N. Engl. J. Med. 335:233-241. [DOI] [PubMed] [Google Scholar]
- 29.Gao, S.-J., L. Kingsley, M. Li, W. Zheng, C. Parravicini, J. Ziegler, R. Newton, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, Y. Chang, and P. S. Moore. 1996. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nat. Med. 2:925-928. [DOI] [PubMed] [Google Scholar]
- 30.Gao, S.-J., Y.-J. Zhang, J.-H. Deng, C. S. Rabkin, O. Flore, and H. B. Jenson. 1999. Molecular polymorphism of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) latent nuclear antigen: evidence for a large repertoire of viral genotypes and dual infection with different viral genotypes. J. Infect. Dis. 180:1466-1476. [DOI] [PubMed] [Google Scholar]
- 31.Gugger, M., and J. C. Reubi. 1999. Gastrin-releasing peptide receptors in non-neoplastic and neoplastic human breast. Am. J. Pathol. 155:2067-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katano, H., Y. Sato, T. Kurata, S. Mori, and T. Sata. 1999. High expression of HHV-8-encoded ORF73 protein in spindle-shaped cells of Kaposi's sarcoma. Am. J. Pathol. 155:47-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kedes, D. H., E. Operskalski, M. Busche, J. Flood, R. Kohn, and D. Ganem. 1996. The seroepidemiology of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus): distribution of infection in KS risk groups for sexual transmission. Nat. Med. 2:918-924. [DOI] [PubMed] [Google Scholar]
- 34.Kirshner, J. R., K. Staskus, A. Haase, M. Lagunoff, and D. Ganem. 1999. Expression of the open reading frame 74 (G-protein-coupled receptor) gene of Kaposi's sarcoma (KS)-associated herpesvirus: implications for KS pathogenesis. J. Virol. 73:6006-6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kliche, S., E. Kremmer, W. Hammerschmidt, U. Koszinowski, and J. Haas. 1998. Persistent infection of Epstein-Barr virus-positive B lymphocytes by human herpesvirus 8. J. Virol. 72:8143-8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krakauer, D. C., and R. J. Payne. 1997. The evolution of virus-induced apoptosis. Proc. R. Soc. Lond. B 264:1757-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacoste, V., J. G. Judde, G. Bestett, J. Cadranel, M. Antoine, F. Valensi, E. Delabesse, E. Macintyre, and A. Gessain. 2000. Virological and molecular characterisation of a new B lymphoid cell line, established from an AIDS patient with primary effusion lymphoma, harbouring both KSHV/HHV8 and EBV viruses. Leuk. Lymphoma 38:401-409. [DOI] [PubMed] [Google Scholar]
- 38.Lagunoff, M., J. Bechtel, E. Venetsanakos, A. M. Roy, N. Abbey, B. Herndier, M. McMahon, and D. Ganem. 2002. De novo infection and serial transmission of Kaposi's sarcoma-associated herpesvirus in cultured endothelial cells. J. Virol. 76:2440-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee, L. F., J. Guan, Y. Qiu, and H. J. Kung. 2001. Neuropeptide-induced androgen independence in prostate cancer cells: roles of nonreceptor tyrosine kinases Etk/Bmx, Src, and focal adhesion kinase. Mol. Cell. Biol. 21:8385-8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu, C., Y. Okruzhnov, H. Li, and J. Nicholas. 2001. Human herpesvirus 8 (HHV-8)-encoded cytokines induce expression of and autocrine signaling by vascular endothelial growth factor (VEGF) in HHV-8-infected primary-effusion lymphoma cell lines and mediate VEGF-independent antiapoptotic effects. J. Virol. 75:10933-10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mesri, E. A., E. Cesarman, L. Arvanitakis, S. Rafii, M. A. S. Moore, D. N. Posnett, D. M. Knowles, and A. S. Asch. 1996. Human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus is a new transmissible virus that infects B cells. J. Exp. Med. 183:2385-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore, P. S., and Y. Chang. 1998. Kaposi's sarcoma-associated herpesvirus-encoded oncogenes and oncogenesis. J. Natl. Cancer. Inst. Monogr. 23:65-71. [DOI] [PubMed] [Google Scholar]
- 43.Moore, P. S., and Y. Chang. 2001. Molecular virology of Kaposi's sarcoma-associated herpesvirus. Phil. Trans. R. Soc. Lond. B 356:499-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore, P. S., S.-J. Gao, G. Dominguez, E. Cesarman, O. Lungu, D. M. Knowles, R. Garber, D. J. McGeoch, P. Pellett, and Y. Chang. 1996. Primary characterization of a herpesvirus-like agent associated with Kaposi's sarcoma. J. Virol. 70:549-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moses, A. V., K. N. Fish, R. Ruhl, P. P. Smith, J. G. Strussenberg, L. Zhu, B. Chandran, and J. A. Nelson. 1999. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J. Virol. 73:6892-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naranatt, P. P., S. M. Akula, C. A. Zien, H. H. Krishnan, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-ζ-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J. Virol. 77:1524-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orenstein, J. M., S. Alkan, A. Blauvelt, K. T. Jeang, M. D. Weinstein, D. Ganem, and B. Herndier. 1997. Visualization of human herpesvirus type 8 in Kaposi's sarcoma by light and transmission electron microscopy.AIDS 11:F35-F45. [DOI] [PubMed] [Google Scholar]
- 48.Panyutich, E. A., J. W. Said, and S. A. Miles. 1998. Infection of primary dermal microvascular endothelial cells by Kaposi's sarcoma-associated herpesvirus. AIDS 12:467-472. [DOI] [PubMed] [Google Scholar]
- 49.Reed, J. A., R. G. Nador, D. Spaulding, Y. Tani, E. Cesarman, and D. M. Knowles. 1998. Demonstration of Kaposi's sarcoma-associated herpes virus cyclin D homolog in cutaneous Kaposi's sarcoma by colorimetric in situ hybridization using a catalyzed signal amplification system. Blood 91:3825-3832. [PubMed] [Google Scholar]
- 50.Renne, R., D. Blackbourn, D. Whitby, J. Levy, and D. Ganem. 1998. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 72:5182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 52.Reubi, J. C., M. Gugger, B. Waser, and J. C. Schaer. 2001. Y(1)-mediated effect of neuropeptide Y in cancer: breast carcinomas as targets. Cancer Res. 61:4636-4641. [PubMed] [Google Scholar]
- 53.Roth, W. K., H. Brandstetter, and M. Sturzl. 1992. Cellular and molecular features of HIV-associated Kaposi's sarcoma. AIDS 6:895-913. [DOI] [PubMed] [Google Scholar]
- 54.Roux, L., A. E. Simon, and J. J. Holland. 1991. Effects of defective interfering viruses on virus replication and pathogenesis in vitro and in vivo. Adv. Virus Res. 40:181-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Russo, J. J., R. A. Bohenzky, M. Chien, K. Chen, D. Maddalena, M. Yan, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of Kaposi's sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schafer, M., N. Ewald, C. Schafer, A. Stapler, H. M. Piper, and T. Noll. 2003. Signaling of hypoxia-induced autonomous proliferation of endothelial cells. FASEB J. 17:449-451. [DOI] [PubMed] [Google Scholar]
- 57.Schluter, K. D., Y. Goldberg, G. Taimor, M. Schafer, and H. M. Piper. 1998. Role of phosphatidylinositol 3-kinase activation in the hypertrophic growth of adult ventricular cardiomyocytes. Cardiovasc. Res. 40:174-181. [DOI] [PubMed] [Google Scholar]
- 58.Shen, Y., and T. E. Shenk. 1995. Viruses and apoptosis. Curr. Opin. Genet. Dev. 5:105-111. [DOI] [PubMed] [Google Scholar]
- 59.Simpson, G. R., T. F. Schulz, D. Whitby, P. M. Cook, C. Boshoff, L. Rainbow, M. R. Howard, S.-J. Gao, R. A. Bohensky, P. Simmonds, C. Lee, A. de Ruiter, A. Hatziakis, R. S. Tedder, I. V. D. Weller, R. A. Weiss, and P. S. Moore. 1996. Prevalence of Kaposi's sarcoma-associated herpesvirus infection measured by antibodies to recombinant capsid protein and latent immunofluorescence antigen. Lancet 348:1133-1138. [DOI] [PubMed] [Google Scholar]
- 60.Stürzl, M., C. Blasig, A. Schreier, F. Neipel, C. Hohenadl, E. Cornali, G. Ascherl, S. Esser, N. H. Brockmeyer, M. Ekman, E. E. Kaaya, E. Tschachler, and P. Biberfeld. 1997. Expression of HHV-8 latency-associated T0.7 RNA in spindle cells and endothelial cells of AIDS-associated, classical and African Kaposi's sarcoma. Int. J. Cancer 72:68-71. [DOI] [PubMed] [Google Scholar]
- 61.Stürzl, M., C. Hohenadl, C. Zietz, E. Castanos-Velez, A. Wunderlich, G. Ascherl, P. Biberfeld, P. Monini, P. J. Browning, and B. Ensoli. 1999. Expression of K13/v-FLIP gene of human herpesvirus 8 and apoptosis in Kaposi's sarcoma spindle cells. J. Natl. Cancer Inst. 91:1725-1733. [DOI] [PubMed] [Google Scholar]
- 62.Uphoff, C. C., A. Carbone, G. Gaidano, and H. G. Drexler. 1998. HHV-8 infection is specific for cell lines derived from primary effusion (body cavity-based) lymphomas. Leukemia 12:1806-1809. [DOI] [PubMed] [Google Scholar]
- 63.Vieira, J., P. O'Hearn, L. Kimball, B. Chandran, and L. Corey. 2001. Activation of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) lytic replication by human cytomegalovirus. J. Virol. 75:1378-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Werner, S., P. H. Hofschneider, and W. K. Roth. 1989. Cells derived from sporadic and AIDS-related Kaposi's sarcoma reveal identical cytochemical and molecular properties in vitro. Int. J. Cancer. 43:1137-1144. [DOI] [PubMed] [Google Scholar]
- 65.Zhou, F. C., Y. J. Zhang, J. H. Deng, X. P. Wang, H. Y. Pan, E. Hettler, and S. J. Gao. 2002. Efficient infection by a recombinant Kaposi's sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis. J. Virol. 76:6185-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]