Abstract
For some time synovial fibroblasts have been regarded simply as innocent synovial cells, mainly responsible for synovial homeostasis. During the past decade, however, a body of evidence has accumulated illustrating that rheumatoid arthritis synovial fibroblasts (RASFs) are active drivers of joint destruction in rheumatoid arthritis. Details regarding the intracellular signalling cascades that result in long-term activation and synthesis of proinflammatory molecules and matrix-degrading enzymes by RASFs have been analyzed. Molecular, cellular and animal studies have identified various interactions with other synovial and inflammatory cells. This expanded knowledge of the distinct role played by RASFs in the pathophysiology of rheumatoid arthritis has moved these fascinating cells to the fore, and work to identify targeted therapies to inhibit their joint destructive potential is underway.
Introduction
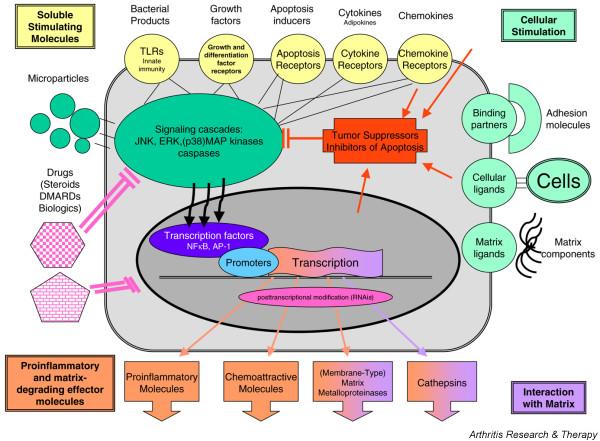
Rheumatoid arthritis synovial fibroblasts (RASFs; also termed fibroblast-like synoviocytes or type B synoviocytes), together with synovial macrophages, are the two leading cell types in the terminal layer of the hyperplastic synovial tissue that invades and degrades adjacent cartilage and bone. In this destructive process, RASFs actively drive inflammation and degradation of the joint by producing inflammatory cytokines and matrix-degrading molecules (Fig. 1).
Figure 1.
Network of interactions of RASFs with cells and matrix. RASFs are sensitive to stimulation and modulation by numerous growth factors, cytokines and chemokines, as well as by direct interaction with immunologically active cells and matrix components within the rheumatoid synovium. DMARDs such as methotrexate and leflunomide can inhibit the activity of RASFs to produce proinflammatory and matrix-degrading enzymes by interfering with their intracellular metabolic pathways. Modified from Müller-Ladner [80]. AP, activator protein; DMARD, disease-modifying antirheumatic drug; ERK, extracellular signal-regulated kinse; JNK, c-jun amino-terminal kinase; MAP kinase, mitogen-activated protein kinase; NF-κB, nuclear factor-κB; RASF, rheumatoid arthritis synovial fibroblast; RNAi, RNA interference; TLR, Toll-like receptor.
In nondiseased tissue, the physiological function of synovial fibroblasts (SFs) is to provide the joint cavity and the adjacent cartilage with nutritive plasma proteins and lubricating molecules such as hyaluronic acid. SFs are also involved in continuous matrix remodeling by producing matrix components such as collagen and hyaluronan as well as a variety of matrix-degrading enzymes. Even though SFs are not primarily part of the immune system and do not express disease-specific HLA-DR molecules, they can develop these properties during the course of rheumatoid arthritis (RA), as outlined here. The variability of SF characteristics is further illustrated by the fact that no RA-specific or synovium-specific fibroblast markers have yet been identified. Currently, the best markers of SFs in flow cytometry and immunohisto-chemistry and cytochemistry are vimentin, prolyl-5-hydroxylase and Thy-1.
Since the first description of an altered RASF phenotype by Fassbender in 1983 [1], data have been gathered that allow us to understand the transition from an innocent mesenchymal cell to a destructive cell that plays a leading role in established RA. Early studies in MRL-lpr/lpr mice that spontaneously develop RA-like arthritis showed that synovial cells proliferate, attach and invade joint structures even before inflammatory cells migrate into the synovium [2]. This inflammation-independent activation of RASFs was corroborated by studies conducted in the severe combined immunodeficient (SCID) mouse model of cartilage destruction, in which implanted human RASFs degrade human co-implanted cartilage in the absence of inflammatory cells [3]. Hence, the most fascinating areas of fibroblast biology and research (as outlined below) are not only the responsiveness of RASFs to distinct extracellular stimuli but also the initial events that result in significant phenotype change, most likely occurring before overt inflammation takes place. This hypothesis recently received further support from findings in innate immunity [4].
RASFs in the pre-inflammatory phase of rheumatoid arthritis
From a functional and therapeutic point of view, the preclinical stage of RA is of great interest. One of the most challenging goals in RASF research is to determine the specific role that these cells play in the early phase of the disease. As outlined below, it appears that, before clinical signs of RA become evident, activation of the innate immune system leads to a distinct upregulation of effector molecules in RASFs. Potential triggers for this early activation are infectious as well as noninfectious agents and their respective (degradation) products [4].
Microbial fragments can stimulate RASFs via highly conserved basic innate immune receptor systems, such as Toll-like receptors (TLRs). From the currently 10 known TLRs in humans, TLR2, TLR3 and TLR4 have thus far been identified as being expressed on RASFs. As functional effects of TLR2 activation in RASFs, induction of vascular endothelial growth factor (VEGF) and IL-8 production were demonstrated after stimulation with bacterial peptidoglycan (a known ligand of TLR2) [5]. Furthermore, TLR2 and TLR4 activation induced synthesis of IL-15 in RASFs via nuclear factor-κB (NF-κB) [6]. In a proinflammatory cycle, cytokines such as IL-1 and tumour necrosis factor (TNF)-α were shown to enhance further the expression of TLR2 in RASFs. A gene expression study [7] revealed that RASFs synthesize various chemokines after stimulation with a TLR2 ligand. Among these chemokines, C-X-C motif ligand (CXCL)2 (gro-2) and C-C motif ligand (CCL)8 (monocyte chemoattractant protein [MCP]-2) probably contribute significantly to the accumulation of inflammatory cells in the rheumatoid synovium. Also, TLR3 appears to play a distinct pathophysiological role in RA synovium, because RNA released from necrotic cells acts as an endogenous TLR3 ligand for the stimulation of pro-inflammatory gene expression in RASFs. Stimulation of cultured RASFs with the TLR3 ligand poly(I-C) resulted in the production of high levels of interferon-β, interferon-γ-inducible protein 10 (CXCL10), CCL5, and IL-6 proteins [8]. Accordingly, regulation of TLR function can be used to down-regulate RASF activity. For example, vasoactive intestinal peptide has exhibited therapeutic effects in arthritis by inhibiting both innate and acquired immune responses. In RASFs vasoactive intestinal peptide was able to down-regulate the lipopolysaccharide-induced but not the constitutive expression of TLR4, followed by a decrease in production of CCL2 and CXCL8 chemokines [9].
Based on these data, it can be hypothesized that a 'sentinel' function of synovial fibroblasts [10] is operative even in the preclinical phase of RA and leads to the initiation and early perpetuation of the disease.
RASFs as effector cells in inflammation
Local and systemic inflammation is one of the hallmarks of RA. Apart from genuine inflammatory cells such as neutrophils and lymphocytes, RASFs contribute significantly to the various proinflammatory pathways within the rheumatoid joint. The 'sentinel' function of RASFs can be extended to (chemo)attraction of leucocytes, which is mandatory for the accumulation of immunomodulatory cells in the rheumatoid synovium. In addition to the above-mentioned chemokine secretion upon stimulation with TLR ligands, the influx of CD4+ T cells into the proliferating synovium is enhanced by RASFs because of their production of CXCL16 [11], the chemoattractive IL-16, and stromal cell derived factor-1 (one of the key factors for migration of T cells toward fibroblasts [pseudoemperipolesis]). Entering a vicious cycle, chemotactic molecules are further released from RASFs after stimulation of the CD40 ligand/CD40 system, for instance by cell-to-cell contact with T lymphocytes. Upon such stimulation, RASFs produce a variety of chemo-attractive molecules. Among them are macrophage inflammatory protein (MIP), MCP, CCL5 (also known as RANTES [regulated on activation, normal T-cell expressed and secreted]) and IL-8. Interleukin-17, a CD4+ T-cell-derived cytokine, further upregulates cytokine production in RASFs and enhances this proinflammatory interaction cascade. In addition, RASFs release MIP-3α after stimulation with IL-1β, IL-18 and TNF-α, which leads to perivascular chemoattraction of mononuclear cells. As mentioned above, cell-to-cell contact enhances these chemoattractive processes; for example, the interaction of RASFs and leucocytes via β2 integrin/vascular cell adhesion molecule (VCAM)-1 resulted in an upregulation of MIP-1α synthesis in polymorphonuclear neutrophils and monocytes from RA synovial fluid [12].
Apart from secretion of chemotactic proteins, RASFs produce a wide range of proinflammatory cytokines and effector molecules. Being the source of cyclo-oxygenase (COX)-2 in the synovial lining, RASFs are linked to a currently intensively discussed system that is involved in regulation of synovial inflammatory pathways, namely the COX-1/COX-2 system. A number of nonselective and selective COX inhibitors, including ibuprofen, diclofenac, meloxicam and rofecoxib, were found to be able to inhibit IL-1-triggered prostaglandin production in RASFs [13]. Interestingly, the selective E2 COX-2 inhibitor celecoxib but no other tested COX-2 inhibitor induced apoptosis in RASFs in vitro [14].
Taken together, because of the ability of RASFs to synthesize a broad range of proinflammatory and chemoattractive molecules, they can be regarded not only as cells that actively drive inflammation in the pathogenesis of RA but also as among the major targets for disease-modifying and anti-inflammatory drugs.
RASFs and matrix degradation
Functional disability of the joints through progressive degradation of cartilage and bone is a hallmark of RA. Known effector molecules in the destruction of articular cartilage and bone are matrix metalloproteinases (MMPs) and cathepsins. RASFs at sites of invasion or within the synovial lining layer are a major source of MMPs and cathepsins, and drive RA joint destruction via these enzymes.
Proteinases
MMPs include collagenases, stromelysin, gelatinases, and membrane-type (MT) MMPs. Of these, collagenase-1 (MMP-1) cleaves collagens I, II, VII and X. Inhibition of MMP-1 synthesis by retroviral over-expression of ribozymes that target MMP-1 mRNA resulted in a significant reduction of the invasiveness of RASFs in the SCID mouse model for RA [15], without affecting the production of other MMPs. Also, the recently discovered membrane-type MMPs are involved in RA and RASF pathophysiology. MT1-MMP (MMP-14) and MT3-MMP (MMP-16) cleave extracellular matrix components and can activate other MMPs. MT1-MMP and MT3-MMP are abundant in RA synovium, with MT3-MMP being expressed by RASFs, and MT1-MMP by RASFs and CD68-positive osteoclasts and macrophages. The proteolytic activity at sites of synovial attachment to cartilage was found to be mediated by a complex consisting of MT1-MMP, tissue inhibitor of matrix metalloproteinase (TIMP)-2 and MMP-2, whereby TIMP-2 promotes the binding of pro-MMP-2 to MT1-MMP, by which it is subsequently activated [16]. The distinct role played by MT1-MMP and MT3-MMP in joint destruction is further supported by their relative over-expression in RA synovium as compared with MT2-MMP (MMP-15) and MT4-MMP (MMP-17) [17].
Of note, recent data emphasized that activation and destruction in RA uses similar pathways as observed in malignant diseases [18]. The metastasis-associated protein S100A4, which promotes the progression of cancer by regulating remodelling of the extracellular matrix, upregulated MMP-3 mRNA and protein in RASFs. Furthermore, expression of MMP-1, MMP-9 and MMP-13 mRNA was induced by S100A4.
In addition to MMPs, RASF-produced cathepsins contribute significantly to the degrading processes in the rheumatoid joint. The production of cathepsin K appears to be the main contribution of RASFs to bone degradation. However, cathepsin L, which degrades collagen types I, II, IX and XI and proteoglycans, was also found to be expressed in RASFs [19]. Cathepsin L mediated cartilage destruction in the SCID mouse model for RA could be reduced by specific ribozymes inhibiting the translation of cathepsin L mRNA into active protein [20].
Cartilage degradation by RASFs is reduced by the MMP-antagonizing family the TIMPs. Gene transfer experiments demonstrate that TIMP-1 specifically inhibits the synovial fibroblast mediated destruction of cartilage in the SCID mouse model. The same effect was shown for TIMP-3, which in addition to MMPs inhibits TNF-α-converting enzyme (a molecule that activates TNF-α synthesis in RA synovium) [21]. Novel metalloproteinase inhibitors such as RECK (reversion inducing cysteine-rich protein with Kazal motifs) have been added to the family of these protective molecules during recent years [22].
Facilitators of osteoclastogenesis
Analysis of the pathways that result in bone degradation has been initiated by numerous research groups. Receptor activator of NF-κB (RANK), a member of the TNF receptor family, primarily initiates a bone-degrading pathway and maturation of osteoclasts via its binding partner RANK ligand (RANKL). In rheumatoid synovium, RANKL was found to be strongly expressed at sites of bone erosion, and RASFs were shown to be part of this RANK/RANKL interaction system by actively producing RANKL [23]. Accordingly, RASFs expressing higher levels of RANKL induced a higher number of osteoclast-like cells than did RASFs expressing only low levels of RANKL [24]. Various disease-modifying anti-rheumatic drugs (DMARDs) used in the treatment of RA act on these pathogenetic pathways. It was shown that methotrexate, sulfasalazine and infliximab inhibit the expression of RANKL in RASFs in a dose-dependent manner, and increase the synthesis of osteoprotegerin, a RANKL antagonist, in RASF supernatants [25].
Proinflammatory cytokines, including TNF-α exert a distinct role in bone remodeling via RASFs. Osteoclastogenesis is stimulated by TNF-α and IL-1-dependent upregulation of bone morphogenetic protein-2 and -6 in these cells [26].
In summary, because of the potency of RASFs in producing cartilage-degrading and bone-degrading enzymes and their stimulatory effect on osteoclasts, RASFs must be regarded as the main effector cells for the activation and stimulation of osteoclasts, which leads to the primary problem in RA: joint destruction.
Induction of the activated phenotype of RASFs
RASFs differ considerably from SFs from healthy joints. This activated phenotype comprises morphological properties and changes in long-term growth and apoptosis, as well as altered response to various stimuli. Furthermore, RASFs attach to cartilage and bone, and drive the pathophysiology of RA by producing matrix-degrading enzymes and proinflammatory cytokines. A main focus of RASF research is to characterize further this RASF phenotype and to find the triggers that initially induce the aggressive behaviour of RASFs.
Cytokines and growth factors
The primary extracellular stimulus for fibroblasts is fibroblast growth factor (FGF). RASFs not only proliferate in response to FGF but they are also part of an autocrine loop by producing FGF themselves, triggering further fibroblast growth. The effect of one of the FGF isoforms, namely FGF-2, is not only restricted to the proliferation of RASFs but is also involved in bone destruction by supporting the maturation of osteoclasts [27]. Another common growth factor for fibroblasts, transforming growth factor (TGF)-β, can be found in RA synovial tissue. Its synthesis requires co-operation with synovial macrophages. TGF-β stimulates collagen production of RASFs when injected directly into the joint cavity, and enhanced the growth of RASFs by modulating the activity of phosphatidylinositol 3-kinase and Akt. In addition, TGF-β can induce IL-6 and VEGF production in RASFs via activation of the transcription factor NF-κB [28]. The stimulatory effect of TGF-β appears to be partly dependent on RASF-matrix interactions, because attachment of RASFs to laminin-111 facilitated TGF-β-induced activation of the p38-mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinse and SMAD2 pathways, resulting in upregulation of MMP-3 [29]. However, TGF-β is a pluripotent molecule. This is illustrated by its ability to suppress articular inflammation by downregulating the chemoattractive and proinflammatory molecule RANTES in RASFs on one hand [30] and to enhance destructive effects by stimulating the synthesis of IL-1 and MMP-1 on the other.
The effects of growth factors on RASFs are further enhanced by recently discovered 'associated' stimulatory molecules such as TNF-like weak inducer of apoptosis (TWEAK), and by the crosstalk between different cytokine-dependent signalling cascades. The importance of TWEAK on synovial pathophysiology was illustrated by the inhibition of TWEAK, which resulted in downregulation of numerous proinflammatory effector molecules such as the chemokines MIP-1β (CCL-4), lymphotactin (XCL-1), CXCL-10, MCP-1 (CCL-2) and RANTES (CCL-5) in articular mesenchymal cells, including fibroblasts [31].
Apart from growth factors, proinflammatory cytokines are the major stimuli of changes in metabolism of RASFs. Release of proinflammatory mediators by RASFs is induced not only by the dominant and well known cytokines in RA pathophysiology (such as TNF-α, IL-6 and IL-1) but also by more recently discovered novel members of this family (IL-17, IL-18, IL-20 and IL-1F8) [32]. Both IL-17 and IL-18 increased the synthesis of the proangiogenic factor VEGF, and IL-20 promoted enhanced chemotaxis via MCP-1 and IL-8 [33-35]. In some cases, only the receptor but not the respective cytokine could be detected in RA synovium. For example, the receptor for IL-21 was found to be expressed on RASFs, but mRNA for IL-21 was neither detectable in RA synovium nor inducible by key proinflammatory cytokines and growth factors such as IL-1, TNF, platelet-derived growth factor and TGF. Accordingly, IL-21 protein was also undetectable in synovial fluid from RA patients [36].
Platelet-derived growth factor, of which numerous isoforms have been shown to be expressed in RA synovium, is a strong stimulator of synovial growth, and is also one of the few cytokines for which a direct proto-oncogene-triggered activation of synovial cells could be demonstrated [37]. The recently licensed platelet-derived growth factor receptor tyrosine kinase inhibitor imatinib was able to downregulate proliferation of RASFs [38,39].
Intracellular signalling
Numerous nuclear transcription factors are involved in the activation of cells in the proliferating rheumatoid synovium. Effector molecule synthesis via the NF-κB pathway is one of the key elements. NF-κB is a dimeric transcription factor that is classically formed by a p50 and a p65 subunit, but also more rare combinations with other subunits (for instance p52) occur. In general, activation of this transcription factor requires upstream proinflammatory stimuli, such as TNF-α. A molecule that blocks NF-κB activation in inactive cells is the inhibitor of NF-κB (IκB). Upon cell stimulation, IκB becomes degraded after phosphorylation by two kinases: IκB kinase-1 and IκB kinase-2. IκB as well as IκB kinase-1 and IκB kinase-2 are present in RA synovium. IκB kinase-2 dominant negative mutant cell populations were found to be resistant to TNF-α-triggered nuclear translocation of NF-κB, and accordingly the presence of IκB kinase-2 was required for cytokine synthesis (IL-6 and IL-8) via NF-κB in RASFs [40]. In contrast, lack of IκB kinase-1 did not modulate this pathway.
Further downstream, NF-κB-dependent processes in RASFs include the transcription of a broad group of target genes, comprising transcription factors such as Ets and ESE, antiapoptotic genes such as BIRC-3, and the FLIP-like gene GG2-1, as well as pro-inflammatory cytokines and effector molecules such as COXs, which catalyze the formation of prostaglandins. Interestingly, recent data showed that B by prostaglandins such as prostaglandin-E2 can inhibit NF-κ stimulating IκB in RASFs [41]. Also, the transcription factor peroxisome proliferation-activated receptor-γ induces a negative regulation of NF-κB followed by a downregulation of numerous cytokines, including TNF-α, IL-1, IL-6 and IL-8, and of MMPs such as MMP-1 and MMP-3.
MAPKs are intracellular effector molecules that are embedded in a signalling cascade that is highly active in RASFs. The MAPK group comprises three members: c-jun amino-terminal kinase, extracellular signal-regulated kinase and p38. Stimulation of MAPK pathways result in the expression of Jun and Fos proteins, which form homodimers and heterdimers to build up the transcription factor activator protein-1. Activator protein-1 DNA binding activity is high in RASFs and leads to expression of a variety of proinflammatory cytokines and MMPs [42,43]. A number of kinases upstream from the MAPK and operative in RASFs have also been identified in recent years. Among them are MAPK kinase-4, c-jun amino-terminal kinase regulating MAPK kinase-7, as well as MAPK kinase-3 and MAPK kinase-6 [44,45]. The majority of these kinases are induced by IL-1 and TNF.
The therapeutic potential of downregulating MAPK pathways was illustrated by the inhibition of IL-6, IL-8, MMP-1 and MMP-3 production in RASFs after application of a specific p38 MAPK inhibitor [46]. In particular, the α and γ isoforms of the p38 MAPK [47,48] appear to modulate several proinflammatory pathways in RASFs and have therefore already been targeted in clinical trials. However, serious adverse effects have prevented further development of therapeutic p38 inhibitors thus far.
Because of increased interest in RASFs as targets of novel therapeutic approaches, analysis of activating and inhibiting mechanisms has entered the focus of numerous research laboratories worldwide. The molecular mechanisms that are the basis of the effects of DMARDs probably affect the doubling time of the RASF population and disrupt pro-inflammatory cytokine loops [49,50]. For instance, leflunomide was found to act on RASFs by downregulating MAPK signalling pathways, resulting in inhibition of the production of MMP-1, MMP-3 and MMP-13, and in increased synthesis of IL-1 receptor antagonist [51,52].
Hypoxia and angiogenetic factors
Every tissue or compartment within a given organism requires an adequate supply with oxygen and nutrients, especially when growing over an extended period of time. In the rheumatoid joint, one of the dominant features is the synovial hyperplasia, which consists mainly of an increase in cell numbers, especially in the synovial lining layer. To facilitate this growth, angiogenesis is mandatory not only for synovial activation but also for subsequent joint destruction [53]. One of the triggering factors appears to be articular hypoxia, which stimulates both synthesis of proangiogenic factors but also the expression of chemotactic factors, MMPs such as MMP-1 and MMP-3 (combined with a downregulation of TIMP-1 in RASFs), and osteoclastogenic factors such as inhibitor of differentiation [54].
Of the key proangiogenic factors, VEGF mRNA and protein as well as its respective receptor flk-1 (KDR) are present in rheumatoid synovium. Co-cultivation of RASFs with inflammatory cells resulted in enhanced VEGF synthesis and neovascularization. Conversely, virus-mediated over-expression of the soluble VEGF receptor sFlt-1 was able to suppress disease activity in collagen-induced arthritis.
Proinflammatory cytokines can upregulate proangiogenic factors in RASFs. This angiogenesis-inducing effect of cytokines could be shown for angiopoietin-1, which is present in RA synovium and is upregulated in RASFs by TNF-α at the mRNA and protein levels. Expression of angiopoietin-1 and angiopoietin-2 in RASFs is directly linked to their respective endothelium-specific tyrosine kinase receptors Tie-1 and Tie-2 [55]. Antiangiogenic molecules such as members of the thrombospondin family (for example, thrombospondin-2) can inhibit RASF-dependent vascularization, because thrombospondin-2 transduced RASFs were able to inhibit local vascularization and inflammation in the SCID mouse model [56].
Cellular interactions
Distinct cellular interactions are required to support further the long-term growth of rheumatoid synovium. Some of them are directly linked to hypoxic conditions, such as the hypoxia-induced upregulation of intercellular adhesion molecule (ICAM)-1 in RASFs, which resulted in adhesion of RASFs to adjacent lymphocytes [57]. Inteferon-γ, IL-1, and TNF-α can further upregulate the expression of ICAM-1, facilitating the interaction of RASFs with T lymphocytes through ligation of ICAM-1 to its binding partner leukocyte function associated antigen-1. Subsequently, ICAM-1-positive RASFs in vivo are surrounded by leukocyte function associated antigen-1-positive T lymphocytes, which are associated with an up-regulation of IL-1 expression by RASFs. Apart from ICAM-1, numerous adhesion molecules and ligands are known to mediate RASF-dependent pannus formation. An important example of the effects of such cell-to-cell interaction is the bidirectional interaction between the adhesion molecule VCAM-1, its ligand very late activation antigen-4, and the matrix component connective segment-1. VCAM-1 is found in RASFs invading articular cartilage and in the synovial microvasculature. Proinflammatory cytokines such as TNF-α, IL-1β, and IL-18 can induce VCAM-1 expression on RASFs.
VCAM-1 binds to the membrane-bound lymphocyte surface antigen, very late activation antigen-4, which also serves as ligand for connective segment-1, an alternatively spliced form of fibronectin. This interaction results in direct multidirectional interaction between RASF, matrix, and lymphocytes.
The interaction of RASFs with matrix proteins can modulate their adherence properties. For example, interactions of RASFs with integral membrane proteins such as cadherin-11 in the lining layer contribute significantly to pannus formation in rheumatoid synovium [58]. Cadherin-11 stimulates the formation of tissue-like sheets and lining-like structures in vitro, and is expressed in a tissue-restricted pattern. Interrupting such an interaction can be used therapeutically; for example, invasion of RASFs into bovine cartilage could be inhibited by antibodies to α4 integrins. Of note, other matrix-RASF interactions such as the interaction of very late activation antigen-5 with fibronectin were able to protect RASF from apoptosis [59].
Proto-oncogenes and tumour suppressors
In untreated RA, the granulation tissue that forms within the synovium (pannus) consists, to a significant degree, of RASFs and grows steadily. Based on the histological finding that fewer than 3% of RASFs undergo apoptosis [60], numerous researchers have addressed the dysbalance of proapoptotic and antiapoptotic factors (for example, proto-oncogenes versus apoptosis-inducing molecules and tumour-suppressors) in these cells. This work has led to accumulation of a body of evidence that the long-term growth and reduced apoptosis of RASFs is based on the upregulation of early response genes and proto-oncogenes, such as egr-1, c-fos, myc and ras. The oncogene ras is predominantly expressed in the synovial lining layer associated with expression of the proteolytic enzyme cathepsin L at sites of invasive growth. Conversely, gene transfer based inhibition experiments of double-negative ras, raf and myc mutants ameliorated inflammation and reduced bone destruction in adjuvant arthritis as well as cartilage destruction and RASF invasiveness in the SCID mouse model of RA [61].
Consistent with the over-expression of proto-oncogenes is the lack or deficiency of tumour-suppressor genes such as p53 and its proapoptotic effector molecule p53-upregulated modulator of apoptosis (PUMA), maspin, and phosphatase and tensin homolog (PTEN) [62].
In RA, lack of PTEN expression, but not mutations within the gene encoding PTEN, participate in the long-term persistence of activated RASFs in the synovial lining at sites of destruction [63]. IκB/NF-κB interactions and negative regulation of other nuclear factors such as Akt (protein kinase B) are dependent on PTEN [64]. Furthermore, it can be speculated that the lack of the tyrosine kinase PTEN in aggressive RASFs contributes to the imbalance of tyrosine kinases and phosphatases in this disease. Interestingly, PTEN has been demonstrated to be downregulated by TGF-β, which at least partly could be responsible for the diminished levels of PTEN in RA [63].
Resistance to apoptosis
A major factor contributing to synovial growth is the resistance of RASF against apoptosis, which can be linked to distinct anti-apoptotic molecules such as FLICE inhibitory protein (FLIP) and sentrin (SUMO-1). FLIP exerts its anti-apoptotic effect via inhibition of the apoptosis-triggering intra-cellular enzyme caspase 8 [65]. Accordingly, antagonizing FLIP by antisense oligonucleotides sensitizes RASFs to Fas-mediated apoptosis [66]. Sentrin interferes with Fas-induced as well as TNF-induced apoptosis, and was shown to be highly expressed in RASFs at sites of synovial invasion [67].
Other potent inhibitors of apoptosis that have been found to be upregulated in RASFs are members of the Bcl family, such as Bcl-2 and Mcl-1. Bcl-2 inhibits one of the terminal steps of apoptosis. Recent data indicate that the regulation of Bcl-2 expression is related to the autocrine activation of IL-15 receptors by SF-derived antiapoptotic IL-15 [68]. Mcl-1 has been shown to counteract the effects of the proapoptotic intracellular factors Bax, Bak and Bim [69]. The expression of Mcl-1 could be induced by treatment with TNF-α or IL-1β in RASFs and knockdown of Mcl-1 by small-interfering-RNA induced apoptosis in RASFs as well as in synovial macrophages [70].
Targeting proapoptotic members of the TNF family, such as TNF-related apoptosis-inducing ligand (TRAIL), revealed that the sensitivity of RASFs to apoptosis might be a highly selective, histone deacetylase-dependent process [71]. Only agonistic antibodies against TRAIL-R2 (DR5), but not TRAIL-R1 (DR4), were able to induce apoptosis in cultured RASFs. Moreover, intra-articular over-expression of TRAIL by viral gene transfer exerted a comparable effect in a rabbit arthritis model. Similarly, nontoxic doses of the proteasome inhibitor lactacystin can also induce RASF apoptosis and might be a strategy for future RASF-targeted therapeutic approaches. Lactastatin induced cytosolic accumulation of p53 and enhanced apoptosis via TRAIL-R2 (DR5) [72]. Also, the osteoprotective molecule osteoprotegerin influences the apoptotic rate of RASFs because OPG reduced the rate of apoptosis of RASFs after incubation with TRAIL, an effect that could be antagonized by anti-osteoprotegerin monoclonal antibodies [73].
In summary, the activated phenotype of RASFs, which is the basis for the long-term growth of the rheumatoid synovium, is characterized by a substantial dysbalance of proapoptotic versus antiapoptotic pathways in favour of the latter.
Cytokine independent pathways of activation
Even though all of the above-mentioned cytokines and growth factors have been shown to play pivotal roles in the activation of RASFs, attempts to induce an aggressive phenotype in normal SFs by incubating them with these stimulating factors have not been successful. Therefore, the search for triggering factors was extended to cytokine independent pathways. Experimental models provided evidence that oncogene-derived or virus-derived gene sequences incorporated into the DNA of RASFs could be such triggers. Retroviral L1 elements expressed in RASFs were found to induce upregulation of intracellular kinases, including p38δ, which is a specific isoform of the p38 MAPKs [74]. Since it was shown that L1 is induced by DNA demethylation, a novel search for epigenetic modifications in RASF has been conducted. Epigenetic modifications are mediated by methylation, deacetylation, ubiquitination, phosphorylation and microRNA. Based on the observation that endogenous retroviral sequences such as L1 can induce specific signalling molecules, including p38δ and galectin-3 binding protein [75], the galectin-3 system has been explored. Galectin-3, which has been shown to be elevated in tumours and metastasis, induces angiogenesis and inhibits apoptosis [76]. Levels of galectin-3 are high in sera and synovial fluid of RA patients and correlate with C-reactive protein levels. Also, galectin-3 binding protein was found to be elevated in joints of RA patients as compared with patient with osteoarthritis and healthy control individuals. Interestingly, high levels of galectin-3 binding protein were associated with high levels of cartilage oligomeric matrix protein, which is a marker of synovial cell activation and joint destruction [77].
These data point to a cytokine-independent pathway operating in the pathogenesis of RA, which could also explain why the disease cannot yet be cured and disease activity recurs after cessation of therapy, such as with anti-TNF blockade.
Conclusion
In addition to the examples outlined above illustrating that targeting RASFs and RASF-dependent effector molecules could yield new effective therapeutic options, it has been demonstrated that the RASF can potentially be used as a drug carrier. In a study conducted in the SCID mouse model of RA, in which the implanted metabolically active cartilage-invading RASFs had taken up methotrexate-albumin conjugates intracellularly before implantation [78], methotrexate and methotrexate-albumin conjugates both inhibited cartilage invasion and degradation with comparable efficiency [79].
All the various studies conducted to address the specific properties of RASFs underline the important role played by these cells in the pathogenesis of RA (Fig. 1). The working hypothesis of a cytokine-independent activation of destructive and inflammatory pathways, which was recently also connected to epigenetic modifications including demethylation [74,75] and hyperacetylation [71], might explain the relatively high number of nonresponders receiving treatment with DMARDs and the failure of these agents to block joint destruction completely. Studies addressing the role played by epigenetic modifications in these cells could shed light on the development of the altered phenotype found in RASFs.
In the years to come, particular attention must be given to the search for therapies specifically designed to inhibit the joint destructive potential of RASFs. Gene transfer experiments with the inhibitors of MMPs, TIMP-1 and TIMP-3 yielded promising results. Over-expression of TIMPs led to a diminution of the destructive potential of RASFs. Molecules such as TIMP-3 that influence the end product of the complex signalling cascades that lead to joint destruction might be novel targets, which may allow us to block both cytokine-dependent and cytokine-independent pathways of joint destruction in RA.
Abbreviations
CCL = C-C motif ligand; COX = cyclo-oxygenase; CXCL = C-X-C motif ligand; DMARD = disease-modifying antirheumatic drug; FGF = fibroblast growth factor; FLIP = FLICE inhibitory protein; ICAM = intercellular adhesion molecule; IκB = inhibitor of nuclear factor-κB; IL = interleukin; MAPK = mitogen-activated protein kinase; MCP = monocyte chemoattractant protein; MIP = macrophage inflammatory protein; MMP = matrix metalloproteinase; MT = membrane-type; NF-κB = nuclear factor-κB; PTEN = phosphatase and tensin homolog; RA = rheumatoid arthritis; RANK(L) = receptor activator of nuclear factor-κB (ligand); RANTES = regulated on activation, normal T-cell expressed and secreted; RASF = rheumatoid arthritis synovial fibroblast; SCID = severe combined immunodeficient; SF = synovial fibroblast; TGF = transforming growth factor; TLR = Toll-like receptor; TNF = tumour necrosis factor; TRAIL = TNF-related apoptosis-inducing ligand; TWEAK = TNF-like weak inducer of apoptosis; VCAM = vascular cell adhesion molecule; VEGF = vascular endothelial growth factor.
Competing interests
The authors declare that they have no competing interests.
Note
This review is part of a series on Cells of the synovium in rheumatoid arthritis edited by Gary Firestein.
Other articles in this series can be found at http://arthritis-research.com/articles/review-series.asp?series=ar_Cells
Acknowledgments
Acknowledgements
The work was supported by the German Research Society (DFG # Mu 1383/3, 1383/10 and 1383/13, Pa 689/2, Pa 689/3 and SFB 492-TP19) and the Swiss National Science Foundation (SNF 3200BO-103691).
Contributor Information
Ulf Müller-Ladner, Email: ladner@kerckhoff-klinik.de.
Steffen Gay, Email: steffen.gay@usz.ch.
Oliver Distler, Email: oliver.distler@usz.ch.
Thomas Pap, Email: thomas.pap@uni-muenster.de.
References
- Fassbender HG. Histomorphological basis of articular cartilage destruction in rheumatoid arthritis. Coll Relat Res. 1983;3:141–155. doi: 10.1016/s0174-173x(83)80040-5. [DOI] [PubMed] [Google Scholar]
- Gay S, Gay RE, Koopman WJ. Molecular and cellular mechanisms of joint destruction in rheumatoid arthritis: two cellular mechanisms explain joint destruction? Ann Rheum Dis. 1993;52(Suppl 1):S39–S47. doi: 10.1136/ard.52.Suppl_1.S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Ladner U, Kriegsmann J, Franklin BN, Matsumoto S, Geiler T, Gay RE, Gay S. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am J Pathol. 1996;149:1607–1615. [PMC free article] [PubMed] [Google Scholar]
- Ospelt C, Kyburz D, Pierer M, Seibl R, Kurowska M, Distler O, Neidhart M, Muller-Ladner U, Pap T, Gay RE, Gay S. Toll-like receptors in rheumatoid arthritis joint destruction mediated by two distinct pathways. Ann Rheum Dis. 2004;63(Suppl 2):ii90–ii91. doi: 10.1136/ard.2004.028324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho ML, Ju JH, Kim HR, Oh HJ, Kang CM, Jhun JY, Lee SY, Park MK, Min JK, Park SH, et al. Toll-like receptor 2 ligand mediates the upregulation of angiogenic factor, vascular endothelial growth factor and interleukin-8/CXCL8 in human rheumatoid synovial fibroblasts. Immunol Lett. 2007;108:121–128. doi: 10.1016/j.imlet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Jung YO, Cho ML, Kang CM, Jhun JY, Park JS, Oh HJ, Min JK, Park SH, Kim HY. Toll-like receptor 2 and 4 combination engagement upregulate IL-15 synergistically in human rheumatoid synovial fibroblasts. Immunol Lett. 2007;109:21–27. doi: 10.1016/j.imlet.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Pierer M, Rethage J, Seibl R, Lauener R, Brentano F, Wagner U, Hantzschel H, Michel BA, Gay RE, Gay S, Kyburz D. Chemokine secretion of rheumatoid arthritis synovial fibroblasts stimulated by Toll-like receptor 2 ligands. J Immunol. 2004;172:1256–1265. doi: 10.4049/jimmunol.172.2.1256. [DOI] [PubMed] [Google Scholar]
- Brentano F, Schorr O, Gay RE, Gay S, Kyburz D. RNA released from necrotic synovial fluid cells activates rheumatoid arthritis synovial fibroblasts via Toll-like receptor 3. Arthritis Rheum. 2005;52:2656–2665. doi: 10.1002/art.21273. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Canas I, Juarranz Y, Santiago B, Arranz A, Martinez C, Galindo M, Paya M, Gomariz RP, Pablos JL. VIP down-regulates TLR4 expression and TLR4-mediated chemokine production in human rheumatoid synovial fibroblasts. Rheumatology (Oxford) 2006;45:527–532. doi: 10.1093/rheumatology/kei219. [DOI] [PubMed] [Google Scholar]
- Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151:317–322. [PMC free article] [PubMed] [Google Scholar]
- Ruth JH, Haas CS, Park CC, Amin MA, Martinez RJ, Haines GK, 3rd, Shahrara S, Campbell PL, Koch AE. CXCL16-mediated cell recruitment to rheumatoid arthritis synovial tissue and murine lymph nodes is dependent upon the MAPK pathway. Arthritis Rheum. 2006;54:765–778. doi: 10.1002/art.21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyuda M, Kasama T, Isozaki T, Matsunawa MM, Yajima N, Miyaoka H, Uchida H, Kameoka Y, Ide H, Adachi M. Activated leucocytes express and secrete macrophage inflammatory protein-1alpha upon interaction with synovial fibroblasts of rheumatoid arthritis via a beta2-integrin/ICAM-1 mechanism. Rheumatology (Oxford) 2003;42:1390–1397. doi: 10.1093/rheumatology/keg391. [DOI] [PubMed] [Google Scholar]
- Kojima F, Naraba H, Sasaki Y, Beppu M, Aoki H, Kawai S. Prostaglandin E2 is an enhancer of interleukin-1beta-induced expression of membrane-associated prostaglandin E synthase in rheumatoid synovial fibroblasts. Arthritis Rheum. 2003;48:2819–2828. doi: 10.1002/art.11261. [DOI] [PubMed] [Google Scholar]
- Kusunoki N, Yamazaki R, Kawai S. Induction of apoptosis in rheumatoid synovial fibroblasts by celecoxib, but not by other selective cyclooxygenase 2 inhibitors. Arthritis Rheum. 2002;46:3159–3167. doi: 10.1002/art.10692. [DOI] [PubMed] [Google Scholar]
- Rutkauskaite E, Zacharias W, Schedel J, Muller-Ladner U, Mawrin C, Seemayer CA, Alexander D, Gay RE, Aicher WK, Michel BA, et al. Ribozymes that inhibit the production of matrix metallo-proteinase 1 reduce the invasiveness of rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2004;50:1448–1456. doi: 10.1002/art.20186. [DOI] [PubMed] [Google Scholar]
- Honda S, Migita K, Hirai Y, Origuchi T, Yamasaki S, Kamachi M, Shibatomi K, Fukuda T, Kita M, Hida A, et al. Expression of membrane-type 1 matrix metalloproteinase in rheumatoid synovial cells. Clin Exp Immunol. 2001;126:131–136. doi: 10.1046/j.1365-2249.2001.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pap T, Shigeyama Y, Kuchen S, Fernihough JK, Simmen B, Gay RE, Billingham M, Gay S. Differential expression pattern of membrane-type matrix metalloproteinases in rheumatoid arthritis. Arthritis Rheum. 2000;43:1226–1232. doi: 10.1002/1529-0131(200006)43:6<1226::AID-ANR5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Senolt L, Grigorian M, Lukanidin E, Simmen B, Michel BA, Pavelka K, Gay RE, Gay S, Neidhart M. S100A4 is expressed at site of invasion in rheumatoid arthritis synovium and modulates production of matrix metalloproteinases. Ann Rheum Dis. 2006;65:1645–1648. doi: 10.1136/ard.2005.047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabandt A, Aicher WK, Gay RE, Sukhatme VP, Nilson-Hamilton M, Hamilton RT, McGhee JR, Fassbender HG, Gay S. Expression of the collagenolytic and Ras-induced cysteine proteinase cathepsin L and proliferation-associated oncogenes in synovial cells of MRL/I mice and patients with rheumatoid arthritis. Matrix. 1990;10:349–361. doi: 10.1016/s0934-8832(11)80142-3. [DOI] [PubMed] [Google Scholar]
- Schedel J, Seemayer CA, Pap T, Neidhart M, Kuchen S, Michel BA, Gay RE, Muller-Ladner U, Gay S, Zacharias W. Targeting cathepsin L (CL) by specific ribozymes decreases CL protein synthesis and cartilage destruction in rheumatoid arthritis. Gene Ther. 2004;11:1040–1047. doi: 10.1038/sj.gt.3302265. [DOI] [PubMed] [Google Scholar]
- van der Laan WH, Quax PH, Seemayer CA, Huisman LG, Pieterman EJ, Grimbergen JM, Verheijen JH, Breedveld FC, Gay RE, Gay S, et al. Cartilage degradation and invasion by rheumatoid synovial fibroblasts is inhibited by gene transfer of TIMP-1 and TIMP-3. Gene Ther. 2003;10:234–242. doi: 10.1038/sj.gt.3301871. [DOI] [PubMed] [Google Scholar]
- van Lent PL, Span PN, Sloetjes AW, Radstake TR, van Lieshout AW, Heuvel JJ, Sweep CG, van den Berg WB. Expression and localisation of the new metalloproteinase inhibitor RECK (reversion inducing cysteine-rich protein with Kazal motifs) in inflamed synovial membranes of patients with rheumatoid arthritis. Ann Rheum Dis. 2005;64:368–374. doi: 10.1136/ard.2004.027870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Jeon HS, Song EK, Han MK, Park SI, Lee SI, Yun HJ, Kim JR, Kim JS, Lee YC, et al. CD40 ligation of rheumatoid synovial fibroblasts regulates RANKL-mediated osteoclastogenesis: evidence of NF-kappaB-dependent, CD40-mediated bone destruction in rheumatoid arthritis. Arthritis Rheum. 2006;54:1747–1758. doi: 10.1002/art.21873. [DOI] [PubMed] [Google Scholar]
- Shigeyama Y, Pap T, Kunzler P, Simmen BR, Gay RE, Gay S. Expression of osteoclast differentiation factor in rheumatoid arthritis. Arthritis Rheum. 2000;43:2523–2530. doi: 10.1002/1529-0131(200011)43:11<2523::AID-ANR20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Lee CK, Lee EY, Chung SM, Mun SH, Yoo B, Moon HB. Effects of disease-modifying antirheumatic drugs and antiinflammatory cytokines on human osteoclastogenesis through interaction with receptor activator of nuclear factor kappaB, osteoprotegerin, and receptor activator of nuclear factor kappaB ligand. Arthritis Rheum. 2004;50:3831–3843. doi: 10.1002/art.20637. [DOI] [PubMed] [Google Scholar]
- Lories RJ, Derese I, Ceuppens JL, Luyten FP. Bone morphogenetic proteins 2 and 6, expressed in arthritic synovium, are regulated by proinflammatory cytokines and differentially modulate fibroblast-like synoviocyte apoptosis. Arthritis Rheum. 2003;48:2807–2818. doi: 10.1002/art.11389. [DOI] [PubMed] [Google Scholar]
- Nakano K, Okada Y, Saito K, Tanaka Y. Induction of RANKL expression and osteoclast maturation by the binding of fibroblast growth factor 2 to heparan sulfate proteoglycan on rheumatoid synovial fibroblasts. Arthritis Rheum. 2004;50:2450–2458. doi: 10.1002/art.20367. [DOI] [PubMed] [Google Scholar]
- Sakuma M, Hatsushika K, Koyama K, Katoh R, Ando T, Watanabe Y, Wako M, Kanzaki M, Takano S, Sugiyama H, et al. TGF-{beta} type I receptor kinase inhibitor down-regulates rheumatoid synoviocytes and prevents the arthritis induced by type II collagen antibody. Int Immunol. 2007;19:117–126. doi: 10.1093/intimm/dxl128. [DOI] [PubMed] [Google Scholar]
- Hoberg M, Rudert M, Pap T, Klein G, Gay S, Aicher WK. Attachment to laminin-111 facilitates transforming growth factor-β induced expression of matrix metalloproteinase-3 in synovial fibroblasts. Ann Rheum Dis. 2007;66:446–451. doi: 10.1136/ard.2006.060228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho ML, Min SY, Chang SH, Kim KW, Heo SB, Lee SH, Park SH, Cho CS, Kim HY. Transforming growth factor beta 1(TGF-beta1) down-regulates TNFalpha-induced RANTES production in rheumatoid synovial fibroblasts through NF-kappaB-mediated transcriptional repression. Immunol Lett. 2006;105:159–166. doi: 10.1016/j.imlet.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Perper SJ, Browning B, Burkly LC, Weng S, Gao C, Giza K, Su L, Tarilonte L, Crowell T, Rajman L, et al. TWEAK is a novel arthritogenic mediator. J Immunol. 2006;177:2610–2620. doi: 10.4049/jimmunol.177.4.2610. [DOI] [PubMed] [Google Scholar]
- Magne D, Palmer G, Barton JL, Mezin F, Talabot-Ayer D, Bas S, Duffy T, Noger M, Guerne PA, Nicklin MJ, Gabay C. The new IL-1 family member IL-1F8 stimulates production of inflammatory mediators by synovial fibroblasts and articular chondrocytes. Arthritis Res Ther. 2006;8:R80. doi: 10.1186/ar1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho ML, Jung YO, Moon YM, Min SY, Yoon CH, Lee SH, Park SH, Cho CS, Jue DM, Kim HY. Interleukin-18 induces the production of vascular endothelial growth factor (VEGF) in rheumatoid arthritis synovial fibroblasts via AP-1-dependent pathways. Immunol Lett. 2006;103:159–166. doi: 10.1016/j.imlet.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Honorati MC, Neri S, Cattini L, Facchini A. Interleukin-17, a regulator of angiogenic factor release by synovial fibroblasts. Osteoarthritis Cartilage. 2006;14:345–352. doi: 10.1016/j.joca.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Hsu YH, Li HH, Hsieh MY, Liu MF, Huang KY, Chin LS, Chen PC, Cheng HH, Chang MS. Function of interleukin-20 as a proinflammatory molecule in rheumatoid and experimental arthritis. Arthritis Rheum. 2006;54:2722–2733. doi: 10.1002/art.22039. [DOI] [PubMed] [Google Scholar]
- Jungel A, Distler JH, Kurowska-Stolarska M, Seemayer CA, Seibl R, Forster A, Michel BA, Gay RE, Emmrich F, Gay S, Distler O. Expression of interleukin-21 receptor, but not interleukin-21, in synovial fibroblasts and synovial macrophages of patients with rheumatoid arthritis. Arthritis Rheum. 2004;50:1468–1476. doi: 10.1002/art.20218. [DOI] [PubMed] [Google Scholar]
- Pohlers D, Huber R, Ukena B, Kinne RW. Expression of platelet-derived growth factors C and D in the synovial membrane of patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 2006;54:788–794. doi: 10.1002/art.21670. [DOI] [PubMed] [Google Scholar]
- Kameda H, Ishigami H, Suzuki M, Abe T, Takeuchi T. Imatinib mesylate inhibits proliferation of rheumatoid synovial fibroblast-like cells and phosphorylation of Gab adapter proteins activated by platelet-derived growth factor. Clin Exp Immunol. 2006;144:335–341. doi: 10.1111/j.1365-2249.2006.03067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler C, Joutsiniemi S, Lindstedt KA, Juutilainen T, Kovanen PT, Eklund KK. Imatinib mesylate inhibits platelet derived growth factor stimulated proliferation of rheumatoid synovial fibroblasts. Biochem Biophys Res Commun. 2006;347:31–35. doi: 10.1016/j.bbrc.2006.06.052. [DOI] [PubMed] [Google Scholar]
- Andreakos E, Smith C, Kiriakidis S, Monaco C, de Martin R, Brennan FM, Paleolog E, Feldmann M, Foxwell BM. Heterogeneous requirement of IkappaB kinase 2 for inflammatory cytokine and matrix metalloproteinase production in rheumatoid arthritis: implications for therapy. Arthritis Rheum. 2003;48:1901–1912. doi: 10.1002/art.11044. [DOI] [PubMed] [Google Scholar]
- Gomez PF, Pillinger MH, Attur M, Marjanovic N, Dave M, Park J, Bingham CO, 3rd, Al-Mussawir H, Abramson SB. Resolution of inflammation: prostaglandin E2 dissociates nuclear trafficking of individual NF-kappaB subunits (p65, p50) in stimulated rheumatoid synovial fibroblasts. J Immunol. 2005;175:6924–6930. doi: 10.4049/jimmunol.175.10.6924. [DOI] [PubMed] [Google Scholar]
- Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Asahara H, Fujisawa K, Kobata T, Hasunuma T, Maeda T, Asanuma M, Ogawa N, Inoue H, Sumida T, Nishioka K. Direct evidence of high DNA binding activity of transcription factor AP-1 in rheumatoid arthritis synovium. Arthritis Rheum. 1997;40:912–918. doi: 10.1002/art.1780400520. [DOI] [PubMed] [Google Scholar]
- Bradley K, Scatizzi JC, Fiore S, Shamiyeh E, Koch AE, Firestein GS, Gorges LL, Kuntsman K, Pope RM, Moore TL, et al. Retinoblastoma suppression of matrix metalloproteinase 1, but not interleukin-6, through a p38-dependent pathway in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2004;50:78–87. doi: 10.1002/art.11482. [DOI] [PubMed] [Google Scholar]
- Inoue T, Hammaker D, Boyle DL, Firestein GS. Regulation of JNK by MKK-7 in fibroblast-like synoviocytes. Arthritis Rheum. 2006;54:2127–2135. doi: 10.1002/art.21919. [DOI] [PubMed] [Google Scholar]
- Westra J, Limburg PC, de Boer P, van Rijswijk MH. Effects of RWJ 6 a p38 mitogen activated protein kinase (MAPK) inhibitor, on the production of inflammatory mediators by rheumatoid synovial fibroblasts. Ann Rheum Dis. 7657;63:1453–1459. doi: 10.1136/ard.2003.013011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb A, Tohidast-Akrad M, Cetin E, Axmann R, Smolen J, Schett G. Differential tissue expression and activation of p38 MAPK alpha, beta, gamma, and delta isoforms in rheumatoid arthritis. Arthritis Rheum. 2006;54:2745–2756. doi: 10.1002/art.22080. [DOI] [PubMed] [Google Scholar]
- Kunisch E, Gandesiri M, Fuhrmann R, Roth A, Winter R, Kinne RW. Predominant activation of MAP kinases and pro-destructive/pro-inflammatory features by TNF-alpha in early-passage, rheumatoid arthritis and osteoarthritis synovial fibroblasts via tumor necrosis factor receptor-1: failure of p38 inhibition to suppress matrix metalloproteinase-1 in rheumatoid arthritis. Ann Rheum Dis. 2007;66:1043–1051. doi: 10.1136/ard.2006.062521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lories RJ, Derese I, De Bari C, Luyten FP. In vitro growth rate of fibroblast-like synovial cells is reduced by methotrexate treatment. Ann Rheum Dis. 2003;62:568–571. doi: 10.1136/ard.62.6.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Carus ME, Balsa A, Benito-Miguel M, Perez de Ayala C, Martin-Mola E. IL-15 and the initiation of cell contact-dependent synovial fibroblast-T lymphocyte cross-talk in rheumatoid arthritis: effect of methotrexate. J Immunol. 2004;173:1463–1476. doi: 10.4049/jimmunol.173.2.1463. [DOI] [PubMed] [Google Scholar]
- Migita K, Miyashita T, Ishibashi H, Maeda Y, Nakamura M, Yatsuhashi H, Ida H, Kawakami A, Aoyagi T, Kawabe Y, Eguchi K. Suppressive effect of leflunomide metabolite (A77 1726) on metalloproteinase production in IL-1beta stimulated rheumatoid synovial fibroblasts. Clin Exp Immunol. 2004;137:612–616. doi: 10.1111/j.1365-2249.2004.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G, Burger D, Mezin F, Magne D, Gabay C, Dayer JM, Guerne PA. The active metabolite of leflunomide, A77 increases the production of IL-1 receptor antagonist in human synovial fibroblasts and articular chondrocytes. Arthritis Res Ther. 2004;6:R181–R189. doi: 10.1186/ar1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler JH, Wenger RH, Gassmann M, Kurowska M, Hirth A, Gay S, Distler O. Physiologic responses to hypoxia and implications for hypoxia-inducible factors in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2004;50:10–23. doi: 10.1002/art.11425. [DOI] [PubMed] [Google Scholar]
- Kurowska-Stolarska M, Distler J, Pap T, Jüngel A, Michel B, Simmen B, Gay R, Maslinski W, Gay S, Distler O. The inhibitor of differentiation-2 (Id-2) induced by hypoxia promotes bone degradation in rheumatoid arthritis (RA) [abstract] Arthritis Rheum. 2004;50:S655. [Google Scholar]
- Takahara K, Iioka T, Furukawa K, Uchida T, Nakashima M, Tsukazaki T, Shindo H. Autocrine/paracrine role of the angiopoietin-1 and -2/Tie2 system in cell proliferation and chemotaxis of cultured fibroblastic synoviocytes in rheumatoid arthritis. Hum Pathol. 2004;35:150–158. doi: 10.1016/j.humpath.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Park YW, Kang YM, Butterfield J, Detmar M, Goronzy JJ, Weyand CM. Thrombospondin 2 functions as an endogenous regulator of angiogenesis and inflammation in rheumatoid arthritis. Am J Pathol. 2004;165:2087–2098. doi: 10.1016/S0002-9440(10)63259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MK, Kim JS, Park BH, Kim JR, Hwang BY, Lee HY, Song EK, Yoo WH. NF-kappaB-dependent lymphocyte hyperadhesiveness to synovial fibroblasts by hypoxia and reoxygenation: potential role in rheumatoid arthritis. J Leukoc Biol. 2003;73:525–529. doi: 10.1189/jlb.0502256. [DOI] [PubMed] [Google Scholar]
- Kiener HP, Lee DM, Agarwal SK, Brenner MB. Cadherin-11 induces rheumatoid arthritis fibroblast-like synoviocytes to form lining layers in vitro. Am J Pathol. 2006;168:1486–1499. doi: 10.2353/ajpath.2006.050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa A, Miura Y, Saura R, Mitani M, Ishikawa H, Hashiramoto A, Yoshiya S, Shiozawa S, Kurosaka M. Anchorage on fibronectin via VLA-5 (alpha5beta1 integrin) protects rheumatoid synovial cells from Fas-induced apoptosis. Ann Rheum Dis. 2006;65:721–727. doi: 10.1136/ard.2005.041707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Muller-Ladner U, Gay RE, Nishioka K, Gay S. Ultra-structural demonstration of apoptosis, Fas and Bcl-2 expression of rheumatoid synovial fibroblasts. J Rheumatol. 1996;23:1345–1352. [PubMed] [Google Scholar]
- Pap T, Nawrath M, Heinrich J, Bosse M, Baier A, Hummel KM, Petrow P, Kuchen S, Michel BA, Gay RE, et al. Cooperation of Ras- and c-Myc-dependent pathways in regulating the growth and invasiveness of synovial fibroblasts in rheumatoid arthritis. Arthritis Rheum. 2004;50:2794–2802. doi: 10.1002/art.20461. [DOI] [PubMed] [Google Scholar]
- Cha HS, Rosengren S, Boyle DL, Firestein GS. PUMA regulation and proapoptotic effects in fibroblast-like synoviocytes. Arthritis Rheum. 2006;54:587–592. doi: 10.1002/art.21631. [DOI] [PubMed] [Google Scholar]
- Pap T, Franz JK, Hummel KM, Jeisy E, Gay R, Gay S. Activation of synovial fibroblasts in rheumatoid arthritis: lack of expression of the tumour suppressor PTEN at sites of invasive growth and destruction. Arthritis Res. 2000;2:59–64. doi: 10.1186/ar69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor AM, Berger S, Narendran A, Keystone EC. Inhibition of protein geranylgeranylation induces apoptosis in synovial fibroblasts. Arthritis Res Ther. 2006;8:R94. doi: 10.1186/ar1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T. The caspase-8 modulator c-FLIP. Crit Rev Immunol. 2005;25:31–58. doi: 10.1615/CritRevImmunol.v25.i1.30. [DOI] [PubMed] [Google Scholar]
- Palao G, Santiago B, Galindo M, Paya M, Ramirez JC, Pablos JL. Down-regulation of FLIP sensitizes rheumatoid synovial fibroblasts to Fas-mediated apoptosis. Arthritis Rheum. 2004;50:2803–2810. doi: 10.1002/art.20453. [DOI] [PubMed] [Google Scholar]
- Franz JK, Pap T, Hummel KM, Nawrath M, Aicher WK, Shigeyama Y, Muller-Ladner U, Gay RE, Gay S. Expression of sentrin, a novel antiapoptotic molecule, at sites of synovial invasion in rheumatoid arthritis. Arthritis Rheum. 2000;43:599–607. doi: 10.1002/1529-0131(200003)43:3<599::AID-ANR17>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Kurowska M, Rudnicka W, Kontny E, Janicka I, Chorazy M, Kowalczewski J, Ziolkowska M, Ferrari-Lacraz S, Strom TB, Maslinski W. Fibroblast-like synoviocytes from rheumatoid arthritis patients express functional IL-15 receptor complex: endogenous IL-15 in autocrine fashion enhances cell proliferation and expression of Bcl-x(L) and Bcl-2. J Immunol. 2002;169:1760–1767. doi: 10.4049/jimmunol.169.4.1760. [DOI] [PubMed] [Google Scholar]
- Liu H, Eksarko P, Temkin V, Haines GK, 3rd, Perlman H, Koch AE, Thimmapaya B, Pope RM. Mcl-1 is essential for the survival of synovial fibroblasts in rheumatoid arthritis. J Immunol. 2005;175:8337–8345. doi: 10.4049/jimmunol.175.12.8337. [DOI] [PubMed] [Google Scholar]
- Liu H, Huang Q, Shi B, Eksarko P, Temkin V, Pope RM. Regulation of Mcl-1 expression in rheumatoid arthritis synovial macrophages. Arthritis Rheum. 2006;54:3174–3181. doi: 10.1002/art.22132. [DOI] [PubMed] [Google Scholar]
- Jungel A, Baresova V, Ospelt C, Simmen BR, Michel BA, Gay RE, Gay S, Seemayer CA, Neidhart M. Trichostatin A sensitises rheumatoid arthritis synovial fibroblasts for TRAIL-induced apoptosis. Ann Rheum Dis. 2006;65:910–912. doi: 10.1136/ard.2005.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Wang J, Liu C, Grizzle WE, Yu S, Zhang S, Barnes S, Koopman WJ, Mountz JD, Kimberly RP, Zhang HG. Cleavage of p53-vimentin complex enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis of rheumatoid arthritis synovial fibroblasts. Am J Pathol. 2005;167:705–719. doi: 10.1016/S0002-9440(10)62045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Kawakami A, Nakashima T, Yamasaki S, Tamai M, Tanaka F, Kamachi M, Ida H, Migita K, Origuchi T, et al. Osteoprotegerin (OPG) acts as an endogenous decoy receptor in tumour necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis of fibroblast-like synovial cells. Clin Exp Immunol. 2004;137:430–436. doi: 10.1111/j.1365-2249.2004.02534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchen S, Seemayer C, Rethage J, von Knoch R, Kuenzler P, Michel B, Gay R, Gay S, Neidhart M. The L1 retroelement-related p40 protein induces p38d MAP kinase. Autoimmunity. 2004;37:57–65. doi: 10.1080/08916930310001637977. [DOI] [PubMed] [Google Scholar]
- Neidhart M, Rethage J, Kuchen S, Kunzler P, Crowl RM, Billingham ME, Gay RE, Gay S. Retrotransposable L1 elements expressed in rheumatoid arthritis synovial tissue: association with genomic DNA hypomethylation and influence on gene expression. Arthritis Rheum. 2000;43:2634–2647. doi: 10.1002/1529-0131(200012)43:12<2634::AID-ANR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Liu FT. Galectins: novel anti-inflammatory drug targets. Expert Opin Ther Targets. 2002;6:461–468. doi: 10.1517/14728222.6.4.461. [DOI] [PubMed] [Google Scholar]
- Ohshima S, Kuchen S, Seemayer CA, Kyburz D, Hirt A, Klinzing S, Michel BA, Gay RE, Liu FT, Gay S, Neidhart M. Galectin 3 and its binding protein in rheumatoid arthritis. Arthritis Rheum. 2003;48:2788–2795. doi: 10.1002/art.11287. [DOI] [PubMed] [Google Scholar]
- Wunder A, Muller-Ladner U, Stelzer EH, Funk J, Neumann E, Stehle G, Pap T, Sinn H, Gay S, Fiehn C. Albumin-based drug delivery as novel therapeutic approach for rheumatoid arthritis. J Immunol. 2003;170:4793–4801. doi: 10.4049/jimmunol.170.9.4793. [DOI] [PubMed] [Google Scholar]
- Fiehn C, Neumann E, Wunder A, Krienke S, Gay S, Muller-Ladner U. Methotrexate (MTX) and albumin coupled with MTX (MTX-HSA) suppress synovial fibroblast invasion and cartilage degradation in vivo. Ann Rheum Dis. 2004;63:884–886. doi: 10.1136/ard.2003.013748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Ladner U, Gay S. The role of fibroblast-like synoviocytes in rheumatoid arthritis. In: Firestein GS, Panayi GS, Wollheim FA, editor. Rheumatoid Arthritis. 2. Oxford: Oxford University Press; 2006. pp. 107–121. [Google Scholar]