Abstract
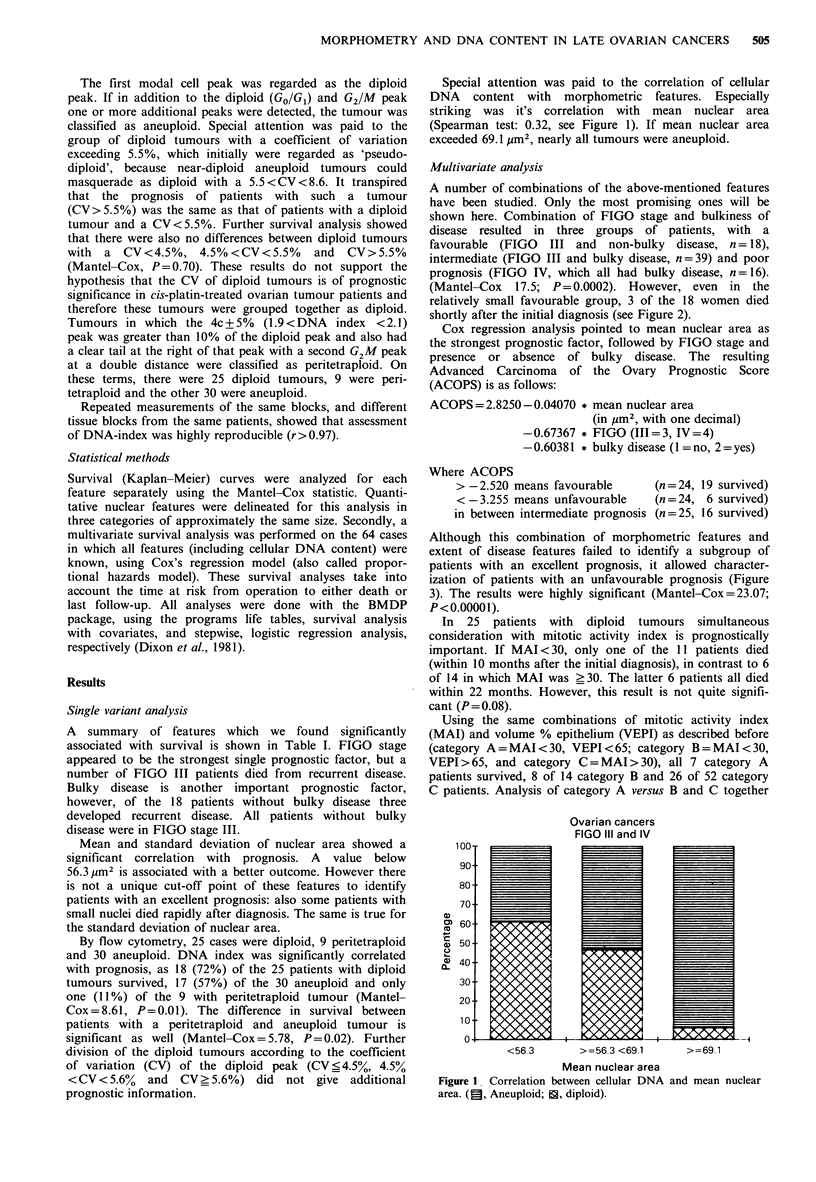
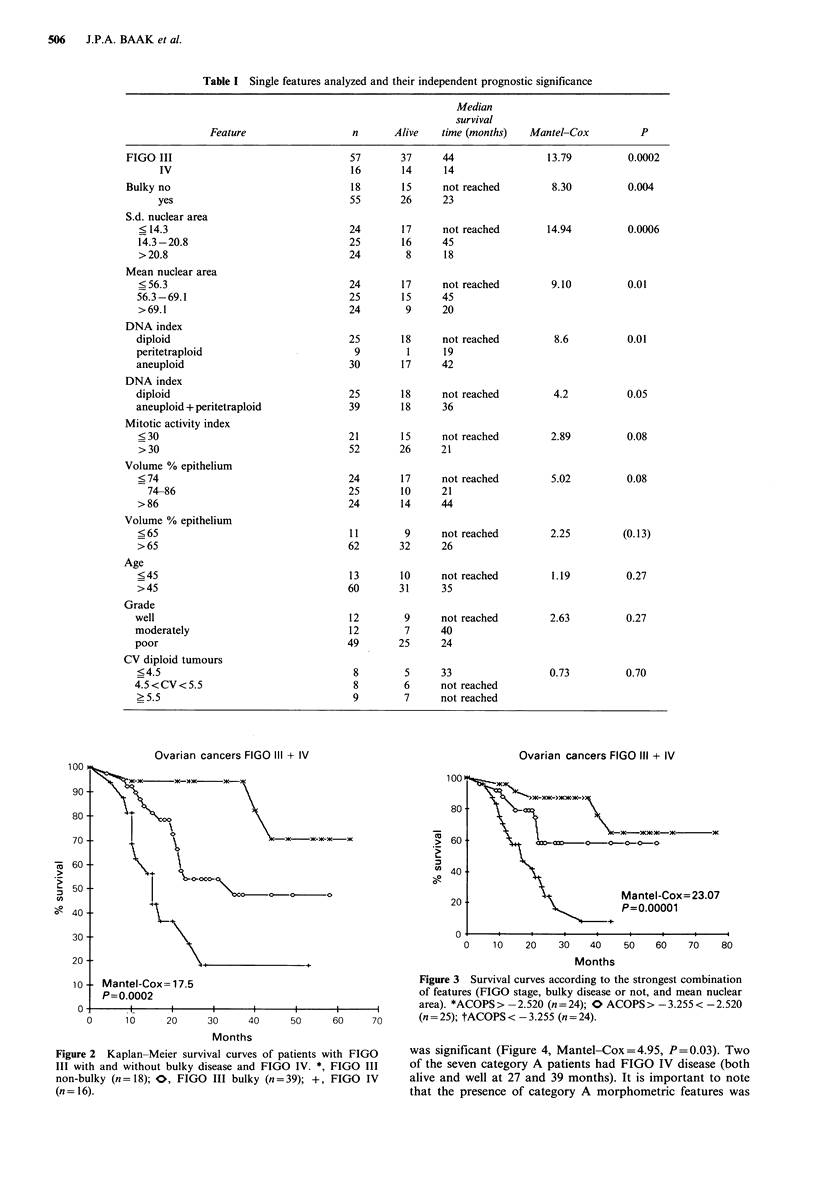
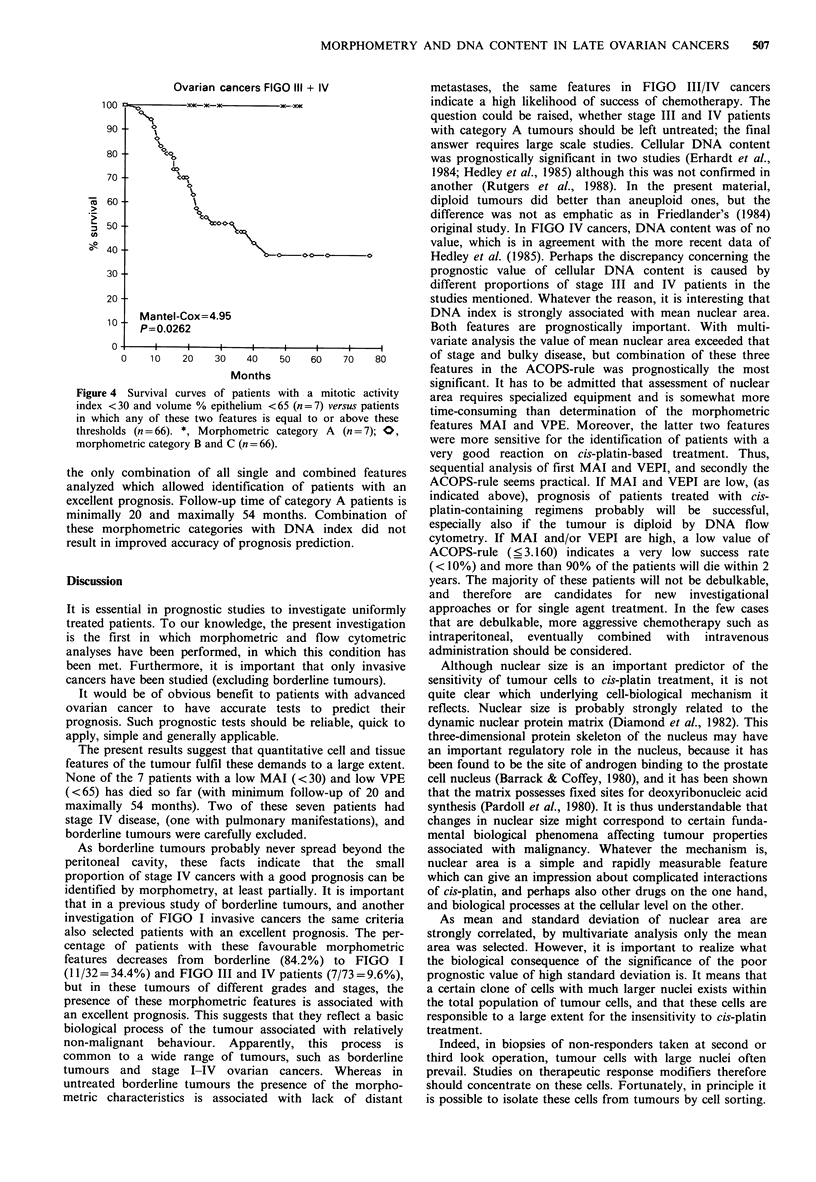
In 73 CAP-1 treated stage III and IV ovarian cancers, the prognostic significance of morphometric features and cellular DNA content has been evaluated in comparison with histologic type, grade of differentiation and a number of clinical characteristics. Borderline tumours were excluded from the study. Median follow-up was 44 months, median survival time 36 months. Single features associated with prognosis were (in order of decreasing significance according to single variate analysis): FIGO stage (P = 0.0002), bulky disease (P = 0.004), standard deviation and mean of nuclear area (P = 0.0006 and P = 0.01), cellular DNA content (P = 0.01), mitotic activity index (P = 0.08) and volume percentage epithelium (P = 0.13, not quite significant). Tumours with a mean nuclear area greater than 70 micron2 (which occurred in 35% of the cases) were nearly all aneuploid. Multivariate analysis showed that the statistically most significant prognostic combination of features consisted of mean nuclear area, presence or absence of bulky disease and FIGO stage (in order of decreasing importance) (Mantel-Cox = 23.07, P less than 0.00001). A low value for the multivariate function of this combination of features was associated with a poor prognosis within 24 months, a high value with a favourable outcome. Another favourable combination of features appeared to be diploid cellular DNA content and a low mitotic activity index (11 patients, one died). However, even with the prognostically most favourable combination of these features, several patients died. Of all combinations of features investigated, only two were associated with an excellent prognosis (low mitotic activity index and low volume percentage epithelium). Cancers of 7 patients (10%) displayed such features, and none of them died during the follow-up period (minimally 20 and maximally 54 months). It is concluded that morphometric and flow cytometric analysis can provide significant and objective information to predict the prognosis of cis-platin-treated advanced ovarian cancer patients.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baak J. P., Blanco A. A., Kurver P. H., Langley F. A., Boon M. E., Lindeman J., Overdiep S. H., Nieuwlaat A., Brekelmans E. Quantitation of borderline and malignant mucinous ovarian tumours. Histopathology. 1981 Jul;5(4):353–360. doi: 10.1111/j.1365-2559.1981.tb01797.x. [DOI] [PubMed] [Google Scholar]
- Baak J. P., Fox H., Langley F. A., Buckley C. H. The prognostic value of morphometry in ovarian epithelial tumors of borderline malignancy. Int J Gynecol Pathol. 1985;4(3):186–191. doi: 10.1097/00004347-198509000-00003. [DOI] [PubMed] [Google Scholar]
- Baak J. P., Wisse-Brekelmans E. C., Langley F. A., Talerman A., Delemarre J. F. Morphometric data to FIGO stage and histological type and grade for prognosis of ovarian tumours. J Clin Pathol. 1986 Dec;39(12):1340–1346. doi: 10.1136/jcp.39.12.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baak J. P., Wisse-Brekelmans E. C., Uyterlinde A. M., Schipper N. W. Evaluation of the prognostic value of morphometric features and cellular DNA content in FIGO I ovarian cancer patients. Anal Quant Cytol Histol. 1987 Aug;9(4):287–290. [PubMed] [Google Scholar]
- Barrack E. R., Coffey D. S. The specific binding of estrogens and androgens to the nuclear matrix of sex hormone responsive tissues. J Biol Chem. 1980 Aug 10;255(15):7265–7275. [PubMed] [Google Scholar]
- Diamond D. A., Berry S. J., Jewett H. J., Eggleston J. C., Coffey D. S. A new method to assess metastatic potential of human prostate cancer: relative nuclear roundness. J Urol. 1982 Oct;128(4):729–734. doi: 10.1016/s0022-5347(17)53158-4. [DOI] [PubMed] [Google Scholar]
- Erhardt K., Auer G., Björkholm E., Forsslund G., Moberger B., Sifverswärd C., Wicksell G., Zetterberg A. Prognostic significance of nuclear DNA content in serous ovarian tumors. Cancer Res. 1984 May;44(5):2198–2202. [PubMed] [Google Scholar]
- Friedlander M. L., Hedley D. W., Taylor I. W., Russell P., Coates A. S., Tattersall M. H. Influence of cellular DNA content on survival in advanced ovarian cancer. Cancer Res. 1984 Jan;44(1):397–400. [PubMed] [Google Scholar]
- Griffiths C. T., Parker L. M., Fuller A. F., Jr Role of cytoreductive surgical treatment in the management of advanced ovarian cancer. Cancer Treat Rep. 1979 Feb;63(2):235–240. [PubMed] [Google Scholar]
- Hedley D. W., Friedlander M. L., Taylor I. W. Application of DNA flow cytometry to paraffin-embedded archival material for the study of aneuploidy and its clinical significance. Cytometry. 1985 Jul;6(4):327–333. doi: 10.1002/cyto.990060409. [DOI] [PubMed] [Google Scholar]
- Hedley D. W., Friedlander M. L., Taylor I. W., Rugg C. A., Musgrove E. A. Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J Histochem Cytochem. 1983 Nov;31(11):1333–1335. doi: 10.1177/31.11.6619538. [DOI] [PubMed] [Google Scholar]
- Kempson R. L. Editorial: Mitosis counting--II. Hum Pathol. 1976 Jul;7(4):482–483. doi: 10.1016/s0046-8177(76)80063-9. [DOI] [PubMed] [Google Scholar]
- Neijt J. P., ten Bokkel Huinink W. W., van der Burg M. E., van Oosterom A. T., Vriesendorp R., Kooyman C. D., van Lindert A. C., Hamerlynck J. V., van Lent M., van Houwelingen J. C. Randomised trial comparing two combination chemotherapy regimens (Hexa-CAF vs CHAP-5) in advanced ovarian carcinoma. Lancet. 1984 Sep 15;2(8403):594–600. doi: 10.1016/s0140-6736(84)90594-4. [DOI] [PubMed] [Google Scholar]
- Pardoll D. M., Vogelstein B., Coffey D. S. A fixed site of DNA replication in eucaryotic cells. Cell. 1980 Feb;19(2):527–536. doi: 10.1016/0092-8674(80)90527-9. [DOI] [PubMed] [Google Scholar]
- Piver M. S. Ovarian carcinoma. A decade of progress. Cancer. 1984 Dec 1;54(11 Suppl):2706–2715. doi: 10.1002/1097-0142(19841201)54:2+<2706::aid-cncr2820541417>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Rutgers D. H., Wils I. S., Schaap A. H., van Lindert A. C. DNA flow cytometry, histological grade, stage, and age as prognostic factors in human epithelial ovarian carcinomas. Pathol Res Pract. 1987 Apr;182(2):207–213. doi: 10.1016/s0344-0338(87)80106-1. [DOI] [PubMed] [Google Scholar]
- Schipper N. W., Smeulders A. W., Baak J. P. Quantification of epithelial volume by image processing applied to ovarian tumors. Cytometry. 1987 Jul;8(4):345–352. doi: 10.1002/cyto.990080402. [DOI] [PubMed] [Google Scholar]
- Wharton J. T., Herson J. Surgery for common epithelial tumors of the ovary. Cancer. 1981 Jul 15;48(2 Suppl):582–589. doi: 10.1002/1097-0142(19810715)48:1+<582::aid-cncr2820481323>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Wils J., Blijham G., Naus A., Belder C., Boschma F., Bron H., Ceelen T., Eekhout A., von Erp J., Geelen P. Primary or delayed debulking surgery and chemotherapy consisting of cisplatin, doxorubicin, and cyclophosphamide in stage III-IV epithelial ovarian carcinoma. J Clin Oncol. 1986 Jul;4(7):1068–1073. doi: 10.1200/JCO.1986.4.7.1068. [DOI] [PubMed] [Google Scholar]
- Zimmerman P. V., Hawson G. A., Bint M. H., Parsons P. G. Ploidy as a prognostic determinant in surgically treated lung cancer. Lancet. 1987 Sep 5;2(8558):530–533. doi: 10.1016/s0140-6736(87)92923-0. [DOI] [PubMed] [Google Scholar]


