Abstract
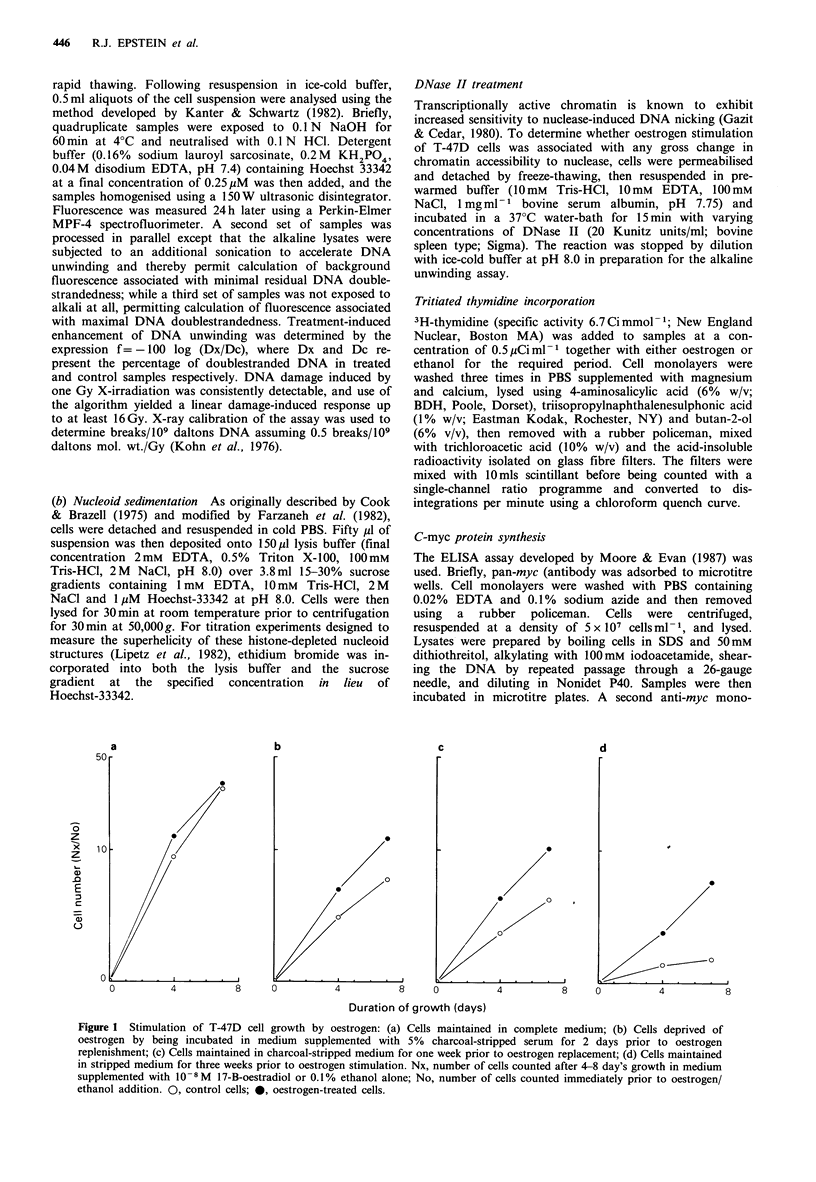
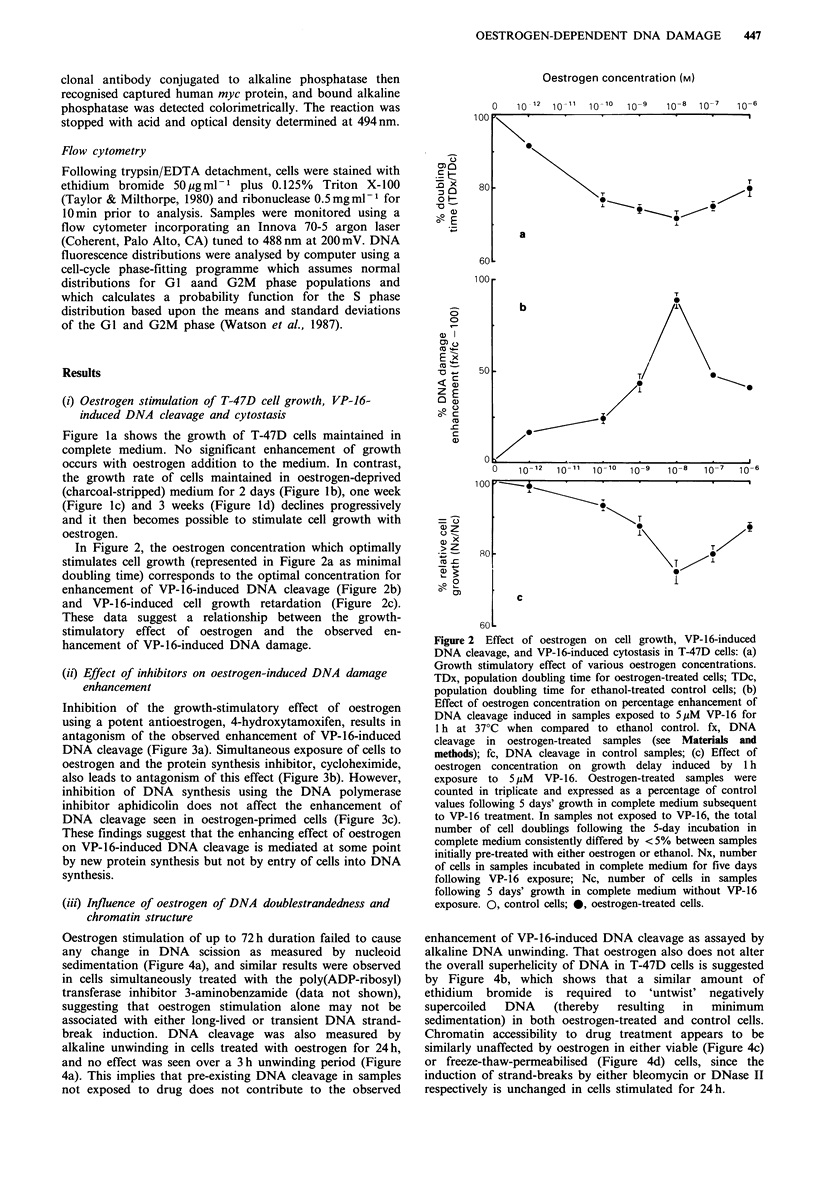
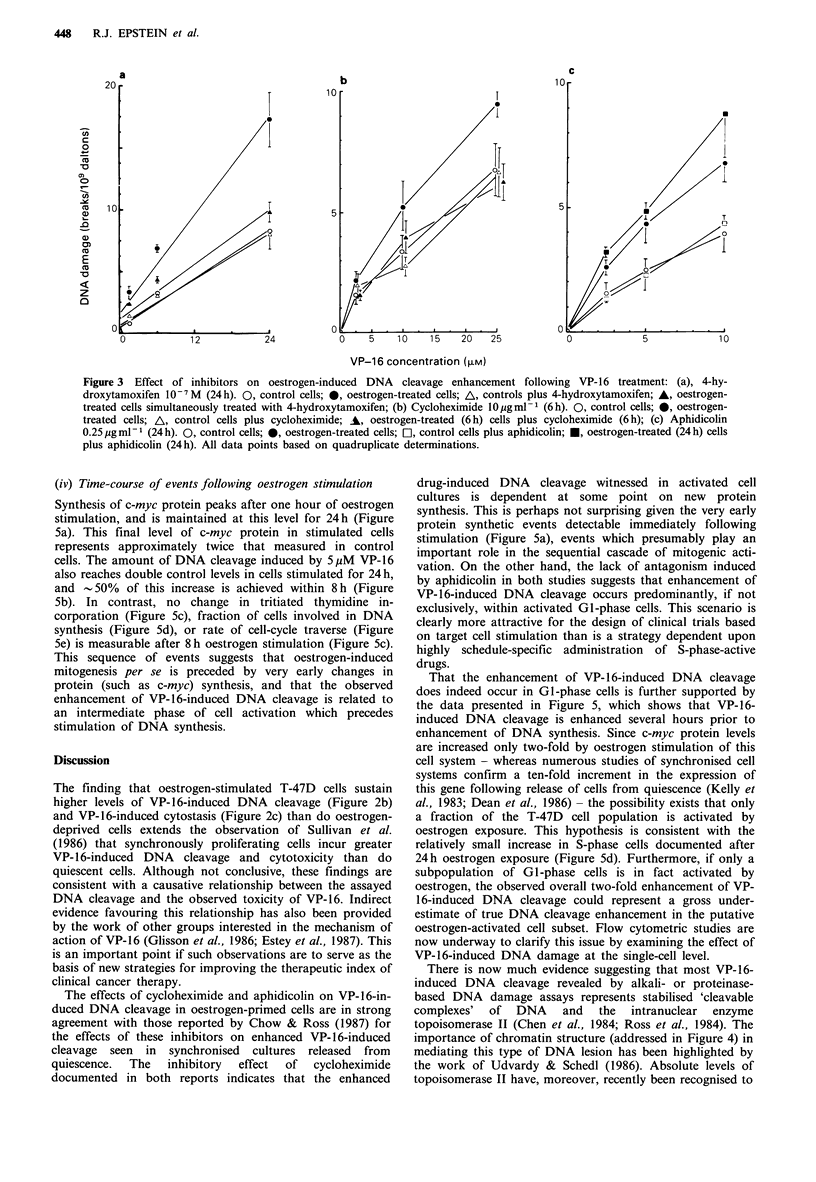
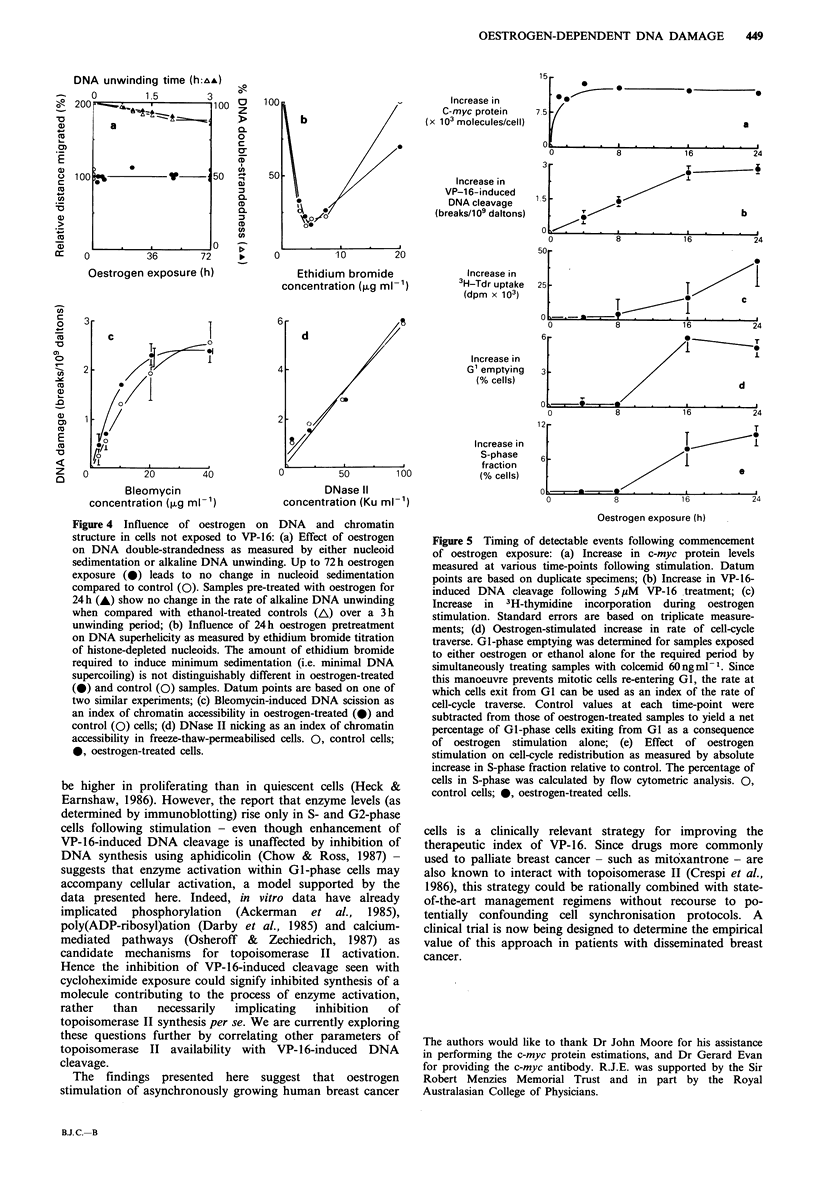
Cycling cells are recognised to be more susceptible than quiescent cells to the cytotoxic action of many commonly used cancer chemotherapeutic agents. We have found that oestrogen stimulation of T-47D human breast cancer cells is accompanied by a two-fold increase in VP-16-induced DNA cleavage as measured by alkaline DNA unwinding, and that this increase in DNA cleavage is accompanied by a corresponding enhancement of drug-induced cytostasis. The enhancement of VP-16-induced DNA cleavage seen with oestrogen exposure is antagonised both by antioestrogen treatment and by cycloheximide, an inhibitor of protein synthesis, but not by the DNA synthesis inhibitor aphidicolin. Increased c-myc protein synthesis is detectable within an hour of oestrogen exposure, while increased VP-16-induced DNA cleavage is detectable within 4h and increased DNA synthesis within 16h. Only small changes in cell-cycle distribution occur with oestrogen stimulation. In the absence of VP-16, oestrogen does not reduce DNA double-strandedness, nor does it induce changes in chromatin structure as measured by alterations in DNA superhelicity or chromatin accessibility. These findings suggest that oestrogen enhances VP-16-induced DNA damage in asynchronously growing G1-phase cells and that this enhancement may be dependent at some point upon de novo protein synthesis. Oestrogen pre-treatment of T-47D human breast cancer cells improves the therapeutic index of VP-16 without the need for cell synchronisation or highly precise drug scheduling.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman P., Glover C. V., Osheroff N. Phosphorylation of DNA topoisomerase II by casein kinase II: modulation of eukaryotic topoisomerase II activity in vitro. Proc Natl Acad Sci U S A. 1985 May;82(10):3164–3168. doi: 10.1073/pnas.82.10.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahnström G., Erixon K. Radiation induced strand breakage in DNA from mammalian cells. Strand separation in alkaline solution. Int J Radiat Biol Relat Stud Phys Chem Med. 1973 Mar;23(3):285–289. doi: 10.1080/09553007314550311. [DOI] [PubMed] [Google Scholar]
- Chen G. L., Yang L., Rowe T. C., Halligan B. D., Tewey K. M., Liu L. F. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Nov 10;259(21):13560–13566. [PubMed] [Google Scholar]
- Chow K. C., Ross W. E. Topoisomerase-specific drug sensitivity in relation to cell cycle progression. Mol Cell Biol. 1987 Sep;7(9):3119–3123. doi: 10.1128/mcb.7.9.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte P. F., Pronzato P., Rubagotti A., Alama A., Amadori D., Demicheli R., Gardin G., Gentilini P., Jacomuzzi A., Lionetto R. Conventional versus cytokinetic polychemotherapy with estrogenic recruitment in metastatic breast cancer: results of a randomized cooperative trial. J Clin Oncol. 1987 Mar;5(3):339–347. doi: 10.1200/JCO.1987.5.3.339. [DOI] [PubMed] [Google Scholar]
- Cook P. R., Brazell I. A. Supercoils in human DNA. J Cell Sci. 1975 Nov;19(2):261–279. doi: 10.1242/jcs.19.2.261. [DOI] [PubMed] [Google Scholar]
- Crespi M. D., Ivanier S. E., Genovese J., Baldi A. Mitoxantrone affects topoisomerase activities in human breast cancer cells. Biochem Biophys Res Commun. 1986 Apr 29;136(2):521–528. doi: 10.1016/0006-291x(86)90471-7. [DOI] [PubMed] [Google Scholar]
- Darby M. K., Schmitt B., Jongstra-Bilen J., Vosberg H. P. Inhibition of calf thymus type II DNA topoisomerase by poly(ADP-ribosylation). EMBO J. 1985 Aug;4(8):2129–2134. doi: 10.1002/j.1460-2075.1985.tb03903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson N. E., Lippman M. E. Stimulation of breast cancer with estrogens: how much clinical value? Eur J Cancer Clin Oncol. 1987 Jul;23(7):897–900. doi: 10.1016/0277-5379(87)90332-4. [DOI] [PubMed] [Google Scholar]
- Dean M., Levine R. A., Ran W., Kindy M. S., Sonenshein G. E., Campisi J. Regulation of c-myc transcription and mRNA abundance by serum growth factors and cell contact. J Biol Chem. 1986 Jul 15;261(20):9161–9166. [PubMed] [Google Scholar]
- Eisenhauer E. A., Bowman D. M., Pritchard K. I., Paterson A. H., Ragaz J., Plenderleith I., Geggie P. H., Maxwell I. Tamoxifen and conjugated estrogens (Premarin) followed by sequenced methotrexate and 5-FU in refractory advanced breast cancer. Cancer Treat Rep. 1984 Nov;68(11):1421–1422. [PubMed] [Google Scholar]
- Estey E. H., Silberman L., Beran M., Andersson B. S., Zwelling L. A. The interaction between nuclear topoisomerase II activity from human leukemia cells, exogenous DNA, and 4'-(9-acridinylamino)methanesulfon-m-anisidide (m-AMSA) or 4-(4,6-O-ethylidene-beta-D-glucopyranoside) (VP-16) indicates the sensitivity of the cells to the drugs. Biochem Biophys Res Commun. 1987 Apr 29;144(2):787–793. doi: 10.1016/s0006-291x(87)80033-5. [DOI] [PubMed] [Google Scholar]
- Farzaneh F., Zalin R., Brill D., Shall S. DNA strand breaks and ADP-ribosyl transferase activation during cell differentiation. Nature. 1982 Nov 25;300(5890):362–366. doi: 10.1038/300362a0. [DOI] [PubMed] [Google Scholar]
- Furr B. J., Jordan V. C. The pharmacology and clinical uses of tamoxifen. Pharmacol Ther. 1984;25(2):127–205. doi: 10.1016/0163-7258(84)90043-3. [DOI] [PubMed] [Google Scholar]
- Gazit B., Cedar H. Nuclease sensitivity of active chromatin. Nucleic Acids Res. 1980 Nov 25;8(22):5143–5155. doi: 10.1093/nar/8.22.5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisson B., Gupta R., Smallwood-Kentro S., Ross W. Characterization of acquired epipodophyllotoxin resistance in a Chinese hamster ovary cell line: loss of drug-stimulated DNA cleavage activity. Cancer Res. 1986 Apr;46(4 Pt 2):1934–1938. [PubMed] [Google Scholar]
- Heck M. M., Earnshaw W. C. Topoisomerase II: A specific marker for cell proliferation. J Cell Biol. 1986 Dec;103(6 Pt 2):2569–2581. doi: 10.1083/jcb.103.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter P. M., Schwartz H. S. A fluorescence enhancement assay for cellular DNA damage. Mol Pharmacol. 1982 Jul;22(1):145–151. [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kuo M. T. Preferential damage of active chromatin by bleomycin. Cancer Res. 1981 Jun;41(6):2439–2443. [PubMed] [Google Scholar]
- Latt S. A., Stetten G. Spectral studies on 33258 Hoechst and related bisbenzimidazole dyes useful for fluorescent detection of deoxyribonucleic acid synthesis. J Histochem Cytochem. 1976 Jan;24(1):24–33. doi: 10.1177/24.1.943439. [DOI] [PubMed] [Google Scholar]
- Leonessa F., Coialbu T., Toma S. Different techniques for drug cytotoxicity evaluation on MCF-7 human breast cancer cell line. Anticancer Res. 1986 Nov-Dec;6(6):1291–1296. [PubMed] [Google Scholar]
- Lipetz P. D., Galsky A. G., Stephens R. E. Relationship of DNA tertiary and quaternary structure to carcinogenic processes. Adv Cancer Res. 1982;36:165–210. doi: 10.1016/s0065-230x(08)60425-x. [DOI] [PubMed] [Google Scholar]
- Lippman M. E., Cassidy J., Wesley M., Young R. C. A randomized attempt to increase the efficacy of cytotoxic chemotherapy in metastatic breast cancer by hormonal synchronization. J Clin Oncol. 1984 Jan;2(1):28–36. doi: 10.1200/JCO.1984.2.1.28. [DOI] [PubMed] [Google Scholar]
- Osheroff N., Zechiedrich E. L. Calcium-promoted DNA cleavage by eukaryotic topoisomerase II: trapping the covalent enzyme-DNA complex in an active form. Biochemistry. 1987 Jul 14;26(14):4303–4309. doi: 10.1021/bi00388a018. [DOI] [PubMed] [Google Scholar]
- Paridaens R., Van der Wijst J. B., Julien J. P., Clarysse A., Ferrazzi E., Rotmensz N., Heuson J. C. Aminoglutethimide and estrogenic stimulation before chemotherapy for treatment of advanced breast cancer. Preliminary results of a phase II study conducted by the E.O.R.T.C. Breast Cancer Cooperative Group. J Steroid Biochem. 1985 Dec;23(6B):1181–1183. doi: 10.1016/0022-4731(85)90041-x. [DOI] [PubMed] [Google Scholar]
- Reddel R. R., Murphy L. C., Sutherland R. L. Factors affecting the sensitivity of T-47D human breast cancer cells to tamoxifen. Cancer Res. 1984 Jun;44(6):2398–2405. [PubMed] [Google Scholar]
- Ross W., Rowe T., Glisson B., Yalowich J., Liu L. Role of topoisomerase II in mediating epipodophyllotoxin-induced DNA cleavage. Cancer Res. 1984 Dec;44(12 Pt 1):5857–5860. [PubMed] [Google Scholar]
- Smith P. J., Anderson C. O., Watson J. V. Predominant role for DNA damage in etoposide-induced cytotoxicity and cell cycle perturbation in human SV40-transformed fibroblasts. Cancer Res. 1986 Nov;46(11):5641–5645. [PubMed] [Google Scholar]
- Sullivan D. M., Glisson B. S., Hodges P. K., Smallwood-Kentro S., Ross W. E. Proliferation dependence of topoisomerase II mediated drug action. Biochemistry. 1986 Apr 22;25(8):2248–2256. doi: 10.1021/bi00356a060. [DOI] [PubMed] [Google Scholar]
- Taylor I. W., Milthorpe B. K. An evaluation of DNA fluorochromes, staining techniques, and analysis for flow cytometry. I. Unperturbed cell populations. J Histochem Cytochem. 1980 Nov;28(11):1224–1232. doi: 10.1177/28.11.6159392. [DOI] [PubMed] [Google Scholar]
- Udvardy A., Schedl P., Sander M., Hsieh T. S. Topoisomerase II cleavage in chromatin. J Mol Biol. 1986 Sep 20;191(2):231–246. doi: 10.1016/0022-2836(86)90260-3. [DOI] [PubMed] [Google Scholar]
- Watson J. V., Chambers S. H., Smith P. J. A pragmatic approach to the analysis of DNA histograms with a definable G1 peak. Cytometry. 1987 Jan;8(1):1–8. doi: 10.1002/cyto.990080101. [DOI] [PubMed] [Google Scholar]
- Weichselbaum R. R., Hellman S., Piro A. J., Nove J. J., Little J. B. Proliferation kinetics of a human breast cancer line in vitro following treatment with 17beta-estradiol and 1-beta-D-arabinofuranosylcytosine. Cancer Res. 1978 Aug;38(8):2339–2342. [PubMed] [Google Scholar]
- Zwelling L. A., Kerrigan D., Lippman M. E. Protein-associated intercalator-induced DNA scission is enhanced by estrogen stimulation in human breast cancer cells. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6182–6186. doi: 10.1073/pnas.80.20.6182. [DOI] [PMC free article] [PubMed] [Google Scholar]


