Abstract
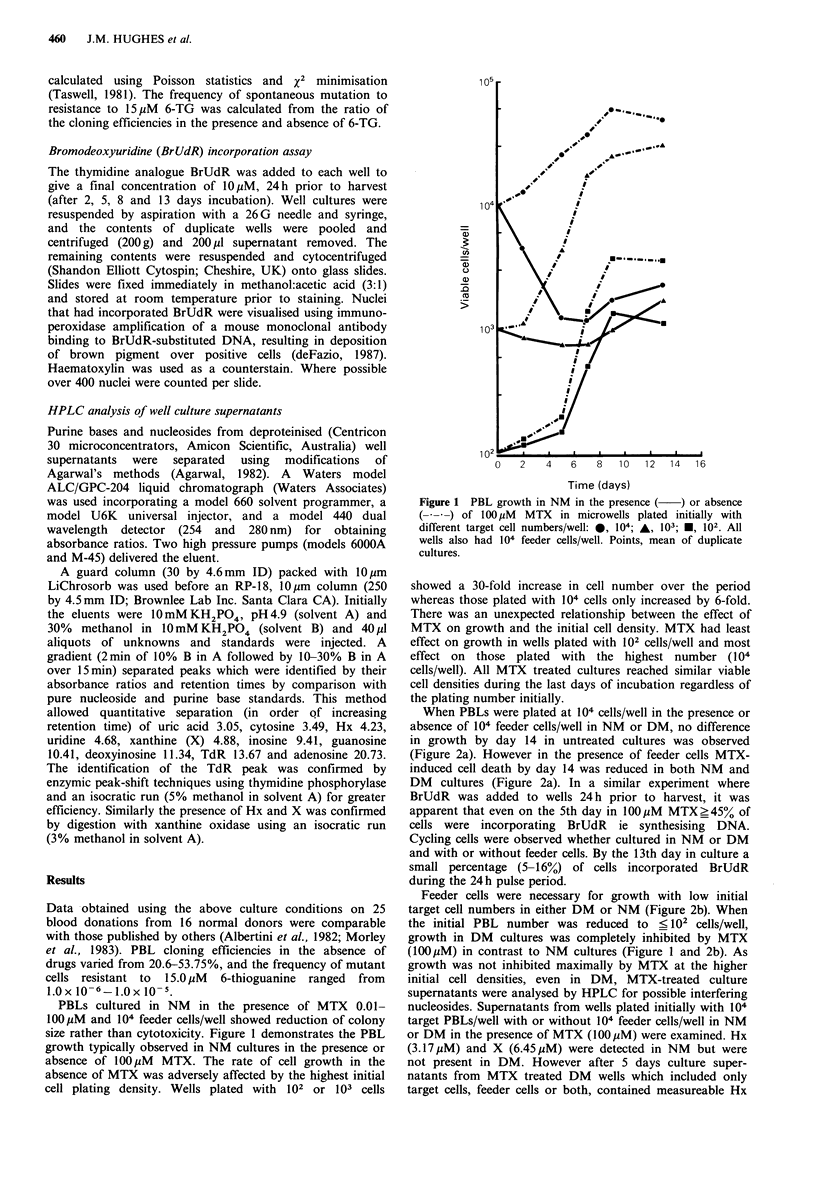
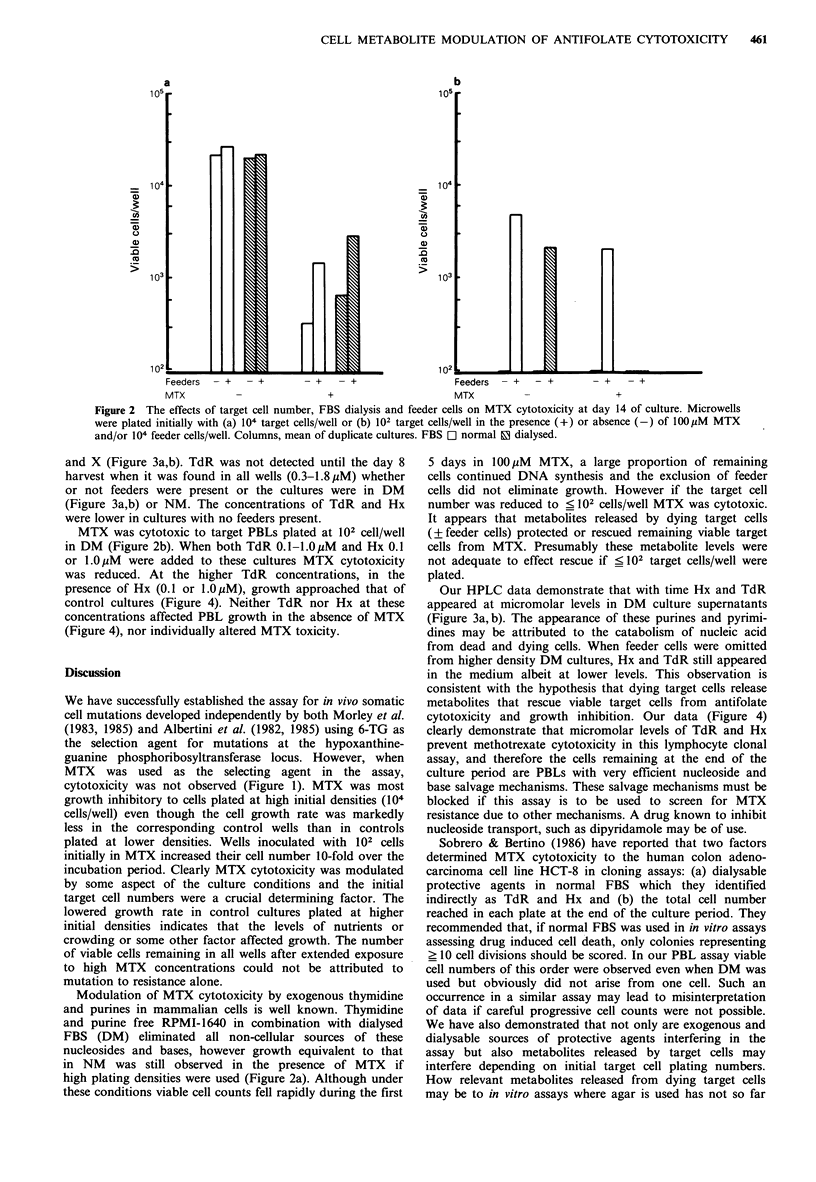
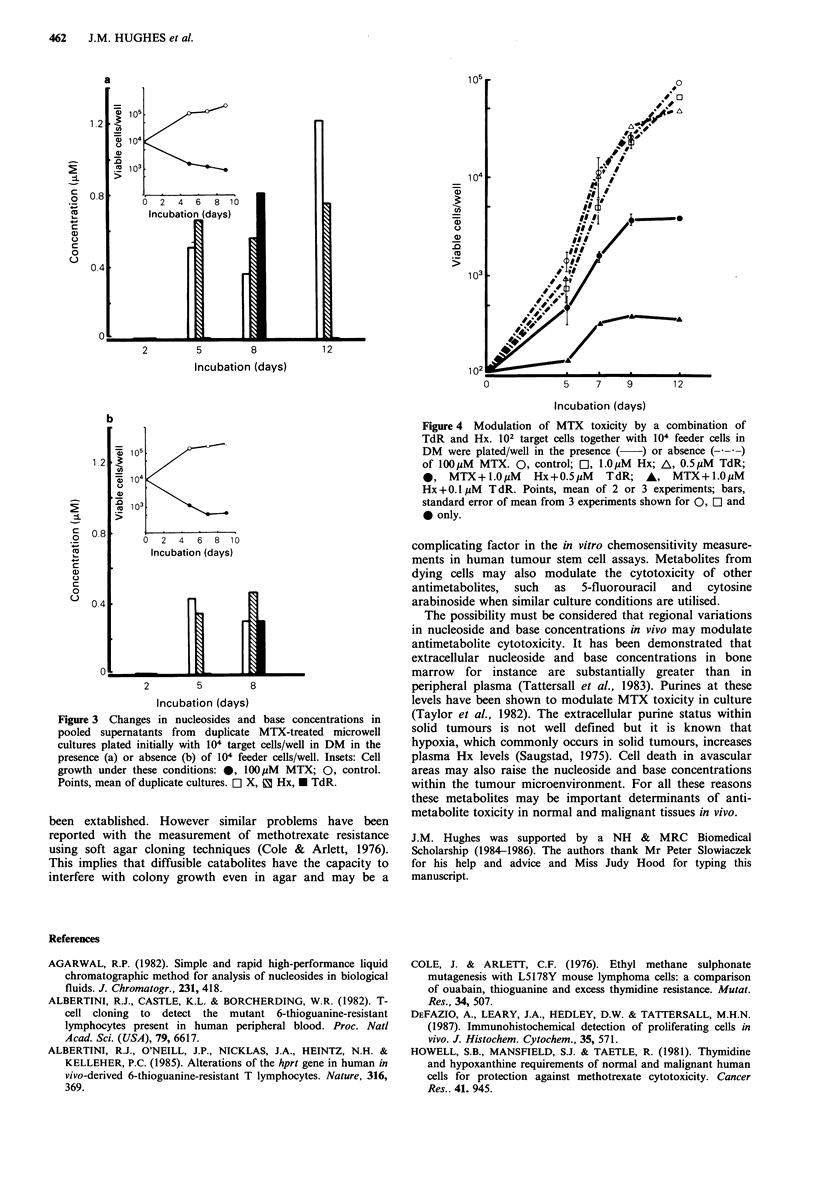
A lymphocyte clonal assay developed to quantitate in vivo somatic cell mutations at the hypoxanthine-guanine phosphoribosyltransferase locus was modified in order to study resistance to methotrexate. Even though nucleoside-free culture conditions were used methotrexate was not lethal to lymphocytes plated into micro-wells at greater than 10(2) cells/well. HPLC analysis of supernatants from wells plated initially with 10(4) cells/well in 100 microM methotrexate revealed the presence of micro-molar levels of hypoxanthine and thymidine by the 5th and 8th day of culture respectively. When lymphocytes were plated at less than or equal to 10(2) cells/well in nucleoside free medium, methotrexate was cytotoxic and micro-molar levels of thymidine together with hypoxanthine protected lymphocytes cultured under these conditions from toxicity. Modulation of nucleic acid antimetabolite cytotoxicity by nucleosides and bases has been recognised for some years. Nucleoside free culture conditions have been advocated for studying cellular sensitivity to antifolates to avoid such interfering factors. However our results indicate that metabolites from dying or damaged cells can prevent methotrexate cytotoxicity, further complicating the development of a suitable clonogenic assay for investigating antifolate sensitivity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal R. P., Major P. P., Kufe D. W. Simple and rapid high-performance liquid chromatographic method for analysis of nucleosides in biological fluids. J Chromatogr. 1982 Sep 10;231(2):418–424. doi: 10.1016/s0378-4347(00)81866-6. [DOI] [PubMed] [Google Scholar]
- Albertini R. J., Castle K. L., Borcherding W. R. T-cell cloning to detect the mutant 6-thioguanine-resistant lymphocytes present in human peripheral blood. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6617–6621. doi: 10.1073/pnas.79.21.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertini R. J., O'Neill J. P., Nicklas J. A., Heintz N. H., Kelleher P. C. Alterations of the hprt gene in human in vivo-derived 6-thioguanine-resistant T lymphocytes. Nature. 1985 Jul 25;316(6026):369–371. doi: 10.1038/316369a0. [DOI] [PubMed] [Google Scholar]
- Cole J., Arlett C. F. Ethyl methanesulphonate mutagenesis with L5178Y mouse lymphoma cells: a comparison of ouabain, thioguanine and excess thymidine resistance. Mutat Res. 1976 Mar;34(3):507–525. doi: 10.1016/0027-5107(76)90226-8. [DOI] [PubMed] [Google Scholar]
- Howell S. B., Mansfield S. J., Taetle R. Thymidine and hypoxanthine requirements of normal and malignant human cells for protection against methotrexate cytotoxicity. Cancer Res. 1981 Mar;41(3):945–950. [PubMed] [Google Scholar]
- Morley A. A., Trainor K. J., Dempsey J. L., Seshadri R. S. Methods for study of mutations and mutagenesis in human lymphocytes. Mutat Res. 1985 Dec;147(6):363–367. doi: 10.1016/0165-1161(85)90005-6. [DOI] [PubMed] [Google Scholar]
- Morley A. A., Trainor K. J., Seshadri R., Ryall R. G. Measurement of in vivo mutations in human lymphocytes. Nature. 1983 Mar 10;302(5904):155–156. doi: 10.1038/302155a0. [DOI] [PubMed] [Google Scholar]
- Saugstad O. D. Hypoxanthine as a measurement of hypoxia. Pediatr Res. 1975 Apr;9(4):158–161. doi: 10.1203/00006450-197504000-00002. [DOI] [PubMed] [Google Scholar]
- Sobrero A. F., Bertino J. R. Endogenous thymidine and hypoxanthine are a source of error in evaluating methotrexate cytotoxicity by clonogenic assays using undialyzed fetal bovine serum. Int J Cell Cloning. 1986 Jan;4(1):51–62. doi: 10.1002/stem.5530040106. [DOI] [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]
- Tattersall M. H., Slowiaczek P., De Fazio A. Regional variation in human extracellular purine levels. J Lab Clin Med. 1983 Sep;102(3):411–420. [PubMed] [Google Scholar]
- Taylor I. W., Slowiaczek P., Francis P. R., Tattersall M. H. Purine modulation of methotrexate cytotoxicity in mammalian cell lines. Cancer Res. 1982 Dec;42(12):5159–5164. [PubMed] [Google Scholar]
- Taylor I. W., Tattersall M. H. Methotrexate cytotoxicity in cultured human leukemic cells studied by flow cytometry. Cancer Res. 1981 Apr;41(4):1549–1558. [PubMed] [Google Scholar]
- deFazio A., Leary J. A., Hedley D. W., Tattersall M. H. Immunohistochemical detection of proliferating cells in vivo. J Histochem Cytochem. 1987 May;35(5):571–577. doi: 10.1177/35.5.3549891. [DOI] [PubMed] [Google Scholar]


