Abstract
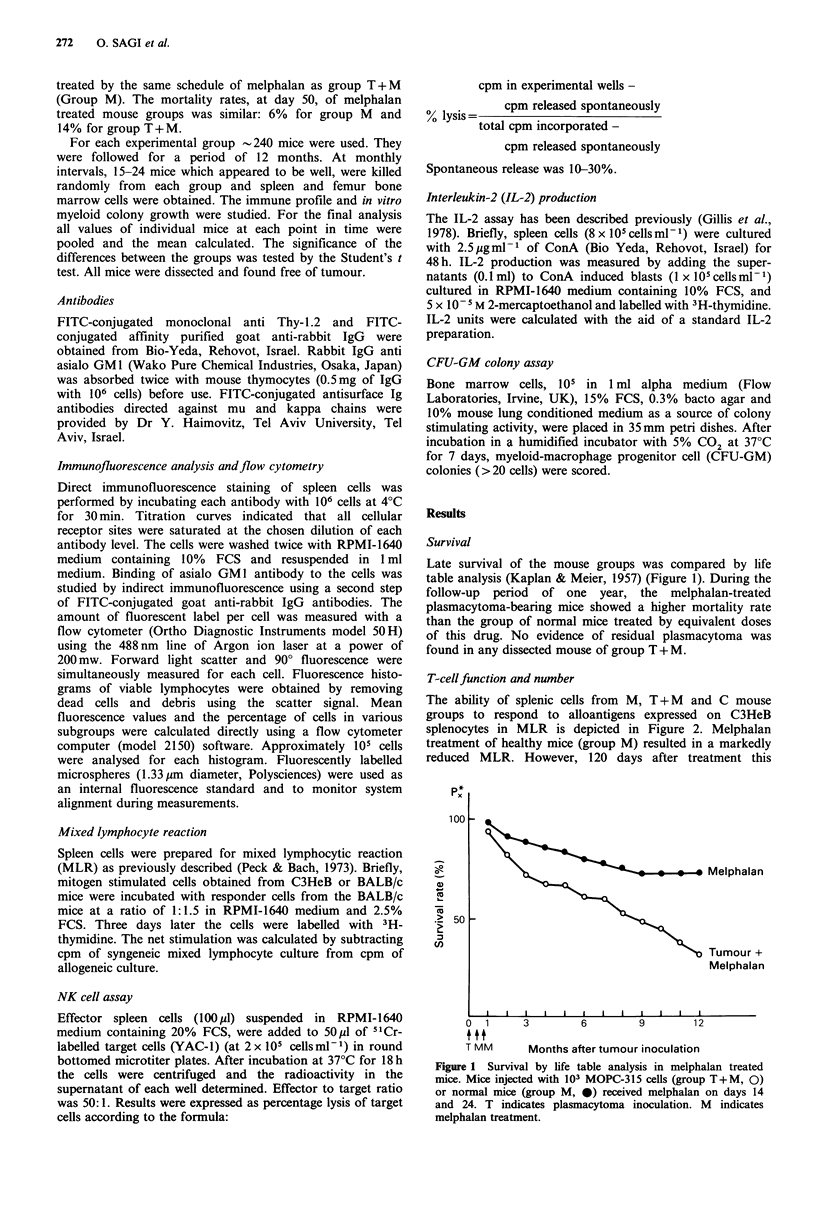
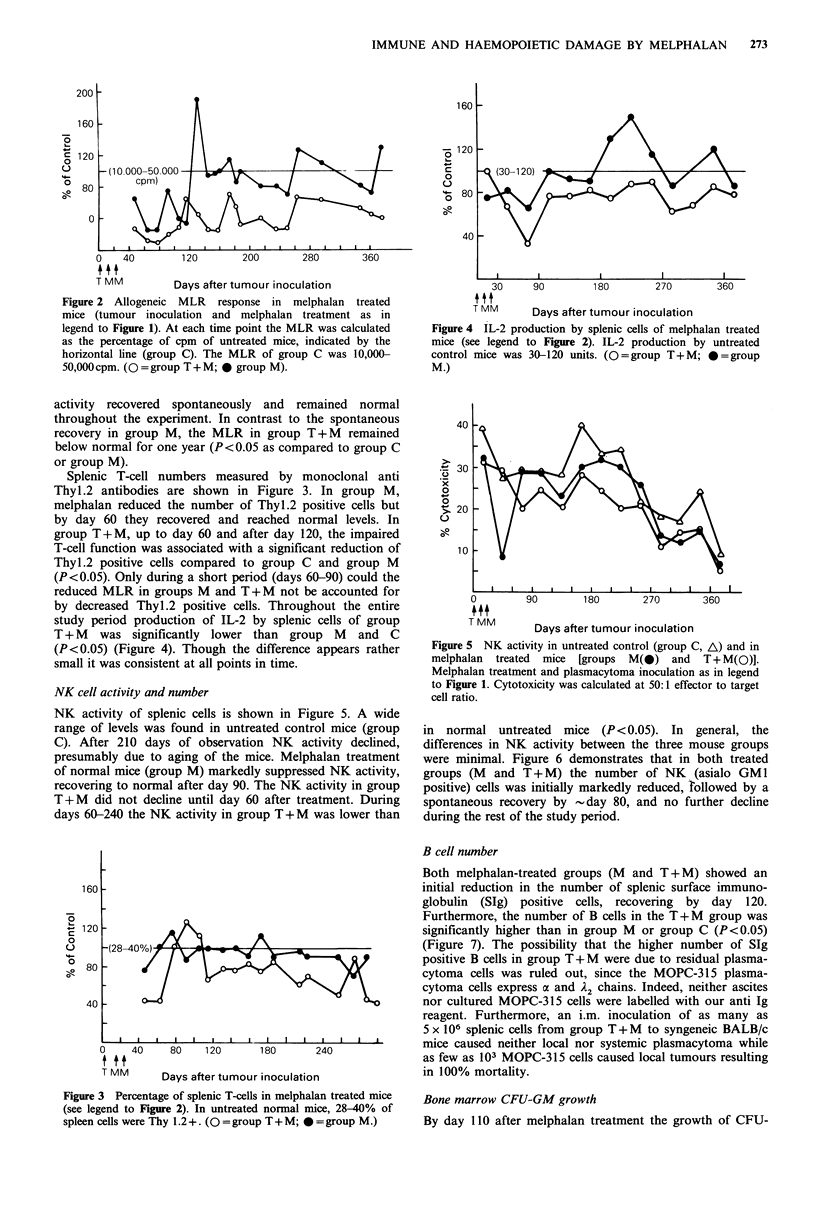
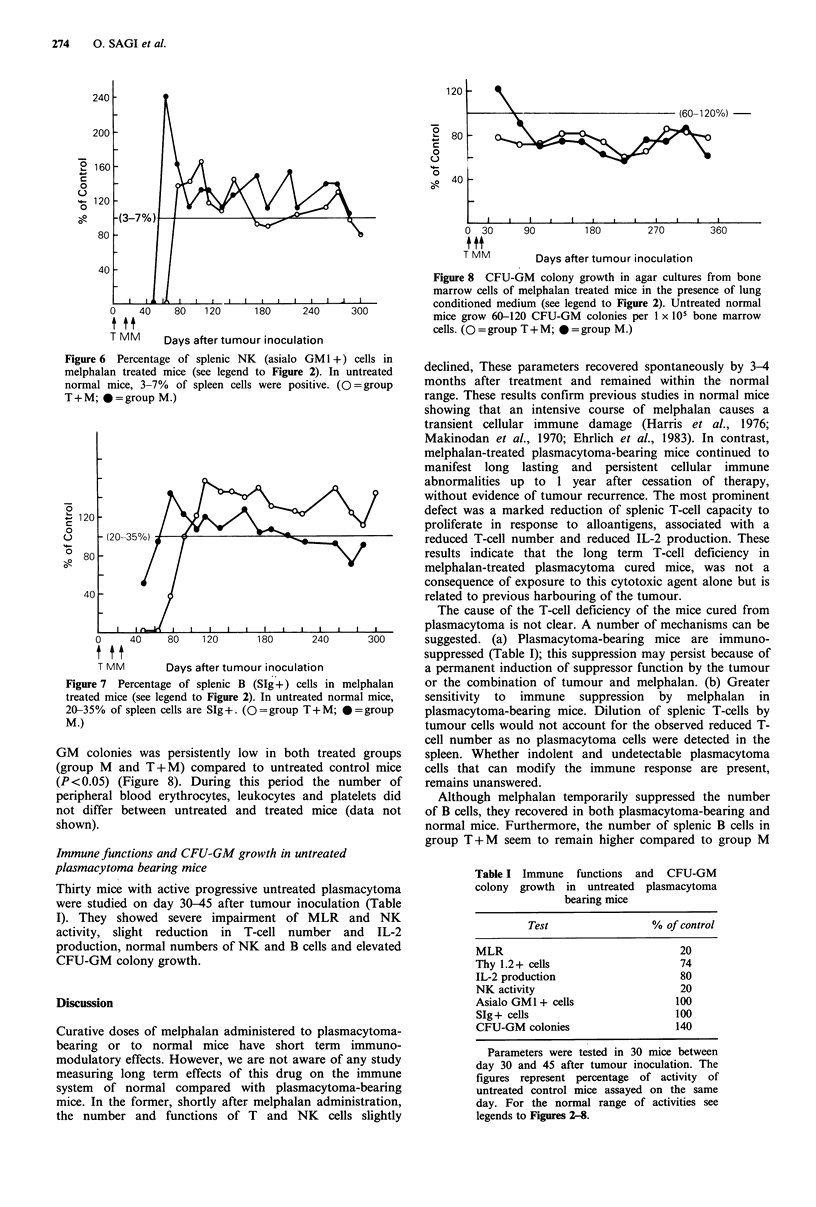
Alkylating agents can cause latent and permanent damage to the bone marrow. We compared the long term effects of melphalan on a number of immune and haemopoietic functions of plasmacytoma bearing BALB/c mice with that of normal mice treated with a similar dose of melphalan. The drug administered orally at a dose of 250 micrograms and 400 micrograms on day 14 and 24 following i.m. inoculation of MOPC-315 plasmacytoma cells resulted in cure of the mice. Their spleen cells showed a permanent impairment of MLR activity, T-cell number and IL-2 production as well as a mild suppression of NK activity for one year after cessation of melphalan therapy. The number of B cells was elevated. In contrast, plasmacytoma-free mice treated with melphalan retained long term normal immune functions, although shortly after melphalan therapy a temporary suppression was noted. On the other hand, melphalan was responsible for bone marrow myeloid stem cell damage since the number of myeloid progenitor cell (CFU-GM) colonies was reduced in both melphalan-treated groups compared to untreated normal controls. Plasmacytoma bearing mice had a shorter survival. These results demonstrate that some late sequelae of alkylating agents are not due to the drug alone; shorter survival and T-cell deficiency are related to the previous presence of the tumour.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Efraim S., Bocian R. C., Mokyr M. B., Dray S. Increase in the effectiveness of melphalan therapy with progression of MOPC-315 plasmacytoma tumor growth. Cancer Immunol Immunother. 1983;15(2):101–107. doi: 10.1007/BF00199699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botnick L. E., Hannon E. C., Hellman S. Multisystem stem cell failure after apparent recovery from alkylating agents. Cancer Res. 1978 Jul;38(7):1942–1947. [PubMed] [Google Scholar]
- Cuzick J., Erskine S., Edelman D., Galton D. A. A comparison of the incidence of the myelodysplastic syndrome and acute myeloid leukaemia following melphalan and cyclophosphamide treatment for myelomatosis. A report to the Medical Research Council's working party on leukaemia in adults. Br J Cancer. 1987 May;55(5):523–529. doi: 10.1038/bjc.1987.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich R., Efrati M., Malatzky E., Shochat L., Bar-Eyal A., Witz I. P. Natural host defence during oncogenesis. NK activity and dimethylbenzanthracene carcinogenesis. Int J Cancer. 1983 Jan 15;31(1):67–73. doi: 10.1002/ijc.2910310112. [DOI] [PubMed] [Google Scholar]
- Fisher B., Rockette H., Fisher E. R., Wickerham D. L., Redmond C., Brown A. Leukemia in breast cancer patients following adjuvant chemotherapy or postoperative radiation: the NSABP experience. J Clin Oncol. 1985 Dec;3(12):1640–1658. doi: 10.1200/JCO.1985.3.12.1640. [DOI] [PubMed] [Google Scholar]
- Fried W., Adler S. Late effects of chemotherapy on hematopoietic progenitor cells. Exp Hematol. 1985;13 (Suppl 16):49–56. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Greenberger J. S., Palaszynski E. W., Pierce J. H., Sakakeeny M. A., Ruscetti S. K., Ihle J. N., Daugherty C. Biologic effects of prolonged melphalan treatment of murine long-term bone marrow cultures and interleukin 3-dependent hematopoietic progenitor cell lines. J Natl Cancer Inst. 1985 Jan;74(1):247–262. [PubMed] [Google Scholar]
- Griffin G. D., Owen B. A., Atchley C. E., Novelli G. D., Solomon A. Decreased immunoglobulin production by a human lymphoid cell line following melphalan treatment. Cancer Res. 1982 Nov;42(11):4505–4510. [PubMed] [Google Scholar]
- Harris C. C. A delayed complication of cancer therapy--cancer. J Natl Cancer Inst. 1979 Aug;63(2):275–277. [PubMed] [Google Scholar]
- Harris J., Sengar D., Stewart T., Hyslop D. The effect of immunosuppressive chemotherapy on immune function in patients with malignant disease. Cancer. 1976 Feb;37(2 Suppl):1058–1069. doi: 10.1002/1097-0142(197602)37:2+<1058::aid-cncr2820370813>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Hays E. F., Hale L., Villarreal B., Fitchen J. H. "Stromal" and hemopoietic stem cell abnormalities in long-term cultures of marrow from busulfan-treated mice. Exp Hematol. 1982 Apr;10(4):383–392. [PubMed] [Google Scholar]
- Makinodan T., Santos G. W., Quinn R. P. Immunosuppressive drugs. Pharmacol Rev. 1970 Jun;22(2):189–247. [PubMed] [Google Scholar]
- Mills K. H., Cawley J. C. Abnormal monoclonal antibody-defined helper/suppressor T-cell subpopulations in multiple myeloma: relationship to treatment and clinical stage. Br J Haematol. 1983 Feb;53(2):271–275. doi: 10.1111/j.1365-2141.1983.tb02021.x. [DOI] [PubMed] [Google Scholar]
- Morley A., Blake J. An animal model of chronic aplastic marrow failure. I. Late marrow failure after busulfan. Blood. 1974 Jul;44(1):49–56. [PubMed] [Google Scholar]
- Morley A., Trainor K., Blake J. A primary stem cell lesion in experimental chronic hypoplastic marrow failure. Blood. 1975 May;45(5):681–688. [PubMed] [Google Scholar]
- Nowell P. C. Mechanisms of tumor progression. Cancer Res. 1986 May;46(5):2203–2207. [PubMed] [Google Scholar]
- Paolucci P., Hayward A. R., Rapson N. T. Pre-B and B cells in children on leukaemia remission maintenance treatment. Clin Exp Immunol. 1979 Aug;37(2):259–266. [PMC free article] [PubMed] [Google Scholar]
- Pearl E. R. Pre-B-cells in normal human bone marrow and in bone marrow from patients with leukemia in remission: persistent quantitative differences and possible expression of cell surface IgM in vitro. Blood. 1983 Mar;61(3):464–468. [PubMed] [Google Scholar]
- Peck A. B., Bach F. H. A miniaturized mouse mixed leukocyte culture in serum-free and mouse serum supplemented media. J Immunol Methods. 1973 Oct;3(2):147–163. doi: 10.1016/0022-1759(73)90030-6. [DOI] [PubMed] [Google Scholar]
- Pilarski L. M., Mant M. J., Ruether B. A., Carayanniotis G., Otto D., Krowka J. F. Abnormal clonogenic potential of T cells from multiple myeloma patients. Blood. 1985 Dec;66(6):1266–1271. [PubMed] [Google Scholar]
- Potter M., Walters J. L. Effect of intraperitoneal pristane on established immunity to the adj-PC-5 plasmacytoma. J Natl Cancer Inst. 1973 Sep;51(3):875–881. doi: 10.1093/jnci/51.3.875. [DOI] [PubMed] [Google Scholar]
- Schofield R. Assessment of cytotoxic injury to bone marrow. Br J Cancer Suppl. 1986;7:115–125. [PMC free article] [PubMed] [Google Scholar]
- Schwarzbard Z., Ophir R., Gotlieb-Stematsky T., Benefraim S. Importance of the concomitant presence of palpable MOPC-315 tumor in stimulation of splenocytes by C-type MOPC-315 virus in vitro. Eur J Cancer Clin Oncol. 1985 Sep;21(9):1069–1075. doi: 10.1016/0277-5379(85)90293-7. [DOI] [PubMed] [Google Scholar]
- Sieber S. M., Adamson R. H. Toxicity of antineoplastic agents in man, chromosomal aberrations antifertility effects, congenital malformations, and carcinogenic potential. Adv Cancer Res. 1975;22:57–155. doi: 10.1016/s0065-230x(08)60176-1. [DOI] [PubMed] [Google Scholar]
- Testa N. G., Hendry J. H., Molineux G. Long-term bone marrow damage in experimental systems and in patients after radiation or chemotherapy. Anticancer Res. 1985 Jan-Feb;5(1):101–110. [PubMed] [Google Scholar]


