Abstract
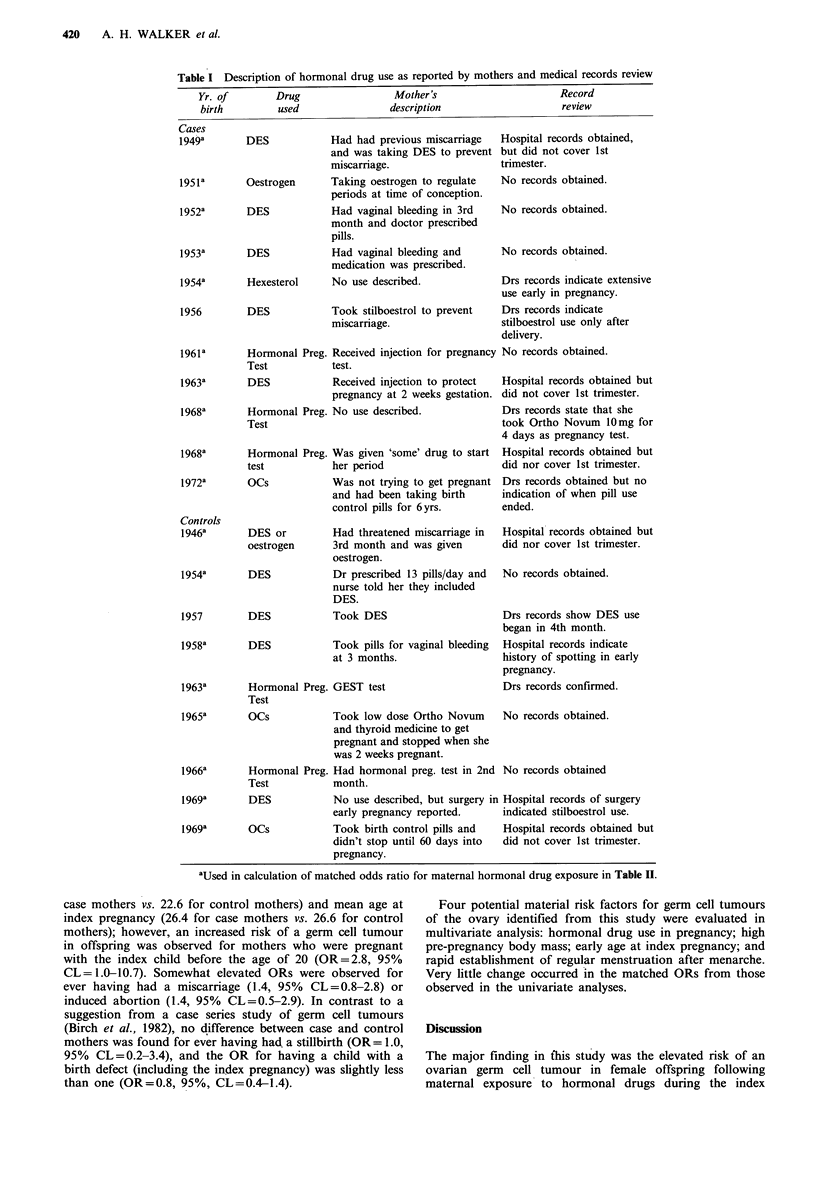
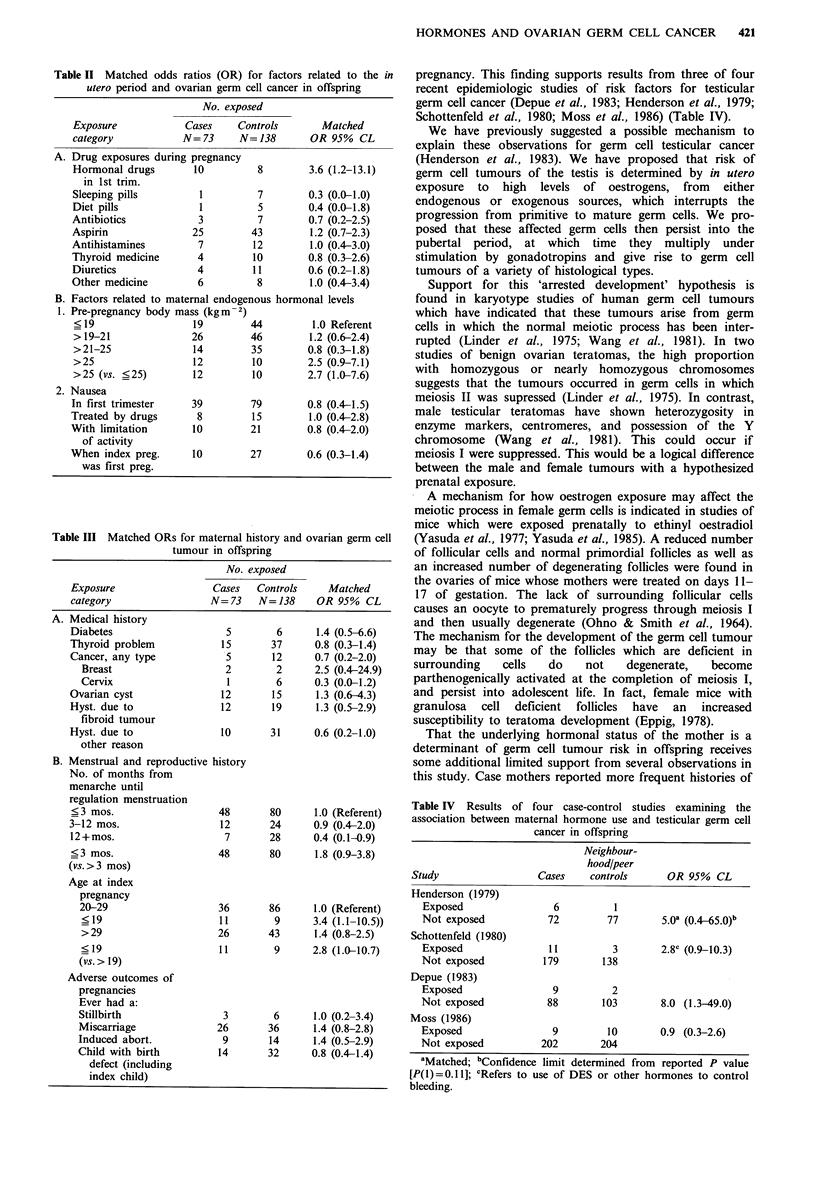
No previous controlled studies of ovarian germ cell tumours have been reported; however the tumour is similar to germ cell testicular cancer in terms of histology, age-specific incidence rates (i.e. highest rates in young adulthood), and secular trends of increasing incidence. The investigation was designed to determine if maternal hormonal factors which have been found to increase the risk of testis cancer in male offspring are also risk factors for the ovarian tumour. The analysis is based on 73 cases diagnosed before age 35 and 138 age-race matched controls. The cases were identified by tumour registries in Los Angeles (1972-84) and Seattle (1974-84) and controls were selected from friends and/or neighbourhood residents. Interviews were conducted on the telephone with mothers of cases and controls. The primary finding was that mother's use of exogenous hormones (including the hormonal pregnancy test, DES or other supportive hormones, and inadvertant use of oral contraceptives after conception) increased risk (Odds ratio, OR = 3.60, 95% CL = 1.2-13.1). Other maternal factors associated with elevated risk were high pre-pregnancy body mass (OR = 2.7, 95% CL = 1.0-7.6), more rapid achievement of regular menstruation after menarche (OR = 1.8, 95% CL = 0.9-3.8), and age at index pregnancy under 20 (OR = 2.8, 95% CL = 1.0-10.7). In conclusion, these results support findings from testis cancer studies regarding a hormonal aetiology for germ cell tumours, and a mechanism by which oestrogen may affect the germ cells is proposed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein L., Depue R. H., Ross R. K., Judd H. L., Pike M. C., Henderson B. E. Higher maternal levels of free estradiol in first compared to second pregnancy: early gestational differences. J Natl Cancer Inst. 1986 Jun;76(6):1035–1039. [PubMed] [Google Scholar]
- Birch J. M., Marsden H. B., Swindell R. Pre-natal factors in the origin of germ cell tumours of childhood. Carcinogenesis. 1982;3(1):75–80. doi: 10.1093/carcin/3.1.75. [DOI] [PubMed] [Google Scholar]
- Depue R. H., Bernstein L., Ross R. K., Judd H. L., Henderson B. E. Hyperemesis gravidarum in relation to estradiol levels, pregnancy outcome, and other maternal factors: a seroepidemiologic study. Am J Obstet Gynecol. 1987 May;156(5):1137–1141. doi: 10.1016/0002-9378(87)90126-8. [DOI] [PubMed] [Google Scholar]
- Depue R. H., Pike M. C., Henderson B. E. Estrogen exposure during gestation and risk of testicular cancer. J Natl Cancer Inst. 1983 Dec;71(6):1151–1155. [PubMed] [Google Scholar]
- Edman C. D., MacDonald P. C. Effect of obesity on conversion of plasma androstenedione to estrone in ovulatory and anovulator young women. Am J Obstet Gynecol. 1978 Feb 15;130(4):456–461. doi: 10.1016/0002-9378(78)90288-0. [DOI] [PubMed] [Google Scholar]
- Eppig J. J. Granulosa cell deficient follicles: occurrence, structure, and relationship to ovarian teratocarcinogenesis in strain LT/Sv mice. Differentiation. 1978;12(2):111–120. [PubMed] [Google Scholar]
- Henderson B. E., Benton B., Jing J., Yu M. C., Pike M. C. Risk factors for cancer of the testis in young men. Int J Cancer. 1979 May 15;23(5):598–602. doi: 10.1002/ijc.2910230503. [DOI] [PubMed] [Google Scholar]
- Linder D., McCaw B. K., Hecht F. Parthenogenic origin of benign ovarian teratomas. N Engl J Med. 1975 Jan 9;292(2):63–66. doi: 10.1056/NEJM197501092920202. [DOI] [PubMed] [Google Scholar]
- Mack T. M. Cancer surveillance program in Los Angeles County. Natl Cancer Inst Monogr. 1977 Dec;47:99–101. [PubMed] [Google Scholar]
- Miettinen O. S. Estimation of relative risk from individually matched series. Biometrics. 1970 Mar;26(1):75–86. [PubMed] [Google Scholar]
- O'Dea J. P., Wieland R. G., Hallberg M. C., Llerena L. A., Zorn E. M., Genuth S. M. Effect of dietery weight loss on sex steroid binding sex steroids, and gonadotropins in obese postmenopausal women. J Lab Clin Med. 1979 Jun;93(6):1004–1008. [PubMed] [Google Scholar]
- OHNO S., SMITH J. B. ROLE OF FETAL FOLLICULAR CELLS IN MEIOSIS OF MAMMALIAN OOECYTES. Cytogenetics. 1964;3:324–333. doi: 10.1159/000129821. [DOI] [PubMed] [Google Scholar]
- Schottenfeld D., Warshauer M. E., Sherlock S., Zauber A. G., Leder M., Payne R. The epidemiology of testicular cancer in young adults. Am J Epidemiol. 1980 Aug;112(2):232–246. doi: 10.1093/oxfordjournals.aje.a112989. [DOI] [PubMed] [Google Scholar]
- Walker A. H., Ross R. K., Pike M. C., Henderson B. E. A possible rising incidence of malignant germ cell tumours in young women. Br J Cancer. 1984 May;49(5):669–672. doi: 10.1038/bjc.1984.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Perkins K. L., Bronson D. L., Fraley E. E. Cytogenetic evidence for premeiotic transformation of human testicular cancers. Cancer Res. 1981 Jun;41(6):2135–2140. [PubMed] [Google Scholar]
- Yasuda Y., Kihara T., Nishimura H. Effect of prenatal treatment with ethinyl estradiol on the mouse uterus and ovary. Am J Obstet Gynecol. 1977 Apr 15;127(8):832–836. doi: 10.1016/0002-9378(77)90114-4. [DOI] [PubMed] [Google Scholar]
- Yasuda Y., Kihara T., Tanimura T., Nishimura H. Gonadal dysgenesis induced by prenatal exposure to ethinyl estradiol in mice. Teratology. 1985 Oct;32(2):219–227. doi: 10.1002/tera.1420320210. [DOI] [PubMed] [Google Scholar]


