Abstract
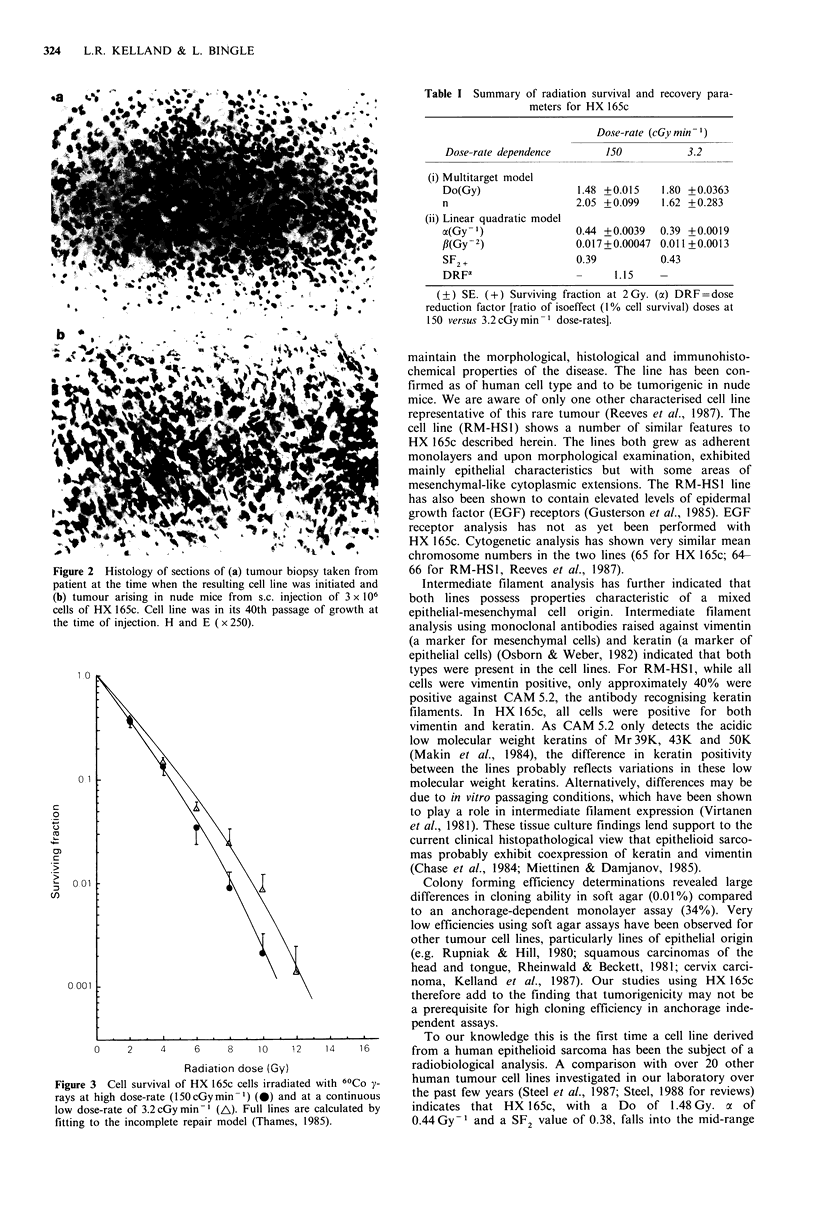
A new human tumour cell line (designated HX 165c) has been established from an epithelioid sarcoma presenting in a 28 year old male. The cells grew as an adherent monolayer with a doubling time of 38 h and had mainly epithelial morphology but with areas of mesenchymal-like cytoplasmic extensions. The mixed epithelial-mesenchymal phenotype was also apparent by intermediate filament analysis which showed reactivity to vimentin and keratin. The cells were tumorigenic in nude mice and aneuploid, possessing a mean chromosome number of 65. In vitro cloning determinations gave colony-forming efficiencies of 0.01% in an anchorage-independent soft agar assay and 34% in a monolayer anchorage-dependent assay. The cells were in the mid-range for radiosensitivity of human tumour cells (surviving fraction at 2 Gy of 0.39). In addition, experiments utilising continuous low dose rate irradiation at 3.2 cGy min-1, showed that the cells possessed only a small capacity to recover from radiation damage (dose reduction factor at 1% cell survival of 1.15 for 150 versus 3.2 cGy min-1). This cell line, being only the second we are aware of to be established from this rare soft tissue sarcoma, should be useful in helping to ascertain the histogenesis of epithelioid sarcoma.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chase D. R., Enzinger F. M., Weiss S. W., Langloss J. M. Keratin in epithelioid sarcoma. An immunohistochemical study. Am J Surg Pathol. 1984 Jun;8(6):435–441. doi: 10.1097/00000478-198406000-00004. [DOI] [PubMed] [Google Scholar]
- Courtenay V. D., Mills J. An in vitro colony assay for human tumours grown in immune-suppressed mice and treated in vivo with cytotoxic agents. Br J Cancer. 1978 Feb;37(2):261–268. doi: 10.1038/bjc.1978.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon J., Peckham M. J., Steel G. G. The radioresponsiveness of human tumours and the initial slope of the cell survival curve. Radiother Oncol. 1984 Dec;2(4):317–323. doi: 10.1016/s0167-8140(84)80074-2. [DOI] [PubMed] [Google Scholar]
- Enzinger F. M. Epitheloid sarcoma. A sarcoma simulating a granuloma or a carcinoma. Cancer. 1970 Nov;26(5):1029–1041. doi: 10.1002/1097-0142(197011)26:5<1029::aid-cncr2820260510>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Fertil B., Malaise E. P. Inherent cellular radiosensitivity as a basic concept for human tumor radiotherapy. Int J Radiat Oncol Biol Phys. 1981 May;7(5):621–629. doi: 10.1016/0360-3016(81)90377-1. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Fu Y. S., Kaye G. I., Lattes R., Majno G. Epithelioid sarcoma. A light and electron microscopic study suggesting a synovial origin. Cancer. 1972 Aug;30(2):486–499. doi: 10.1002/1097-0142(197208)30:2<486::aid-cncr2820300229>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Gusterson B., Cowley G., McIlhinney J., Ozanne B., Fisher C., Reeves B. Evidence for increased epidermal growth factor receptors in human sarcomas. Int J Cancer. 1985 Dec 15;36(6):689–693. doi: 10.1002/ijc.2910360612. [DOI] [PubMed] [Google Scholar]
- Kelland L. R., Burgess L., Steel G. G. Characterization of four new cell lines derived from human squamous carcinomas of the uterine cervix. Cancer Res. 1987 Sep 15;47(18):4947–4952. [PubMed] [Google Scholar]
- Kelland L. R., Burgess L., Steel G. G. Radiation damage repair capacity of a human germ-cell tumour cell line: inhibition by 3-aminobenzamide. Int J Radiat Biol Relat Stud Phys Chem Med. 1987 Feb;51(2):227–241. doi: 10.1080/09553008714550731. [DOI] [PubMed] [Google Scholar]
- Kelland L. R., Steel G. G. Dose-rate effects in the radiation response of four human tumour xenografts. Radiother Oncol. 1986 Nov;7(3):259–268. doi: 10.1016/s0167-8140(86)80037-8. [DOI] [PubMed] [Google Scholar]
- Lindberg R. D., Martin R. G., Romsdahl M. M., Barkley H. T., Jr Conservative surgery and postoperative radiotherapy in 300 adults with soft-tissue sarcomas. Cancer. 1981 May 15;47(10):2391–2397. doi: 10.1002/1097-0142(19810515)47:10<2391::aid-cncr2820471012>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Makin C. A., Bobrow L. G., Bodmer W. F. Monoclonal antibody to cytokeratin for use in routine histopathology. J Clin Pathol. 1984 Sep;37(9):975–983. doi: 10.1136/jcp.37.9.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. B., Bedford J. S., Bailey S. M. Dose-rate effects in mammalian cells in culture III. Comparison of cell killing and cell proliferation during continuous irradiation for six different cell lines. Radiat Res. 1979 Sep;79(3):537–551. [PubMed] [Google Scholar]
- Mitchell J. B., Bedord J. S., Bailey S. M. Dose-rate effects on the cell cycle and survival of S3 HeLa and V79 cells. Radiat Res. 1979 Sep;79(3):520–536. [PubMed] [Google Scholar]
- Osborn M., Weber K. Intermediate filaments: cell-type-specific markers in differentiation and pathology. Cell. 1982 Dec;31(2 Pt 1):303–306. doi: 10.1016/0092-8674(82)90122-2. [DOI] [PubMed] [Google Scholar]
- Reeves B. R., Fisher C., Smith S., Courtenay V. D., Robertson D. Ultrastructural, immunocytochemical, and cytogenetic characterization of a human epithelioid sarcoma cell line (RM-HS1). J Natl Cancer Inst. 1987 Jan;78(1):7–18. doi: 10.1093/jnci/78.1.7. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Beckett M. A. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 1981 May;41(5):1657–1663. [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975 Nov;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Rupniak H. T., Hill B. T. The poor cloning ability in agar of human tumour cells from biopsies of primary tumours. Cell Biol Int Rep. 1980 May;4(5):479–486. doi: 10.1016/0309-1651(80)90035-1. [DOI] [PubMed] [Google Scholar]
- Steel G. G., Deacon J. M., Duchesne G. M., Horwich A., Kelland L. R., Peacock J. H. The dose-rate effect in human tumour cells. Radiother Oncol. 1987 Aug;9(4):299–310. doi: 10.1016/s0167-8140(87)80151-2. [DOI] [PubMed] [Google Scholar]
- Steel G. G., Down J. D., Peacock J. H., Stephens T. C. Dose-rate effects and the repair of radiation damage. Radiother Oncol. 1986 Apr;5(4):321–331. doi: 10.1016/s0167-8140(86)80181-5. [DOI] [PubMed] [Google Scholar]
- Svensson H. The 6th Klaas Breur memorial lecture, 1987. The Chernobyl accident--impact Western Europe. Radiother Oncol. 1988 May;12(1):1–13. doi: 10.1016/0167-8140(88)90187-9. [DOI] [PubMed] [Google Scholar]
- Thames H. D. An 'incomplete-repair' model for survival after fractionated and continuous irradiations. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Mar;47(3):319–339. doi: 10.1080/09553008514550461. [DOI] [PubMed] [Google Scholar]




