Abstract
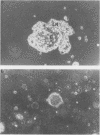
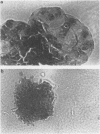
Results are presented which show that mesothelial cells (MC) from ovarian cancer patients can both stimulate and inhibit the clonogenic growth of ovarian tumour cells (TC) in a dose-dependent fashion. TC lines from both non-ovarian and ovarian tumours were variable in their response to MC. Colony formation was rarely induced when the TC population was non-clonogenic and a bladder cell line showed inhibition of colony formation in the presence of MC. Primary tumour cultures from ovarian cancer patients also showed a variable response to MC. Fibroblasts from malignant, benign and non-neoplastic sources were significantly less effective in stimulating the clonogenic growth of responsive cell lines. Conditioned medium was a poor substitute for the presence of intact viable cells, and distance between feeder cell and TC was an important factor in determining the magnitude of response. A significant relationship between the feeder effect of MC and their proliferation in soft agar was observed when epidermal growth factor was used in the medium. The relevance of the findings in the context of the pattern of spread of ovarian cancer is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brattain M. G., Brattain D. E., Sarrif A. M., McRae L. J., Fine W. D., Hawkins J. G. Enhancement of growth of human colon tumor cell lines by feeder layers of murine fibroblasts. J Natl Cancer Inst. 1982 Oct;69(4):767–771. [PubMed] [Google Scholar]
- Broxterman H. J., Sprenkels-Schotte C., Engelen P., Leyva A., Pinedo H. M. Analysis of human ascites effect on clonogenic growth of human tumor cell lines and NRK-49F cells in soft agar. Int J Cell Cloning. 1987 Mar;5(2):158–169. doi: 10.1002/stem.5530050208. [DOI] [PubMed] [Google Scholar]
- Buick R. N., Fry S. E., Salmon S. E. Effect of host-cell interactions on clonogenic carcinoma cells in human malignant effusions. Br J Cancer. 1980 May;41(5):695–704. doi: 10.1038/bjc.1980.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M. L., Jaffe N. D., Hamburger A. W., Lindblad A. L., Banda F. P., Yenson A., Nathan K. A., Cohen M. H. Improvement of human tumor cloning assay by suspension of fibroblasts into the bottom layer of agarose. Cancer. 1986 Jun 15;57(12):2357–2362. doi: 10.1002/1097-0142(19860615)57:12<2357::aid-cncr2820571220>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Connell N. D., Rheinwald J. G. Regulation of the cytoskeleton in mesothelial cells: reversible loss of keratin and increase in vimentin during rapid growth in culture. Cell. 1983 Aug;34(1):245–253. doi: 10.1016/0092-8674(83)90155-1. [DOI] [PubMed] [Google Scholar]
- Epenetos A. A., Canti G., Taylor-Papadimitriou J., Curling M., Bodmer W. F. Use of two epithelium-specific monoclonal antibodies for diagnosis of malignancy in serous effusions. Lancet. 1982 Nov 6;2(8306):1004–1006. doi: 10.1016/s0140-6736(82)90047-2. [DOI] [PubMed] [Google Scholar]
- Gallie B. L., Holmes W., Phillips R. A. Reproducible growth in tissue culture of retinoblastoma tumor specimens. Cancer Res. 1982 Jan;42(1):301–305. [PubMed] [Google Scholar]
- Ghosh A. K., Gatter K. C., Dunnill M. S., Mason D. Y. Immunohistological staining of reactive mesothelium, mesothelioma, and lung carcinoma with a panel of monoclonal antibodies. J Clin Pathol. 1987 Jan;40(1):19–25. doi: 10.1136/jcp.40.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger A. W., White C. P., Brown R. W. Effect of epidermal growth factor on proliferation of human tumor cells in soft agar. J Natl Cancer Inst. 1981 Oct;67(4):825–830. [PubMed] [Google Scholar]
- Hamburger A. W., White C. P., Lurie K., Kaplan R. Monocyte-derived growth factors for human tumor clonogenic cells. J Leukoc Biol. 1986 Oct;40(4):381–392. doi: 10.1002/jlb.40.4.381. [DOI] [PubMed] [Google Scholar]
- Kirk D., Kagawa S., Vener G. Comparable growth regulation of five human tumor cell lines by neonatal human lung fibroblasts in semisolid culture media. Cancer Res. 1983 Aug;43(8):3754–3758. [PubMed] [Google Scholar]
- Kirk D., Szalay M. F., Kaighn M. E. Modulation of growth of a human prostatic cancer cell line (PC-3) in agar culture by normal human lung fibroblasts. Cancer Res. 1981 Mar;41(3):1100–1103. [PubMed] [Google Scholar]
- Koopman P., Cotton R. G. A factor produced by feeder cells which inhibits embryonal carcinoma cell differentiation. Characterization and partial purification. Exp Cell Res. 1984 Sep;154(1):233–242. doi: 10.1016/0014-4827(84)90683-9. [DOI] [PubMed] [Google Scholar]
- Laboisse C. L., Augeron C., Potet F. Growth and differentiation of human gastrointestinal adenocarcinoma stem cells in soft agarose. Cancer Res. 1981 Jan;41(1):310–315. [PubMed] [Google Scholar]
- Mouriquand J., Mouriquand C., Petitpas E., Mermet M. A. Long-term tissue cultures of human pleural effusions: a cytological follow-up. In Vitro. 1978 Jul;14(7):591–600. doi: 10.1007/BF02617918. [DOI] [PubMed] [Google Scholar]
- Peehl D. M., Ham R. G. Growth and differentiation of human keratinocytes without a feeder layer or conditioned medium. In Vitro. 1980 Jun;16(6):516–525. doi: 10.1007/BF02626465. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Beckett M. A. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 1981 May;41(5):1657–1663. [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature. 1977 Feb 3;265(5593):421–424. doi: 10.1038/265421a0. [DOI] [PubMed] [Google Scholar]
- Rosenstraus M. J., Sterman B., Carr A., Brand L. Fibroblast feeder layers inhibit differentiation of retinoic acid-treated embryonal carcinoma cells by increasing the probability of stem cell renewal. Exp Cell Res. 1984 Jun;152(2):378–389. doi: 10.1016/0014-4827(84)90639-6. [DOI] [PubMed] [Google Scholar]
- Sinna G. A., Beckman G., Lundgren E., Nordenson I., Roos G. Characterization of two human ovarian carcinoma cell lines. Gynecol Oncol. 1979 Jun;7(3):267–280. doi: 10.1016/0090-8258(79)90104-5. [DOI] [PubMed] [Google Scholar]
- Stanley M. A., Parkinson E. K. Growth requirements of human cervical epithelial cells in culture. Int J Cancer. 1979 Oct 15;24(4):407–414. doi: 10.1002/ijc.2910240406. [DOI] [PubMed] [Google Scholar]
- Tarin D., Price J. E., Kettlewell M. G., Souter R. G., Vass A. C., Crossley B. Mechanisms of human tumor metastasis studied in patients with peritoneovenous shunts. Cancer Res. 1984 Aug;44(8):3584–3592. [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Peterson J. A., Arklie J., Burchell J., Ceriani R. L., Bodmer W. F. Monoclonal antibodies to epithelium-specific components of the human milk fat globule membrane: production and reaction with cells in culture. Int J Cancer. 1981 Jul 15;28(1):17–21. doi: 10.1002/ijc.2910280104. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Shearer M., Stoker M. G. Growth requirements of human mammary epithelial cells in culture. Int J Cancer. 1977 Dec 15;20(6):903–908. doi: 10.1002/ijc.2910200613. [DOI] [PubMed] [Google Scholar]
- Ueda G., Inoue M., Saito J., Shimizu H., Shimizu C., Sasagawa H., Tanizawa O. Expression of vimentin in common epithelial tumors of the ovary. Nihon Sanka Fujinka Gakkai Zasshi. 1987 Jul;39(7):1151–1152. [PubMed] [Google Scholar]
- Uitendaal M. P., Hubers H. A., McVie J. G., Pinedo H. M. Human tumour clonogenicity in agar is improved by cell-free ascites. Br J Cancer. 1983 Jul;48(1):55–59. doi: 10.1038/bjc.1983.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B. G., Lowe D. G., Shepherd J. H. Patterns of expression of a tumor associated antigen, defined by the monoclonal antibody HMFG2, in human epithelial ovarian carcinoma. Comparison with expression of the HMFG1, AUA1 and F36/22 antigens. Cancer. 1987 Aug 15;60(4):787–793. doi: 10.1002/1097-0142(19870815)60:4<787::aid-cncr2820600414>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Welander C. E., Natale R. B., Lewis J. L., Jr In vitro growth stimulation of human ovarian cancer cells by xenogeneic peritoneal macrophages. J Natl Cancer Inst. 1982 Nov;69(5):1039–1047. [PubMed] [Google Scholar]
- Whitehead R. H., Hughes L. E. Tissue culture studies of malignant effusions. Br J Cancer. 1975 Oct;32(4):512–518. doi: 10.1038/bjc.1975.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. P. Characterization of a cell line derived from the ascites of a patient with papillary serous cystadenocarcinoma of the ovary. J Natl Cancer Inst. 1984 Mar;72(3):513–521. [PubMed] [Google Scholar]
- Wilson A. P., Ford C. H., Newman C. E., Howell A. cis-platinum and ovarian carcinoma. In vitro chemosensitivity of cultured tumour cells from patients receiving high dose cis-platinum as first line treatment. Br J Cancer. 1987 Dec;56(6):763–773. doi: 10.1038/bjc.1987.285. [DOI] [PMC free article] [PubMed] [Google Scholar]