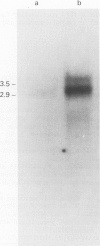
Abstract
The c-fgr protooncogene is a member of the c-src family of tyrosine kinases. Expression of c-fgr was studied in a series of Epstein-Barr virus (EBV) negative Burkitt's lymphoma cell lines and their EBV-converted derivatives. Two transcripts, of 2.9 kb and 3.5 kb, were present at dramatically elevated levels following EBV-conversion. The structure of the c-fgr transcripts was studied by the isolation and nucleotide sequence analysis of cDNA clones. This indicated that the c-fgr protein encoded by the mature mRNA would contain 529 amino acids and have a molecular weight of approximately 58,000. The N-terminus of the predicted c-fgr protein has low amino acid homology with the N-termini of other members of this family of proteins, suggesting a cell specific function for the N-terminal domain. Analysis of the c-fgr cDNA clones also revealed the presence of alternative functional polyadenylation signals, although the use of these does not account for the size difference between the two major c-fgr transcripts.
Full text
PDF

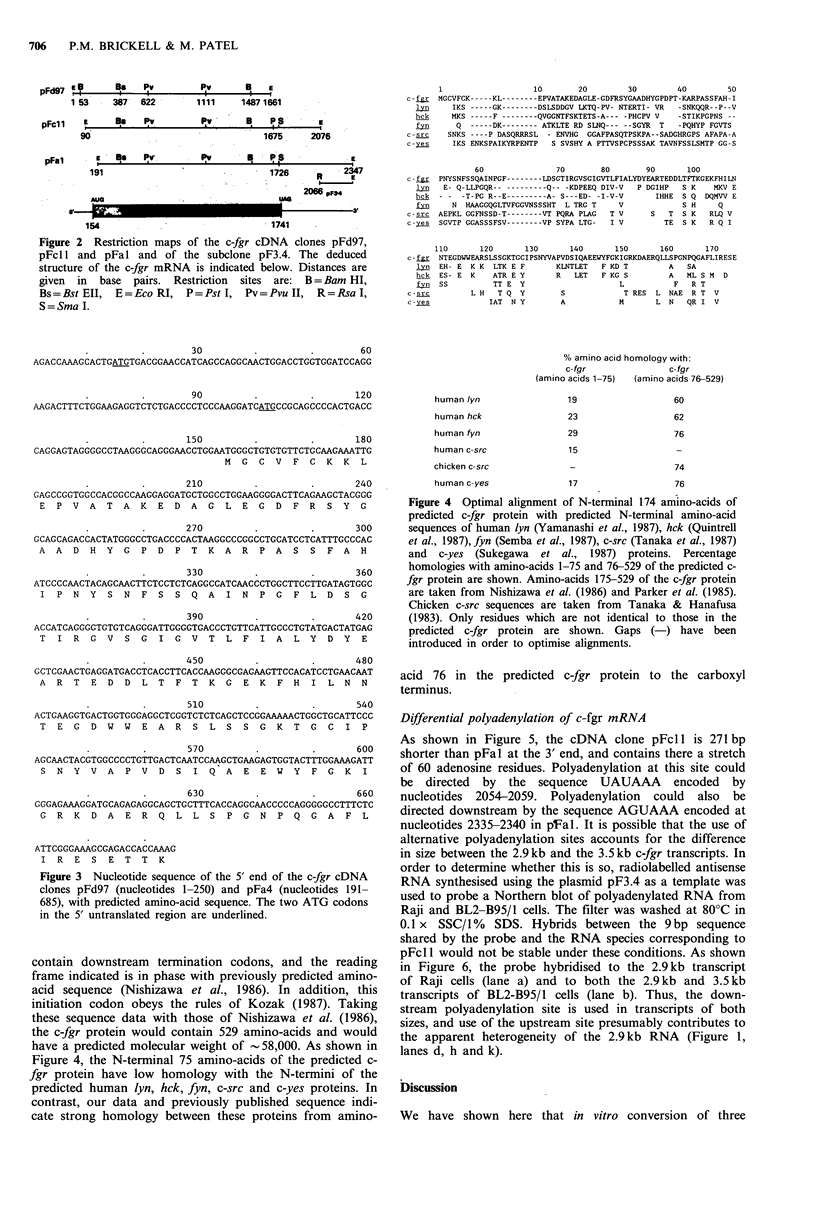



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Calender A., Billaud M., Aubry J. P., Banchereau J., Vuillaume M., Lenoir G. M. Epstein-Barr virus (EBV) induces expression of B-cell activation markers on in vitro infection of EBV-negative B-lymphoma cells. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8060–8064. doi: 10.1073/pnas.84.22.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah M. S., Ley T. J., Tronick S. R., Robbins K. C. fgr proto-oncogene mRNA induced in B lymphocytes by Epstein-Barr virus infection. Nature. 1986 Jan 16;319(6050):238–240. doi: 10.1038/319238a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Craig R. K., Brown P. A., Harrison O. S., McIlreavy D., Campbell P. N. Guinea-pig milk-protein synthesis. Isolation and characterization of messenger ribonucleic acids from lactating mammary gland and identification of caseins and pre-alpha-lactalbumin as translation products in heterologous cell-free systems. Biochem J. 1976 Oct 15;160(1):57–74. doi: 10.1042/bj1600057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman J. C., Ellis L., Blacher R. W., Roth R. A., Rutter W. J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature. 1985 Sep 19;317(6034):267–270. doi: 10.1038/317267a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Higgs D. R., Goodbourn S. E., Lamb J., Clegg J. B., Weatherall D. J., Proudfoot N. J. Alpha-thalassaemia caused by a polyadenylation signal mutation. Nature. 1983 Nov 24;306(5941):398–400. doi: 10.1038/306398a0. [DOI] [PubMed] [Google Scholar]
- Humphries S. E., Whittall R., Minty A., Buckingham M., Williamson R. There are approximately 20 actin gene in the human genome. Nucleic Acids Res. 1981 Oct 10;9(19):4895–4908. doi: 10.1093/nar/9.19.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Ikawa S., Semba K., Sukegawa J., Yamamoto T., Toyoshima K. Isolation and sequencing of cDNA clones homologous to the v-fgr oncogene from a human B lymphocyte cell line, IM-9. Oncogene. 1987;1(3):301–304. [PubMed] [Google Scholar]
- Katamine S., Notario V., Rao C. D., Miki T., Cheah M. S., Tronick S. R., Robbins K. C. Primary structure of the human fgr proto-oncogene product p55c-fgr. Mol Cell Biol. 1988 Jan;8(1):259–266. doi: 10.1128/mcb.8.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Kitagawa H., Fukushima D., Takagaki Y., Miyata T., Nakanishi S. Structural organization of the human kininogen gene and a model for its evolution. J Biol Chem. 1985 Jul 15;260(14):8610–8617. [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Overell R. W., Meier K. E., Krebs E. G., Perlmutter R. M. Translational activation of the lck proto-oncogene. Nature. 1988 Mar 10;332(6160):171–173. doi: 10.1038/332171a0. [DOI] [PubMed] [Google Scholar]
- McLauchlan J., Gaffney D., Whitton J. L., Clements J. B. The consensus sequence YGTGTTYY located downstream from the AATAAA signal is required for efficient formation of mRNA 3' termini. Nucleic Acids Res. 1985 Feb 25;13(4):1347–1368. doi: 10.1093/nar/13.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa M., Semba K., Yoshida M. C., Yamamoto T., Sasaki M., Toyoshima K. Structure, expression, and chromosomal location of the human c-fgr gene. Mol Cell Biol. 1986 Feb;6(2):511–517. doi: 10.1128/mcb.6.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. C., Mardon G., Lebo R. V., Varmus H. E., Bishop J. M. Isolation of duplicated human c-src genes located on chromosomes 1 and 20. Mol Cell Biol. 1985 Apr;5(4):831–838. doi: 10.1128/mcb.5.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellman D., Garber E. A., Cross F. R., Hanafusa H. An N-terminal peptide from p60src can direct myristylation and plasma membrane localization when fused to heterologous proteins. 1985 Mar 28-Apr 3Nature. 314(6009):374–377. doi: 10.1038/314374a0. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintrell N., Lebo R., Varmus H., Bishop J. M., Pettenati M. J., Le Beau M. M., Diaz M. O., Rowley J. D. Identification of a human gene (HCK) that encodes a protein-tyrosine kinase and is expressed in hemopoietic cells. Mol Cell Biol. 1987 Jun;7(6):2267–2275. doi: 10.1128/mcb.7.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom J. T., Harris L. K., Cambier J. C. Anti-Ig induces release of inositol 1,4,5-trisphosphate, which mediates mobilization of intracellular Ca++ stores in B lymphocytes. J Immunol. 1986 Jul 15;137(2):708–714. [PubMed] [Google Scholar]
- Reynolds G. A., Basu S. K., Osborne T. F., Chin D. J., Gil G., Brown M. S., Goldstein J. L., Luskey K. L. HMG CoA reductase: a negatively regulated gene with unusual promoter and 5' untranslated regions. Cell. 1984 Aug;38(1):275–285. doi: 10.1016/0092-8674(84)90549-x. [DOI] [PubMed] [Google Scholar]
- Rogers J., Early P., Carter C., Calame K., Bond M., Hood L., Wall R. Two mRNAs with different 3' ends encode membrane-bound and secreted forms of immunoglobulin mu chain. Cell. 1980 Jun;20(2):303–312. doi: 10.1016/0092-8674(80)90616-9. [DOI] [PubMed] [Google Scholar]
- Rowe M., Rooney C. M., Edwards C. F., Lenoir G. M., Rickinson A. B. Epstein-Barr virus status and tumour cell phenotype in sporadic Burkitt's lymphoma. Int J Cancer. 1986 Mar 15;37(3):367–373. doi: 10.1002/ijc.2910370307. [DOI] [PubMed] [Google Scholar]
- Semba K., Nishizawa M., Miyajima N., Yoshida M. C., Sukegawa J., Yamanashi Y., Sasaki M., Yamamoto T., Toyoshima K. yes-related protooncogene, syn, belongs to the protein-tyrosine kinase family. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5459–5463. doi: 10.1073/pnas.83.15.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebhardt K., Mullins J. I., Bruck C., Rübsamen-Waigmann H. Additional member of the protein-tyrosine kinase family: the src- and lck-related protooncogene c-tkl. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8778–8782. doi: 10.1073/pnas.84.24.8778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendeman S., Thorley-Lawson D. A. The activation antigen BLAST-2, when shed, is an autocrine BCGF for normal and transformed B cells. EMBO J. 1987 Jun;6(6):1637–1642. doi: 10.1002/j.1460-2075.1987.tb02412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Gibbs C. P., Arthur R. R., Anderson S. K., Kung H. J., Fujita D. J. DNA sequence encoding the amino-terminal region of the human c-src protein: implications of sequence divergence among src-type kinase oncogenes. Mol Cell Biol. 1987 May;7(5):1978–1983. doi: 10.1128/mcb.7.5.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. B., Craig R. K., Beale D., Ketterer B. Construction and characterization of a plasmid containing complementary DNA to mRNA encoding the N-terminal amino acid sequence of the rat glutathione transferase Ya subunit. Biochem J. 1984 Apr 1;219(1):223–231. doi: 10.1042/bj2190223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Mann K. P. Early events in Epstein-Barr virus infection provide a model for B cell activation. J Exp Med. 1985 Jul 1;162(1):45–59. doi: 10.1084/jem.162.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick S. R., Popescu N. C., Cheah M. S., Swan D. C., Amsbaugh S. C., Lengel C. R., DiPaolo J. A., Robbins K. C. Isolation and chromosomal localization of the human fgr protooncogene, a distinct member of the tyrosine kinase gene family. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6595–6599. doi: 10.1073/pnas.82.19.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronova A. F., Sefton B. M. Expression of a new tyrosine protein kinase is stimulated by retrovirus promoter insertion. Nature. 1986 Feb 20;319(6055):682–685. doi: 10.1038/319682a0. [DOI] [PubMed] [Google Scholar]
- Willman C. L., Stewart C. C., Griffith J. K., Stewart S. J., Tomasi T. B. Differential expression and regulation of the c-src and c-fgr protooncogenes in myelomonocytic cells. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4480–4484. doi: 10.1073/pnas.84.13.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanashi Y., Fukushige S., Semba K., Sukegawa J., Miyajima N., Matsubara K., Yamamoto T., Toyoshima K. The yes-related cellular gene lyn encodes a possible tyrosine kinase similar to p56lck. Mol Cell Biol. 1987 Jan;7(1):237–243. doi: 10.1128/mcb.7.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]