Abstract
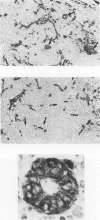
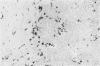
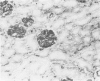
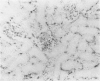
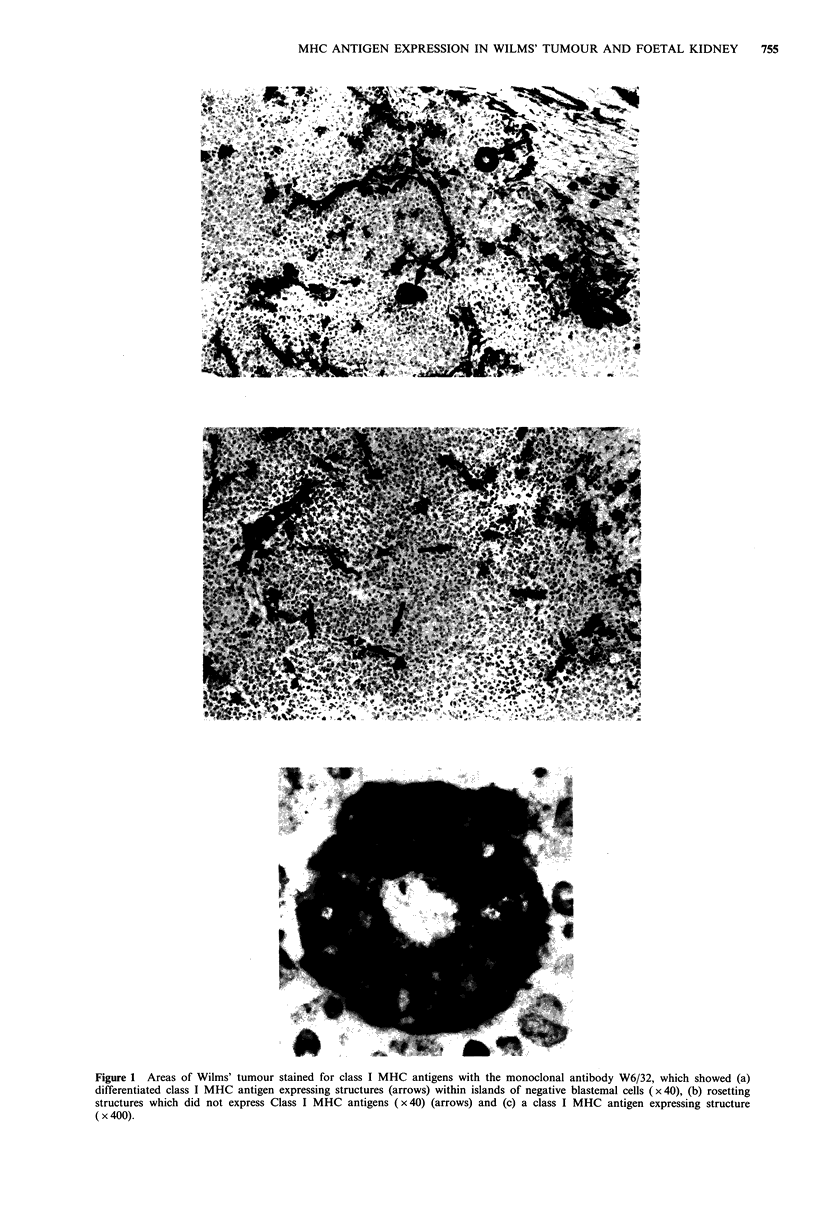
The Wilms' tumour is a solid childhood tumour of the kidney, consisting of blastema, tubules and mesenchyme. Embryonic tumours, such as Wilms', may arise as a result of a developmental disturbance in differentiation. The expression of class I and II major histocompatibility complex (MHC) antigens was investigated on 6 Wilms' tumours and related to that in the developing human kidney in this immunohistological study, using a panel of monoclonal antibodies. The Wilms' tumour blastemal cells were class I MHC antigen negative, but differentiated structures were positive. Class II MHC antigens were not observed in Wilms' tumours. In the developing human kidney class I MHC antigen expression was observed on glomeruli from 8 weeks and on tubules from 13 weeks gestational age. Class II MHC antigen expression was observed on glomeruli from 11 weeks and on tubules from 13 weeks gestation. These results suggest that the blastemal cells within the Wilms' tumour may reflect an early stage of development with respect to the expression of MHC antigens.
Full text
PDF

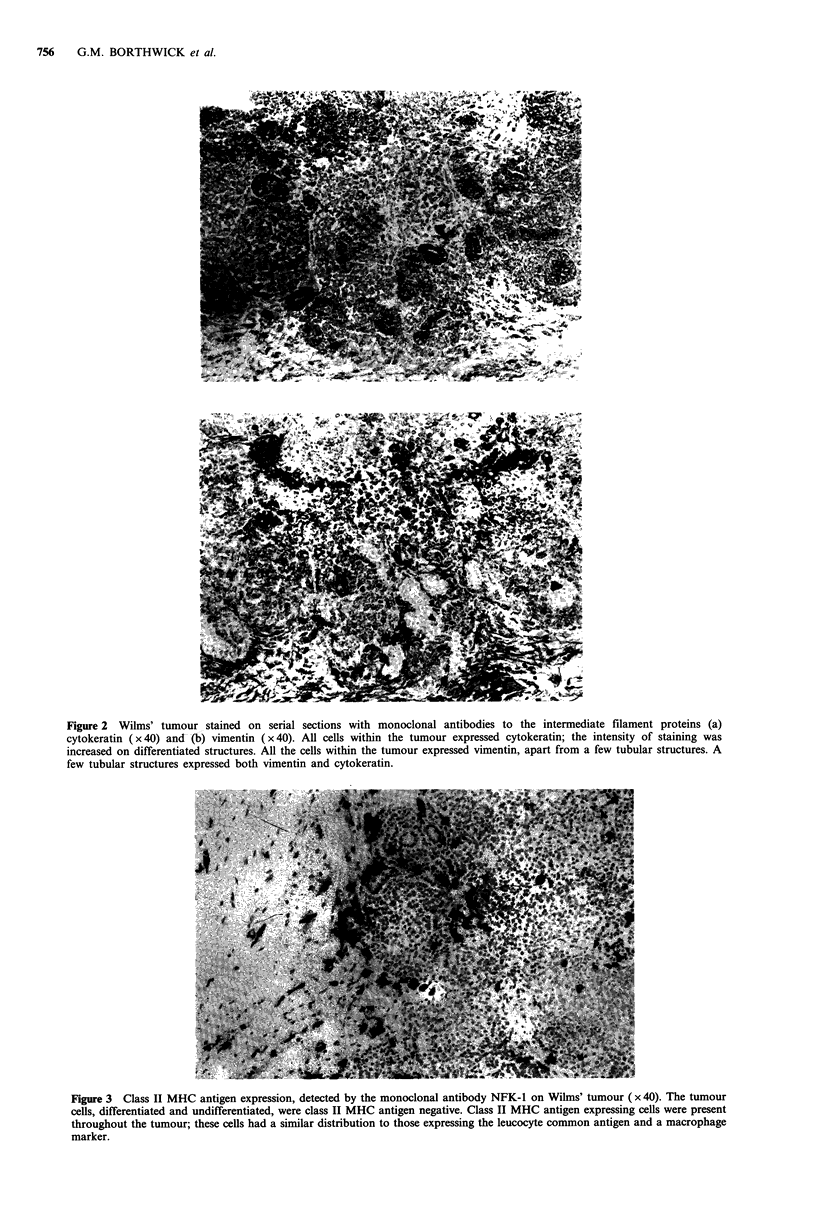
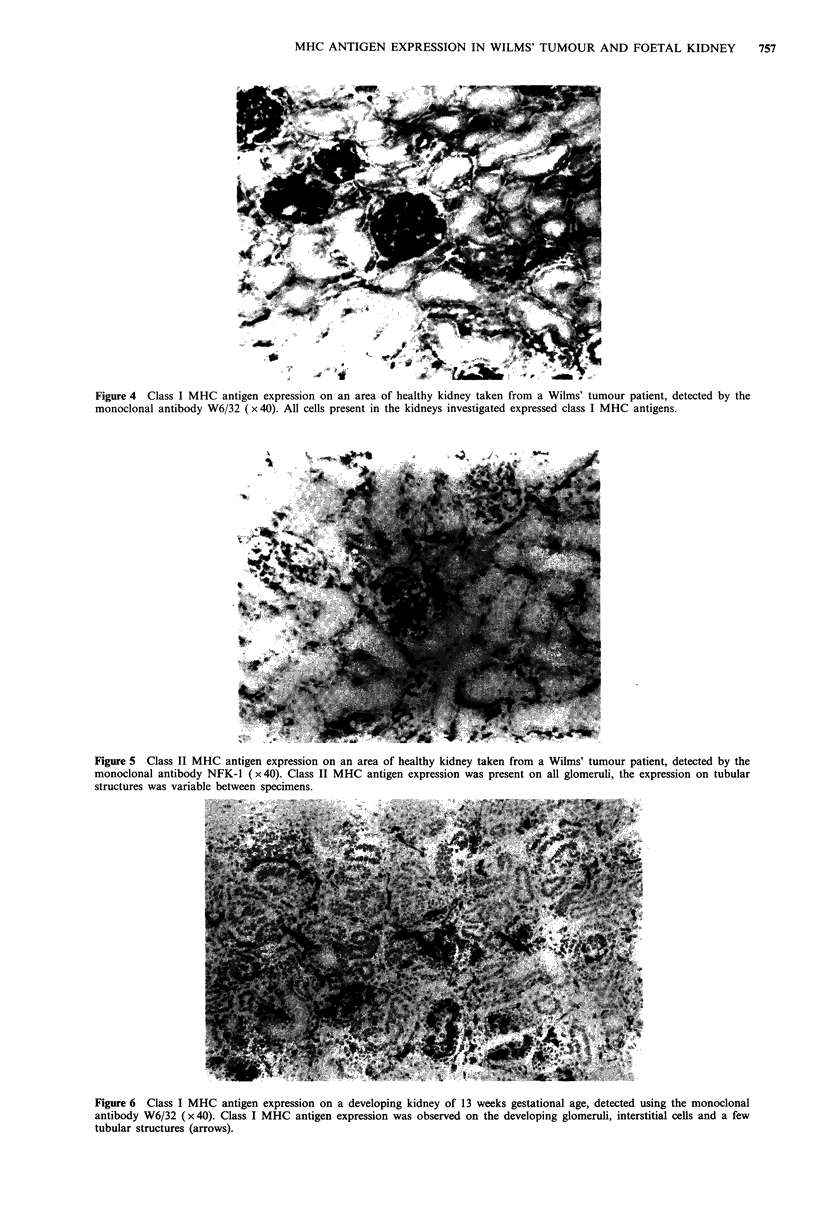
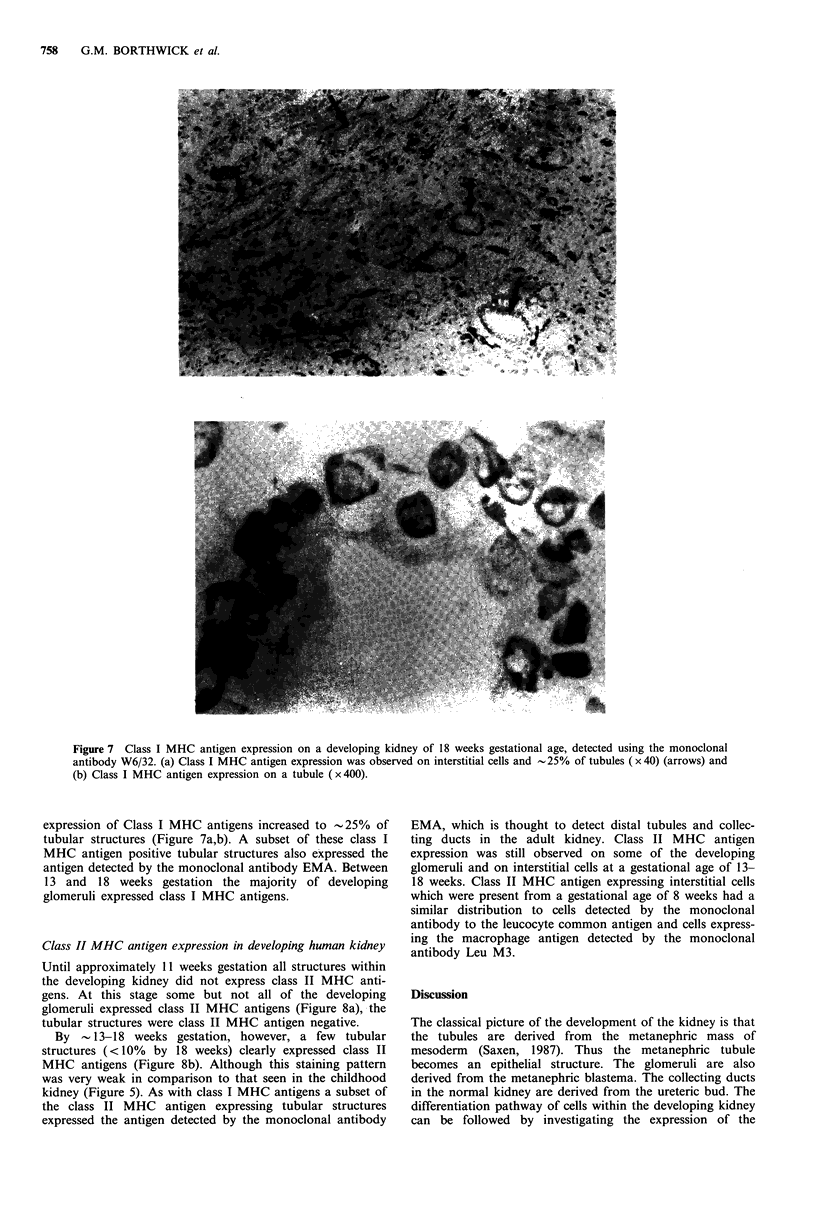
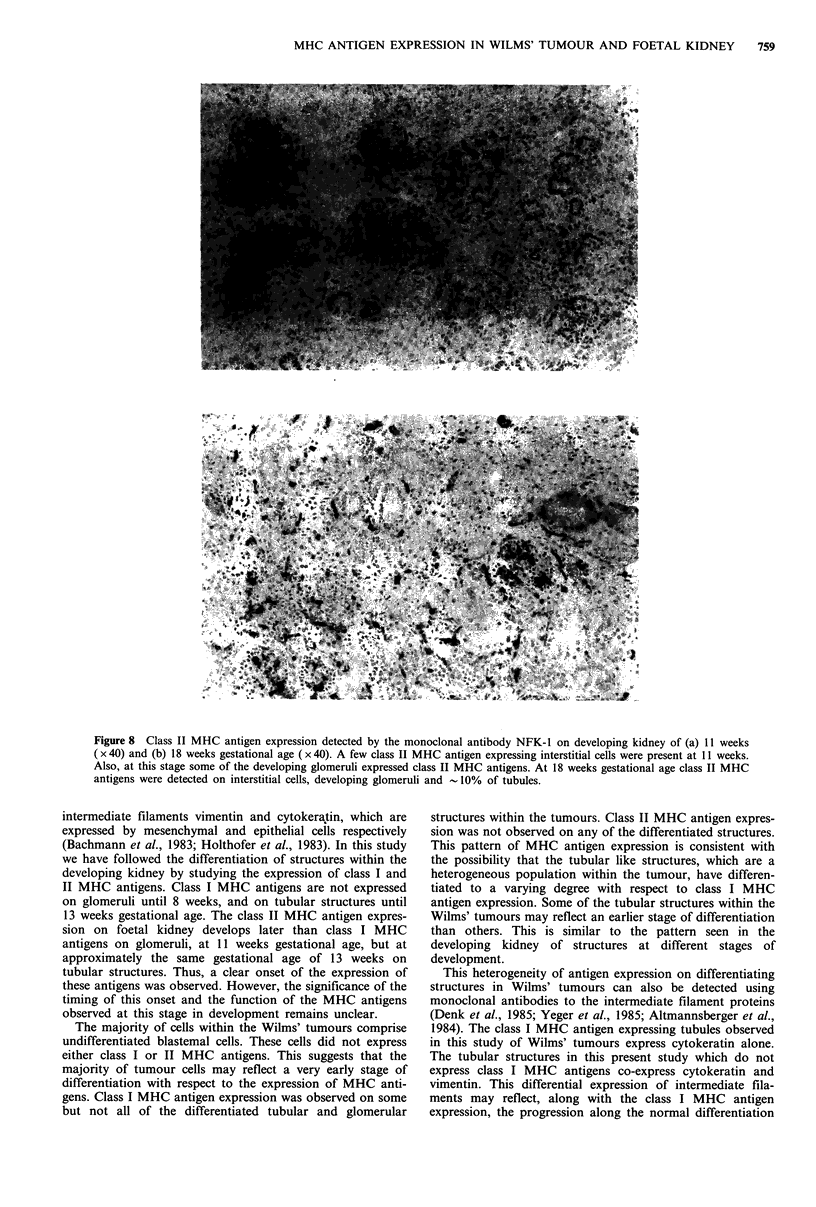


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmannsberger M., Osborn M., Schäfer H., Schauer A., Weber K. Distinction of nephroblastomas from other childhood tumors using antibodies to intermediate filaments. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45(2):113–124. doi: 10.1007/BF02889858. [DOI] [PubMed] [Google Scholar]
- Bachmann S., Kriz W., Kuhn C., Franke W. W. Differentiation of cell types in the mammalian kidney by immunofluorescence microscopy using antibodies to intermediate filament proteins and desmoplakins. Histochemistry. 1983;77(3):365–394. doi: 10.1007/BF00490899. [DOI] [PubMed] [Google Scholar]
- Bernards R., Dessain S. K., Weinberg R. A. N-myc amplification causes down-modulation of MHC class I antigen expression in neuroblastoma. Cell. 1986 Dec 5;47(5):667–674. doi: 10.1016/0092-8674(86)90509-x. [DOI] [PubMed] [Google Scholar]
- Brickell P. M., Latchman D. S., Murphy D., Willison K., Rigby P. W. Activation of a Qa/Tla class I major histocompatibility antigen gene is a general feature of oncogenesis in the mouse. Nature. 1983 Dec 22;306(5945):756–760. doi: 10.1038/306756a0. [DOI] [PubMed] [Google Scholar]
- Csiba A., Whitwell H. L., Moore M. Distribution of histocompatibility and leucocyte differentiation antigens in normal human colon and in benign and malignant colonic neoplasms. Br J Cancer. 1984 Nov;50(5):699–709. doi: 10.1038/bjc.1984.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daar A. S., Fuggle S. V., Fabre J. W., Ting A., Morris P. J. The detailed distribution of HLA-A, B, C antigens in normal human organs. Transplantation. 1984 Sep;38(3):287–292. doi: 10.1097/00007890-198409000-00018. [DOI] [PubMed] [Google Scholar]
- Daar A. S., Fuggle S. V., Fabre J. W., Ting A., Morris P. J. The detailed distribution of MHC Class II antigens in normal human organs. Transplantation. 1984 Sep;38(3):293–298. doi: 10.1097/00007890-198409000-00019. [DOI] [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human leukocyte-specific membrane glycoprotein probably homologous to the leukocyte-common (L-C) antigen of the rat. Eur J Immunol. 1980 Oct;10(10):737–744. doi: 10.1002/eji.1830101003. [DOI] [PubMed] [Google Scholar]
- Dimitriu-Bona A., Burmester G. R., Waters S. J., Winchester R. J. Human mononuclear phagocyte differentiation antigens. I. Patterns of antigenic expression on the surface of human monocytes and macrophages defined by monoclonal antibodies. J Immunol. 1983 Jan;130(1):145–152. [PubMed] [Google Scholar]
- Ellis S. A., Taylor C., McMichael A. Recognition of HLA-B27 and related antigen by a monoclonal antibody. Hum Immunol. 1982 Aug;5(1):49–59. doi: 10.1016/0198-8859(82)90030-1. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B., Feinberg A. P. Somatic deletion and duplication of genes on chromosome 11 in Wilms' tumours. Nature. 1984 May 10;309(5964):176–178. doi: 10.1038/309176a0. [DOI] [PubMed] [Google Scholar]
- Ferguson A., Moore M., Fox H. Expression of MHC products and leucocyte differentiation antigens in gynaecological neoplasms: an immunohistological analysis of the tumour cells and infiltrating leucocytes. Br J Cancer. 1985 Oct;52(4):551–563. doi: 10.1038/bjc.1985.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuggle S. V., Errasti P., Daar A. S., Fabre J. W., Ting A., Morris P. J. Localization of major histocompatibility complex (HLA-ABC and DR) antigens in 46 kidneys. Differences in HLA-DR staining of tubules among kidneys. Transplantation. 1983 Apr;35(4):385–390. doi: 10.1097/00007890-198304000-00024. [DOI] [PubMed] [Google Scholar]
- Heinemann D., Smith P. J., Symes M. O. Expression of histocompatibility antigens and characterisation of mononuclear cell infiltrates in human renal cell carcinomas. Br J Cancer. 1987 Oct;56(4):433–437. doi: 10.1038/bjc.1987.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyderman E., Strudley I., Powell G., Richardson T. C., Cordell J. L., Mason D. Y. A new monoclonal antibody to epithelial membrane antigen (EMA)-E29. A comparison of its immunocytochemical reactivity with polyclonal anti-EMA antibodies and with another monoclonal antibody, HMFG-2. Br J Cancer. 1985 Sep;52(3):355–361. doi: 10.1038/bjc.1985.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthöfer H., Miettinen A., Lehto V. P., Lehtonen E., Virtanen I. Expression of vimentin and cytokeratin types of intermediate filament proteins in developing and adult human kidneys. Lab Invest. 1984 May;50(5):552–559. [PubMed] [Google Scholar]
- Katzav S., Segal S., Feldman M. Immuno-selection in vivo of H-2D phenotypic variants from a metastatic clone of sarcoma cells results in cell lines of altered metastatic competence. Int J Cancer. 1984 Mar 15;33(3):407–415. doi: 10.1002/ijc.2910330320. [DOI] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Copeland N. G., Jenkins N. A., Lampkin B. C., Cavenee W. K. Loss of heterozygosity in three embryonal tumours suggests a common pathogenetic mechanism. Nature. 1985 Jul 25;316(6026):330–334. doi: 10.1038/316330a0. [DOI] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Lampkin B. C., Workman M. L., Copeland N. G., Jenkins N. A., Cavenee W. K. Loss of alleles at loci on human chromosome 11 during genesis of Wilms' tumour. Nature. 1984 May 10;309(5964):170–172. doi: 10.1038/309170a0. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Shew J. Y., Hong F. D., Sery T. W., Donoso L. A., Young L. J., Bookstein R., Lee E. Y. The retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activity. Nature. 1987 Oct 15;329(6140):642–645. doi: 10.1038/329642a0. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Parham P., Rust N., Brodsky F. A monoclonal antibody that recognizes an antigenic determinant shared by HLA A2 and B17. Hum Immunol. 1980 Sep;1(2):121–129. doi: 10.1016/0198-8859(80)90099-3. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Goldman D. S., Sallan S. E. Development of homozygosity for chromosome 11p markers in Wilms' tumour. Nature. 1984 May 10;309(5964):172–174. doi: 10.1038/309172a0. [DOI] [PubMed] [Google Scholar]
- Osborn M., Debus E., Weber K. Monoclonal antibodies specific for vimentin. Eur J Cell Biol. 1984 May;34(1):137–143. [PubMed] [Google Scholar]
- Osborn M., Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983 Apr;48(4):372–394. [PubMed] [Google Scholar]
- Platt J. L., LeBien T. W., Michael A. F. Stages of renal ontogenesis identified by monoclonal antibodies reactive with lymphohemopoietic differentiation antigens. J Exp Med. 1983 Jan 1;157(1):155–172. doi: 10.1084/jem.157.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve A. E., Housiaux P. J., Gardner R. J., Chewings W. E., Grindley R. M., Millow L. J. Loss of a Harvey ras allele in sporadic Wilms' tumour. Nature. 1984 May 10;309(5964):174–176. doi: 10.1038/309174a0. [DOI] [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Klempnauer K. H., Varmus H. E., Bishop J. M., Gilbert F., Brodeur G., Goldstein M., Trent J. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983 Sep 15;305(5931):245–248. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- Wallich R., Bulbuc N., Hämmerling G. J., Katzav S., Segal S., Feldman M. Abrogation of metastatic properties of tumour cells by de novo expression of H-2K antigens following H-2 gene transfection. Nature. 1985 May 23;315(6017):301–305. doi: 10.1038/315301a0. [DOI] [PubMed] [Google Scholar]
- Whitwell H. L., Hughes H. P., Moore M., Ahmed A. Expression of major histocompatibility antigens and leucocyte infiltration in benign and malignant human breast disease. Br J Cancer. 1984 Feb;49(2):161–172. doi: 10.1038/bjc.1984.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeger H., Baumal R., Bailey D., Pawlin G., Phillips M. J. Histochemical and immunohistochemical characterization of surgically resected and heterotransplanted Wilms' tumor. Cancer Res. 1985 May;45(5):2350–2357. [PubMed] [Google Scholar]
- Zola H., McNamara P. J., Moore H. A., Smart I. J., Brooks D. A., Beckman I. G., Bradley J. Maturation of human B lymphocytes--studies with a panel of monoclonal antibodies against membrane antigens. Clin Exp Immunol. 1983 Jun;52(3):655–664. [PMC free article] [PubMed] [Google Scholar]