Abstract
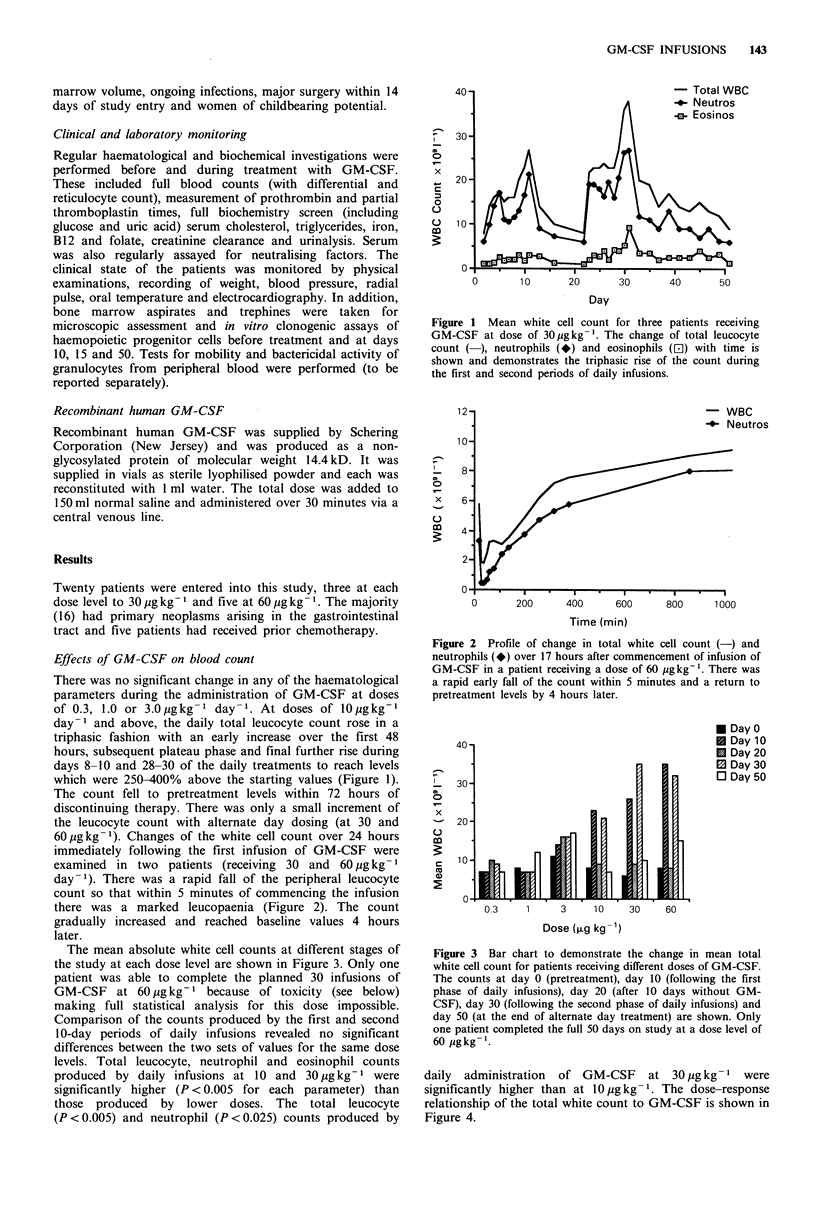
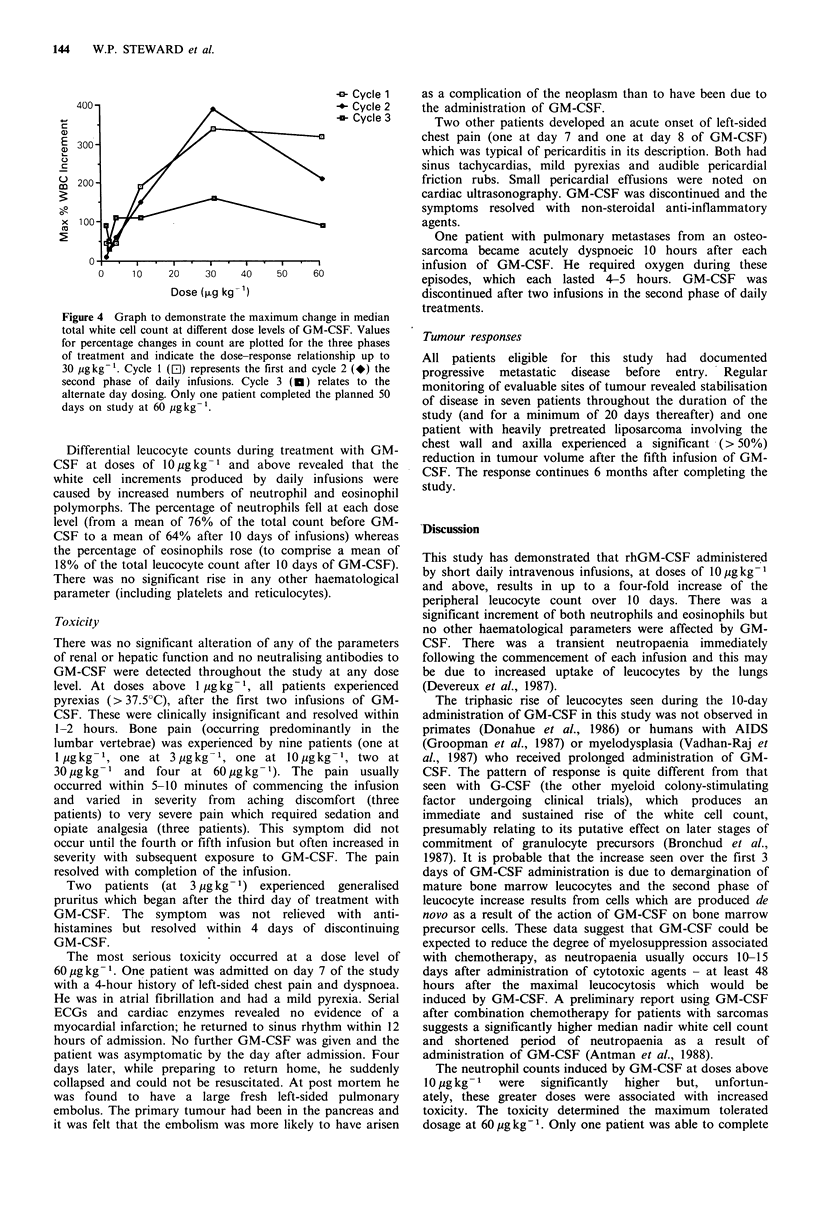
Twenty patients with progressive metastatic solid tumours were entered into a study to evaluate the biological effects and toxicity of recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF). GM-CSF was given as half-hour intravenous infusions during two 10-day phases of daily treatments (separated by 10 days without GM-CSF) and over a final phase of 20 days of alternate day infusions. Doses were escalated in steps from 0.3 to 60 micrograms kg-1 day-1 between successive patient groups. Significant increases (P less than 0.005) of total leucocyte, neutrophil and eosinophil polymorph counts were seen over the periods of daily infusions (up to four-fold rises of total white count) at dose levels of 10 micrograms kg-1 and above. Counts produced at 30 micrograms kg-1 were significantly higher than at 10 micrograms kg-1 (P less than 0.025). Toxic side effects of GM-CSF included mild transient pyrexias, bone pain and pruritus. The maximum tolerated dose was 60 micrograms kg-1, which produced severe toxicity in 80% of patients. The toxicity at this dose included pericarditis and dyspnoea ascribed to a 'capillary-leak' syndrome. One patient receiving 60 micrograms kg-1 died as a result of a pulmonary embolus. Seven patients with previously rapidly progressive metastatic tumours experienced stabilisation of disease while receiving GM-CSF and one patient with a previously heavily pretreated metastatic soft tissue sarcoma underwent a greater than 50% reduction of tumour volume. Patients undergoing chemotherapy may benefit both from a reduction of the myelosuppressive effects of cytotoxic agents and from an antitumour effect if GM-CSF is incorporated into future regimens.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Brandt S. J., Peters W. P., Atwater S. K., Kurtzberg J., Borowitz M. J., Jones R. B., Shpall E. J., Bast R. C., Jr, Gilbert C. J., Oette D. H. Effect of recombinant human granulocyte-macrophage colony-stimulating factor on hematopoietic reconstitution after high-dose chemotherapy and autologous bone marrow transplantation. N Engl J Med. 1988 Apr 7;318(14):869–876. doi: 10.1056/NEJM198804073181401. [DOI] [PubMed] [Google Scholar]
- Bronchud M. H., Scarffe J. H., Thatcher N., Crowther D., Souza L. M., Alton N. K., Testa N. G., Dexter T. M. Phase I/II study of recombinant human granulocyte colony-stimulating factor in patients receiving intensive chemotherapy for small cell lung cancer. Br J Cancer. 1987 Dec;56(6):809–813. doi: 10.1038/bjc.1987.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell M. A., Anderson D., Cerretti D. P., Price V., McKereghan K., Tushinski R. J., Mochizuki D. Y., Larsen A., Grabstein K., Gillis S. Cloning, sequence, and expression of a human granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6250–6254. doi: 10.1073/pnas.82.18.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux S., Linch D. C., Campos Costa D., Spittle M. F., Jelliffe A. M. Transient leucopenia induced by granulocyte-macrophage colony-stimulating factor. Lancet. 1987 Dec 26;2(8574):1523–1524. doi: 10.1016/s0140-6736(87)92654-7. [DOI] [PubMed] [Google Scholar]
- Donahue R. E., Wang E. A., Stone D. K., Kamen R., Wong G. G., Sehgal P. K., Nathan D. G., Clark S. C. Stimulation of haematopoiesis in primates by continuous infusion of recombinant human GM-CSF. 1986 Jun 26-Jul 2Nature. 321(6073):872–875. doi: 10.1038/321872a0. [DOI] [PubMed] [Google Scholar]
- Gasson J. C., Weisbart R. H., Kaufman S. E., Clark S. C., Hewick R. M., Wong G. G., Golde D. W. Purified human granulocyte-macrophage colony-stimulating factor: direct action on neutrophils. Science. 1984 Dec 14;226(4680):1339–1342. doi: 10.1126/science.6390681. [DOI] [PubMed] [Google Scholar]
- Grabstein K. H., Urdal D. L., Tushinski R. J., Mochizuki D. Y., Price V. L., Cantrell M. A., Gillis S., Conlon P. J. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 1986 Apr 25;232(4749):506–508. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- Groopman J. E., Mitsuyasu R. T., DeLeo M. J., Oette D. H., Golde D. W. Effect of recombinant human granulocyte-macrophage colony-stimulating factor on myelopoiesis in the acquired immunodeficiency syndrome. N Engl J Med. 1987 Sep 3;317(10):593–598. doi: 10.1056/NEJM198709033171003. [DOI] [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Cuthbertson R. A., Lyons I., Stanley E., Kelso A., Kannourakis G., Williamson D. J., Klintworth G. K., Gonda T. J. Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell. 1987 Nov 20;51(4):675–686. doi: 10.1016/0092-8674(87)90136-x. [DOI] [PubMed] [Google Scholar]
- Lopez A. F., Nicola N. A., Burgess A. W., Metcalf D., Battye F. L., Sewell W. A., Vadas M. Activation of granulocyte cytotoxic function by purified mouse colony-stimulating factors. J Immunol. 1983 Dec;131(6):2983–2988. [PubMed] [Google Scholar]
- Metcalf D., Burgess A. W. Clonal analysis of progenitor cell commitment of granulocyte or macrophage production. J Cell Physiol. 1982 Jun;111(3):275–283. doi: 10.1002/jcp.1041110308. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The granulocyte-macrophage colony-stimulating factors. Science. 1985 Jul 5;229(4708):16–22. doi: 10.1126/science.2990035. [DOI] [PubMed] [Google Scholar]
- Nienhuis A. W., Donahue R. E., Karlsson S., Clark S. C., Agricola B., Antinoff N., Pierce J. E., Turner P., Anderson W. F., Nathan D. G. Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) shortens the period of neutropenia after autologous bone marrow transplantation in a primate model. J Clin Invest. 1987 Aug;80(2):573–577. doi: 10.1172/JCI113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley I. J., Burgess A. W. Granulocyte macrophage-colony stimulating factor stimulates the synthesis of membrane and nuclear proteins in murine neutrophils. J Cell Biochem. 1983;23(1-4):241–258. doi: 10.1002/jcb.240230121. [DOI] [PubMed] [Google Scholar]
- Vadhan-Raj S., Keating M., LeMaistre A., Hittelman W. N., McCredie K., Trujillo J. M., Broxmeyer H. E., Henney C., Gutterman J. U. Effects of recombinant human granulocyte-macrophage colony-stimulating factor in patients with myelodysplastic syndromes. N Engl J Med. 1987 Dec 17;317(25):1545–1552. doi: 10.1056/NEJM198712173172501. [DOI] [PubMed] [Google Scholar]


