Abstract
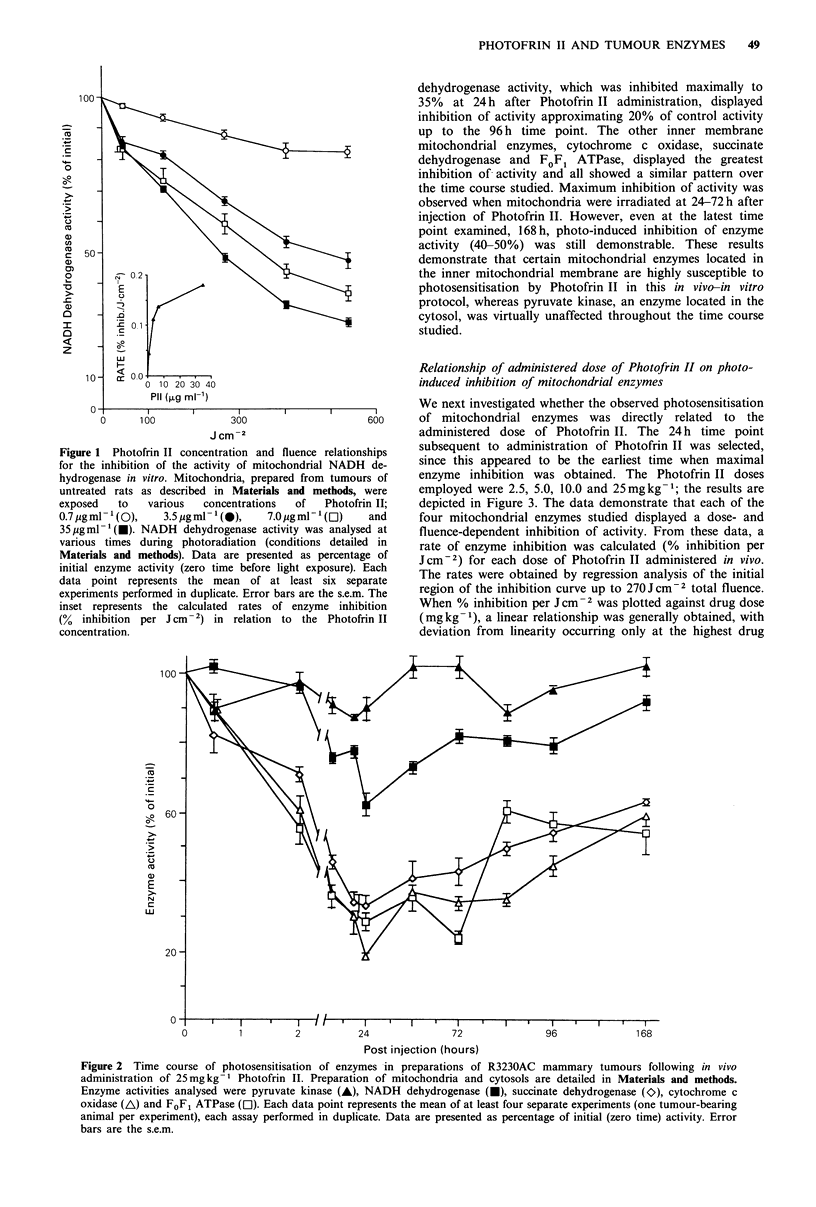
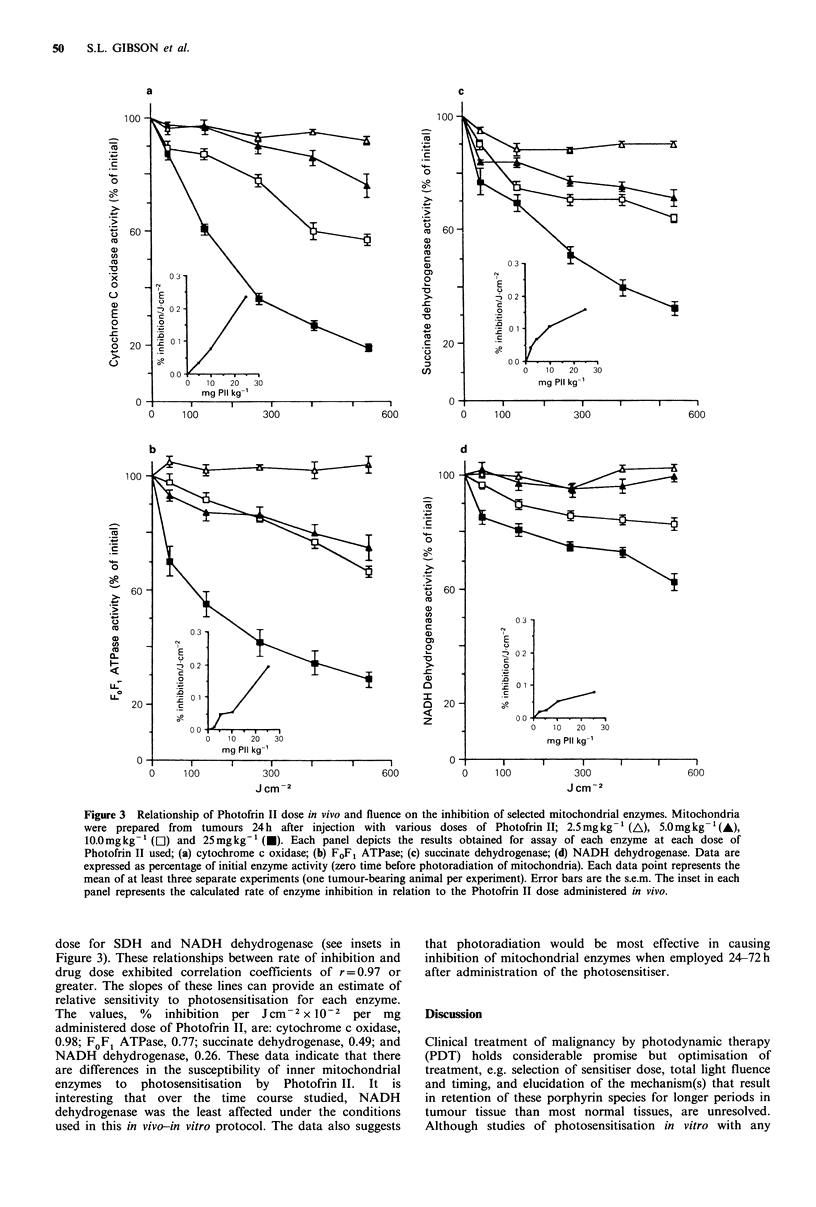
The ability of injected Photofrin II, a preparation enriched in hydrophobic dihaematoporphyrin ethers and esters, to photosensitize selected mitochondrial and cytosolic enzymes during illumination in vitro was examined. Preparations of R3230AC mammary tumours, obtained at designated times after a single dose of Photofrin II, displayed a time-dependent photosensitivity. Maximum inhibition of mitochondrial enzymes occurred at 24 hours post-treatment, whereas no inhibition of the cytosolic enzyme, pyruvate kinase, was observed over the 168 hour time course. At the selected 24 hour time point, mitochondrial enzyme photosensitisation was found to be drug dose (5.25 mg kg-1 Photofrin II) and light dose dependent, the rank order of inhibition being cytochrome c oxidase greater than F0F1 ATPase greater than succinate dehydrogenase greater than NADH dehydrogenase. We conclude that porphyrin species contained in Photofrin II accumulate in mitochondria of tumour cells in vivo and produce maximum photosensitisation at 24-72 hours after administration to tumour-bearing animals. The time course observed here with Photofrin II is similar to that seen previously with the more heterogenous haematoporphyrin derivative preparation in this in vivo-in vitro model.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzi A. Cytochrome c oxidase. Towards a clarification of its structure, interactions and mechanism. Biochim Biophys Acta. 1980 Dec;594(4):231–252. doi: 10.1016/0304-4173(80)90002-6. [DOI] [PubMed] [Google Scholar]
- Berenbaum M. C., Bonnett R., Scourides P. A. In vivo biological activity of the components of haematoporphyrin derivative. Br J Cancer. 1982 Apr;45(4):571–581. doi: 10.1038/bjc.1982.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns M. W., Dahlman A., Johnson F. M., Burns R., Sperling D., Guiltinan M., Siemens A., Walter R., Wright W., Hammer-Wilson M. In vitro cellular effects of hematoporphyrin derivative. Cancer Res. 1982 Jun;42(6):2325–2329. [PubMed] [Google Scholar]
- Byrne C. J., Marshallsay L. V., Ward A. D. The structure of the active material in hematoporphyrin derivative. Photochem Photobiol. 1987 Nov;46(5):575–580. doi: 10.1111/j.1751-1097.1987.tb04816.x. [DOI] [PubMed] [Google Scholar]
- Böhmer R. M., Morstyn G. Uptake of hematoporphyrin derivative by normal and malignant cells: effect of serum, pH, temperature, and cell size. Cancer Res. 1985 Nov;45(11 Pt 1):5328–5334. [PubMed] [Google Scholar]
- De Meis L., Tuena de Gómez Puyou M., Gómez Puyou A. Inhibition of mitochondrial F1 ATPase and sarcoplasmic reticulum ATPase by hydrophobic molecules. Eur J Biochem. 1988 Jan 15;171(1-2):343–349. doi: 10.1111/j.1432-1033.1988.tb13796.x. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J. Studies on the structure of porphyrins contained in Photofrin II. Photochem Photobiol. 1987 Nov;46(5):569–573. doi: 10.1111/j.1751-1097.1987.tb04815.x. [DOI] [PubMed] [Google Scholar]
- Fingar V. H., Henderson B. W. Drug and light dose dependence of photodynamic therapy: a study of tumor and normal tissue response. Photochem Photobiol. 1987 Nov;46(5):837–841. doi: 10.1111/j.1751-1097.1987.tb04856.x. [DOI] [PubMed] [Google Scholar]
- Freitas I., Novarina A. Dark effects of hematoporphyrin derivative on lactate dehydrogenase activity and distribution in HeLa cells: cytochemical evaluation. Photochem Photobiol. 1987 Nov;46(5):699–706. doi: 10.1111/j.1751-1097.1987.tb04835.x. [DOI] [PubMed] [Google Scholar]
- Gibson S. L., Cohen H. J., Hilf R. Evidence against the production of superoxide by photoirradiation of hematoporphyrin derivative. Photochem Photobiol. 1984 Oct;40(4):441–448. doi: 10.1111/j.1751-1097.1984.tb04615.x. [DOI] [PubMed] [Google Scholar]
- Gibson S. L., Hilf R. Photosensitization of mitochondrial cytochrome c oxidase by hematoporphyrin derivative and related porphyrins in vitro and in vivo. Cancer Res. 1983 Sep;43(9):4191–4197. [PubMed] [Google Scholar]
- Gibson S. L., Murant R. S., Hilf R. Photosensitizing effects of hematoporphyrin derivative and photofrin II on the plasma membrane enzymes 5'-nucleotidase, Na+K+-ATPase, and Mg2+-ATPase in R3230AC mammary adenocarcinomas. Cancer Res. 1988 Jun 15;48(12):3360–3366. [PubMed] [Google Scholar]
- Gibson S. L., Murant R. S., Hilf R. Photosensitizing effects of hematoporphyrin derivative immobilized on sepharose. Photochem Photobiol. 1987 Jan;45(1):93–104. doi: 10.1111/j.1751-1097.1987.tb08409.x. [DOI] [PubMed] [Google Scholar]
- Gomer C. J., Dougherty T. J. Determination of [3H]- and [14C]hematoporphyrin derivative distribution in malignant and normal tissue. Cancer Res. 1979 Jan;39(1):146–151. [PubMed] [Google Scholar]
- HILF R., MICHEL I., BELL C., FREEMAN J. J., BORMAN A. BIOCHEMICAL AND MORPHOLOGIC PROPERTIES OF A NEW LACTATING MAMMARY TUMOR LINE IN THE RAT. Cancer Res. 1965 Apr;25:286–299. [PubMed] [Google Scholar]
- Hilf R., Leakey P. B., Sollott S. J., Gibson S. L. Photodynamic inactivation of R3230AC mammary carcinoma in vitro with hematoporphyrin derivative: effects of dose, time, and serum on uptake and phototoxicity. Photochem Photobiol. 1983 Jun;37(6):633–642. doi: 10.1111/j.1751-1097.1983.tb04532.x. [DOI] [PubMed] [Google Scholar]
- Hilf R., Smail D. B., Murant R. S., Leakey P. B., Gibson S. L. Hematoporphyrin derivative-induced photosensitivity of mitochondrial succinate dehydrogenase and selected cytosolic enzymes of R3230AC mammary adenocarcinomas of rats. Cancer Res. 1984 Apr;44(4):1483–1488. [PubMed] [Google Scholar]
- KING T. E., HOWARD R. L. The preparation and some properties of a reduced diphosphopyridine nucleotide dehydrogenase from the snake venom digest of a heartmuscle preparation. J Biol Chem. 1962 May;237:1686–1698. [PubMed] [Google Scholar]
- Kessel D., Chou T. H. Tumor-localizing components of the porphyrin preparation hematoporphyrin derivative. Cancer Res. 1983 May;43(5):1994–1999. [PubMed] [Google Scholar]
- Kessel D., Thompson P., Musselman B., Chang C. K. Chemistry of hematoporphyrin-derived photosensitizers. Photochem Photobiol. 1987 Nov;46(5):563–568. doi: 10.1111/j.1751-1097.1987.tb04814.x. [DOI] [PubMed] [Google Scholar]
- LIPSON R. L., BALDES E. J., OLSEN A. M. The use of a derivative of hematoporhyrin in tumor detection. J Natl Cancer Inst. 1961 Jan;26:1–11. [PubMed] [Google Scholar]
- Moan J., Christensen T., Sommer S. The main photosensitizing components of hematoporphyrin derivative. Cancer Lett. 1982 Feb;15(2):161–166. doi: 10.1016/0304-3835(82)90046-5. [DOI] [PubMed] [Google Scholar]
- Moan J., McGhie J., Jacobsen P. B. Photodynamic effects on cells in vitro exposed to hematoporphyrin derivative and light. Photochem Photobiol. 1983 Jun;37(6):599–604. doi: 10.1111/j.1751-1097.1983.tb04527.x. [DOI] [PubMed] [Google Scholar]
- Moan J., Rimington C., Sommer S. Cellular uptake and photosensitizing properties of hematoporphyrin di-ethers with similar chromatographic properties as the tumorlocalizing fraction of hematoporphyrin derivative. Cancer Lett. 1987 Mar;34(3):283–289. doi: 10.1016/0304-3835(87)90178-9. [DOI] [PubMed] [Google Scholar]
- Moreno G., Atlante A., Salet C., Santus R., Vinzens F. Photosensitivity of DNA replication and respiration to haematoporphyrin derivative (photofrin II) in mammalian CV-1 cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1987 Aug;52(2):213–222. doi: 10.1080/09553008714551681. [DOI] [PubMed] [Google Scholar]
- Murant R. S., Gibson S. L., Hilf R. Photosensitizing effects of Photofrin II on the site-selected mitochondrial enzymes adenylate kinase and monoamine oxidase. Cancer Res. 1987 Aug 15;47(16):4323–4328. [PubMed] [Google Scholar]
- Salet C., Moreno G., Vinzens F. Effects of photodynamic action on energy coupling of Ca2+ uptake in liver mitochondria. Biochem Biophys Res Commun. 1983 Aug 30;115(1):76–81. doi: 10.1016/0006-291x(83)90970-1. [DOI] [PubMed] [Google Scholar]
- Singh G., Jeeves W. P., Wilson B. C., Jang D. Mitochondrial photosensitization by Photofrin II. Photochem Photobiol. 1987 Nov;46(5):645–649. doi: 10.1111/j.1751-1097.1987.tb04826.x. [DOI] [PubMed] [Google Scholar]
- Star W. M., Marijnissen H. P., van den Berg-Blok A. E., Versteeg J. A., Franken K. A., Reinhold H. S. Destruction of rat mammary tumor and normal tissue microcirculation by hematoporphyrin derivative photoradiation observed in vivo in sandwich observation chambers. Cancer Res. 1986 May;46(5):2532–2540. [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]


