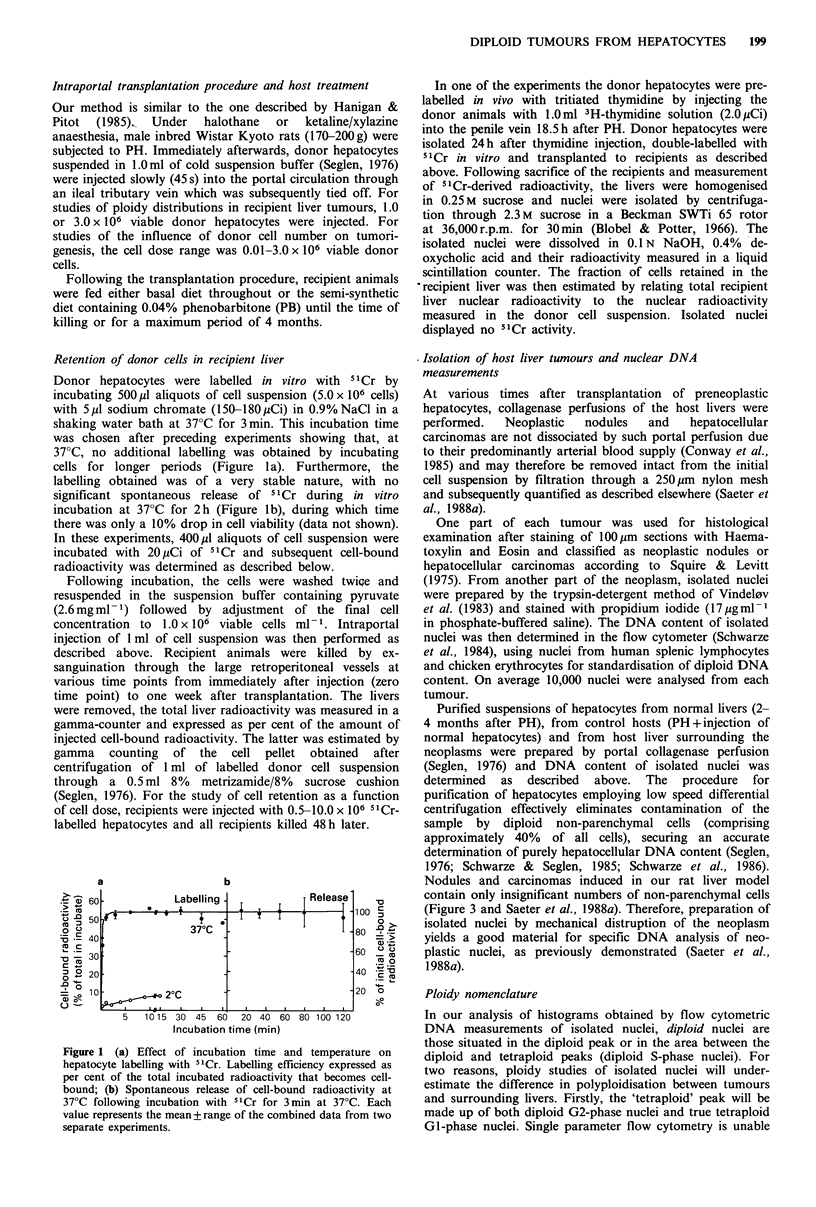
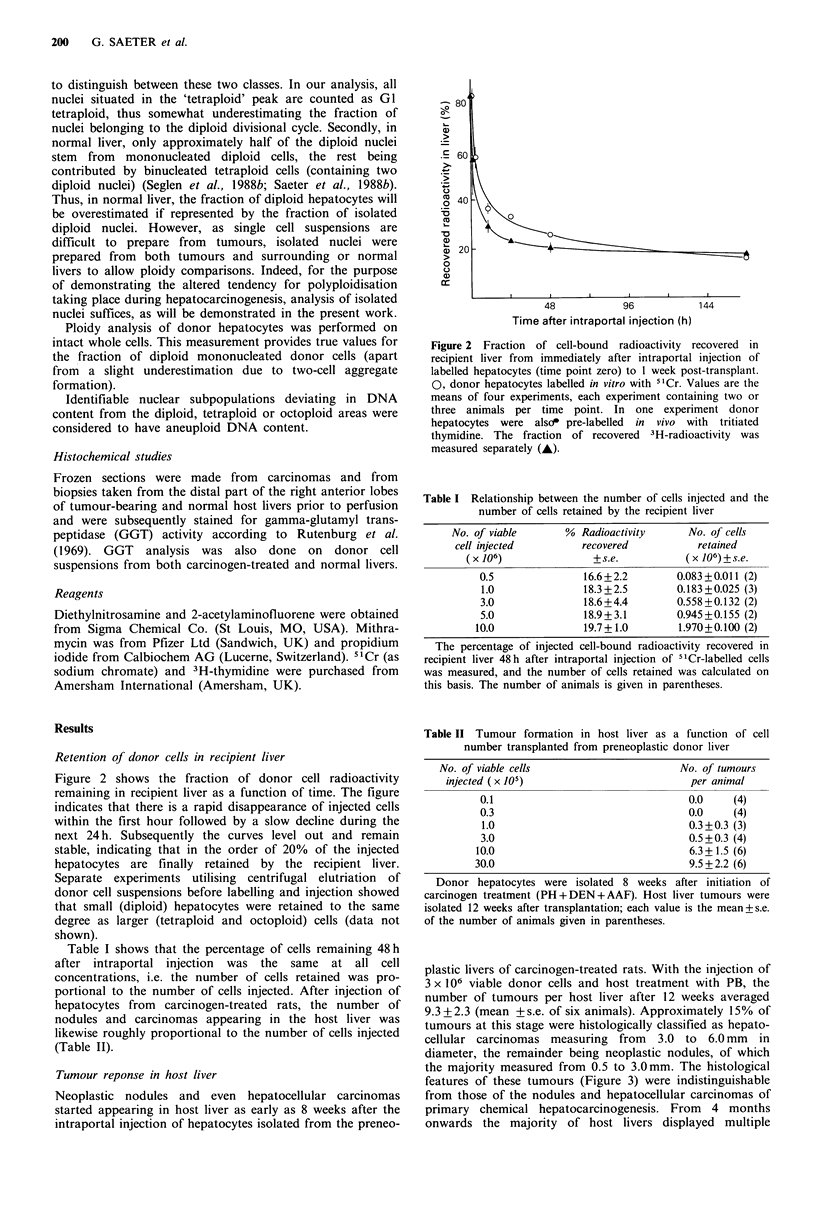
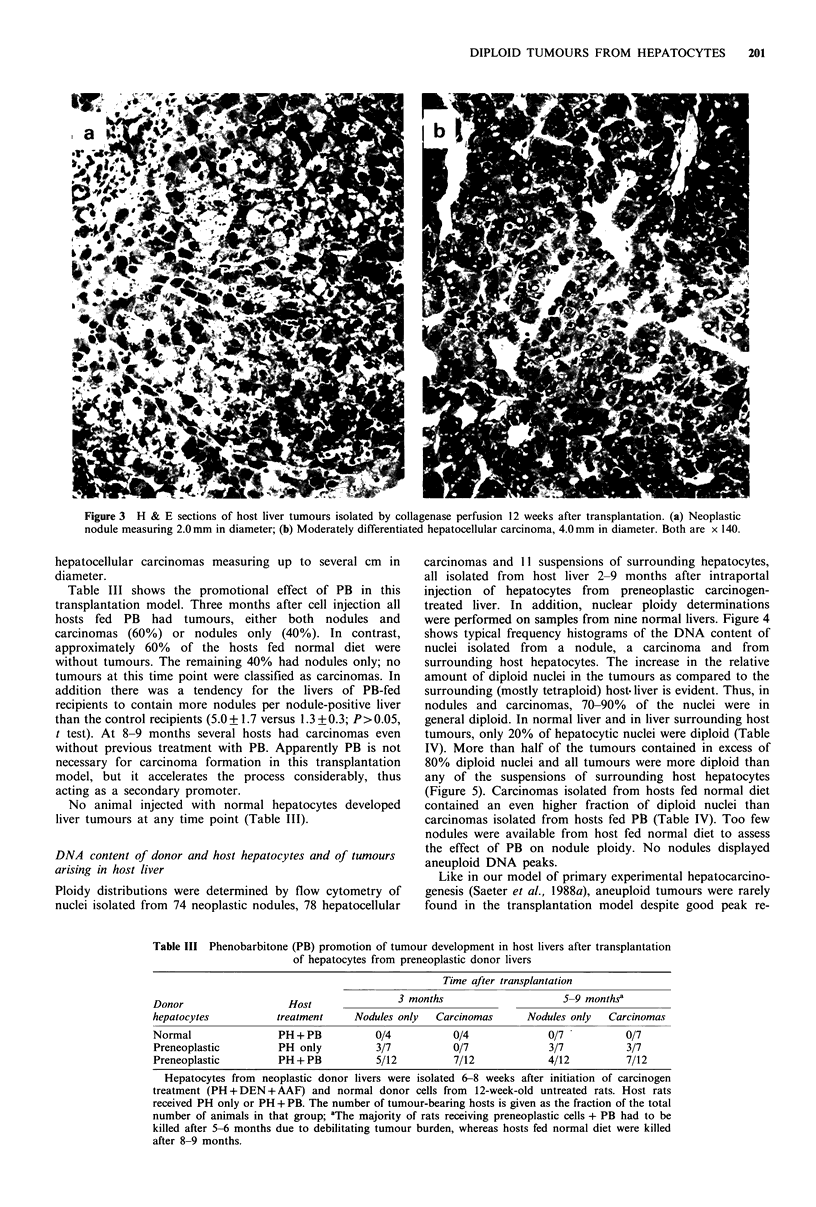
Abstract
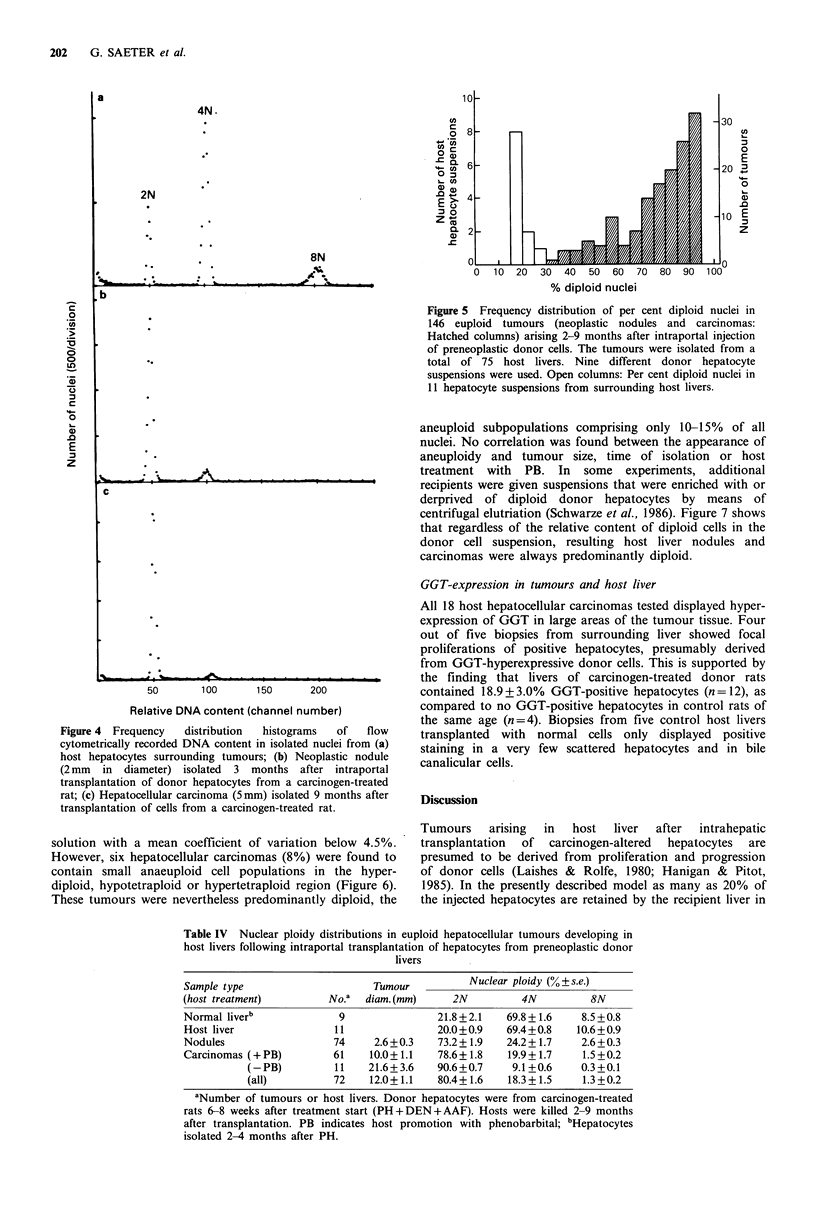
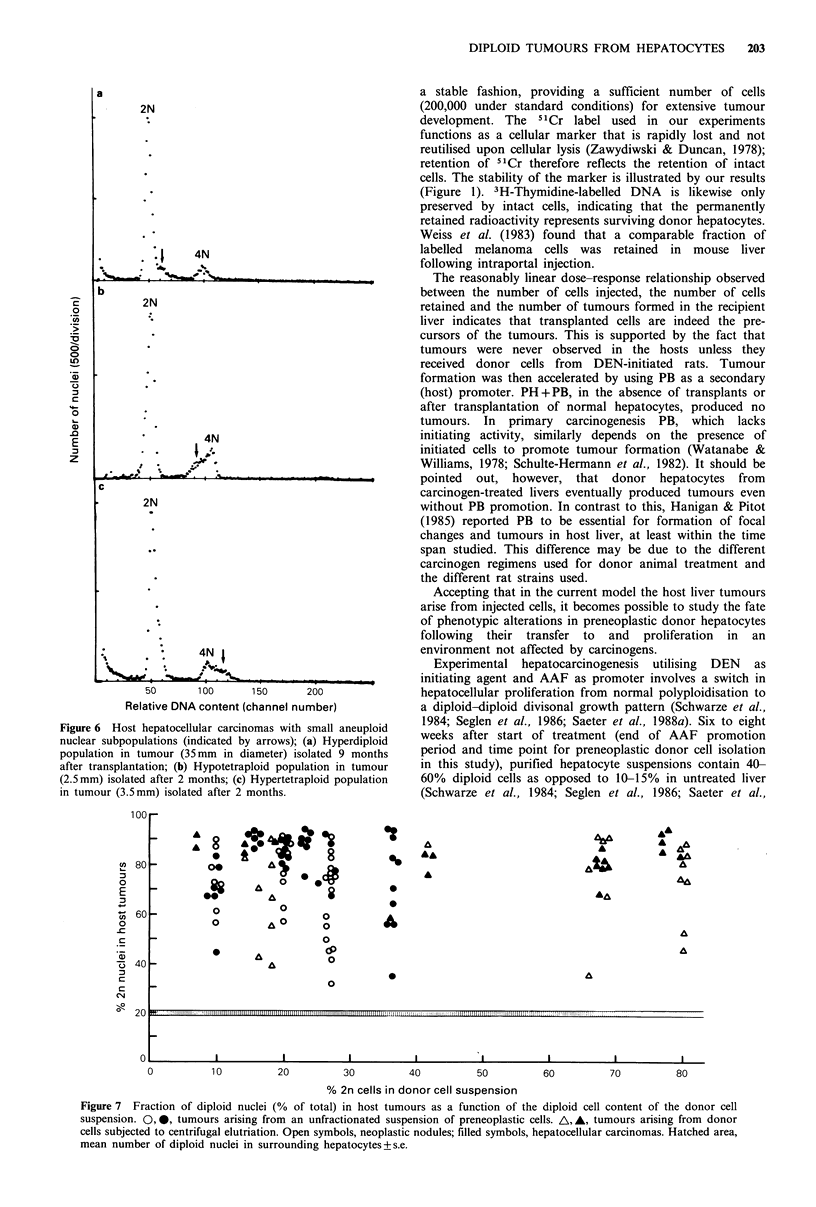
Hepatocyte suspensions were transplanted to the livers of syngeneic Wistar Kyoto rats by means of intraportal injection. Labelling of the donor cells with 51Cr or tritiated thymidine showed that 20% of the cells survived the transplantation procedure and were permanently retained by the recipient liver. Hepatocytes transplanted from normal livers produced no tumours, whereas donor cells from preneoplastic livers of rats treated with the carcinogens diethylnitrosamine and 2-acetylaminofluorene produced neoplastic nodules and hepatocellular carcinomas in the recipients. The number of tumours per host liver was proportional to the number of hepatocytes transplanted. Treatment of the host rats with phenobarbitone accelerated tumour development, causing liver cancer in the majority of the animals within three months. As opposed to the polyploid surrounding liver, both phenobarbitone-promoted and unpromoted host tumours contained predominantly (70-90%) diploid cells, regardless of the wide range of transplant ploidies (10-80% diploid cells) achieved by means of centrifugal elutriation. The results indicate that all host tumours arise from diploid donor hepatocytes and that the acquisition of a constitutive, predominantly non-polyploidising growth pattern may be a characteristic property of hepatocellular tumours.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Brodsky W. Y., Uryvaeva I. V. Cell polyploidy: its relation to tissue growth and function. Int Rev Cytol. 1977;50:275–332. doi: 10.1016/s0074-7696(08)60100-x. [DOI] [PubMed] [Google Scholar]
- Carriere R. The growth of liver parenchymal nuclei and its endocrine regulation. Int Rev Cytol. 1969;25:201–277. doi: 10.1016/s0074-7696(08)60204-1. [DOI] [PubMed] [Google Scholar]
- Conway J. G., Popp J. A., Thurman R. G. Microcirculation of hepatic nodules from diethylnitrosamine-treated rats. Cancer Res. 1985 Aug;45(8):3620–3625. [PubMed] [Google Scholar]
- Deleener A., Castelain P., Preat V., de Gerlache J., Alexandre H., Kirsch-Volders M. Changes in nucleolar transcriptional activity and nuclear DNA content during the first steps of rat hepatocarcinogenesis. Carcinogenesis. 1987 Feb;8(2):195–201. doi: 10.1093/carcin/8.2.195. [DOI] [PubMed] [Google Scholar]
- Finkelstein S. D., Lee G., Medline A., Tatematsu M., Makowka L., Farber E. An experimental method for rapid growth of liver in spleen. The survival and proliferation of chemically induced preneoplastic hepatocytes in spleen. Am J Pathol. 1983 Feb;110(2):119–126. [PMC free article] [PubMed] [Google Scholar]
- Hanigan M. H., Pitot H. C. Growth of carcinogen-altered rat hepatocytes in the liver of syngeneic recipients promoted with phenobarbital. Cancer Res. 1985 Dec;45(12 Pt 1):6063–6070. [PubMed] [Google Scholar]
- Hunt J. M., Buckley M. T., Onnink P. A., Rolfe P. B., Laishes B. A. Liver cell membrane alloantigens as cellular markers in genotypic mosaic rat livers undergoing chemically induced hepatocarcinogenesis. Cancer Res. 1982 Jan;42(1):227–236. [PubMed] [Google Scholar]
- Irving C. C., Roszell J. A., Fredi J. L. Effects of chronic feeding of 2-acetylaminofluorene on nuclear populations in rat liver. Adv Enzyme Regul. 1977 Oct 3;16:365–377. doi: 10.1016/0065-2571(78)90083-3. [DOI] [PubMed] [Google Scholar]
- Laishes B. A., Fink L., Carr B. I. A liver colony assay for a new hepatocyte phenotype as a step toward purifying new cellular phenotypes that arise during hepatocarcinogenesis. Ann N Y Acad Sci. 1980;349:373–382. doi: 10.1111/j.1749-6632.1980.tb29540.x. [DOI] [PubMed] [Google Scholar]
- Laishes B. A., Rolfe P. B. Quantitative assessment of liver colony formation and hepatocellular carcinoma incidence in rats receiving intravenous injections of isogeneic liver cells isolated during hepatocarcinogenesis. Cancer Res. 1980 Nov;40(11):4133–4143. [PubMed] [Google Scholar]
- Neal G. E., Judah D. J., Butler W. H. Some effects of acute and chronic dosing with aflatoxin B1 on rat liver nuclei. Cancer Res. 1976 May;36(5):1771–1778. [PubMed] [Google Scholar]
- Roomi M. W., Ho R. K., Sarma D. S., Farber E. A common biochemical pattern in preneoplastic hepatocyte nodules generated in four different models in the rat. Cancer Res. 1985 Feb;45(2):564–571. [PubMed] [Google Scholar]
- Rutenburg A. M., Kim H., Fischbein J. W., Hanker J. S., Wasserkrug H. L., Seligman A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969 Aug;17(8):517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- Saeter G., Schwarze E., Seglen O. Shift from polyploidizing to nonpolyploidizing growth in carcinogen-treated rat liver. J Natl Cancer Inst. 1988 Aug 17;80(12):950–958. doi: 10.1093/jnci/80.12.950. [DOI] [PubMed] [Google Scholar]
- Saeter G., Schwarze P. E., Nesland J. M., Juul N., Pettersen E. O., Seglen P. O. The polyploidizing growth pattern of normal rat liver is replaced by divisional, diploid growth in hepatocellular nodules and carcinomas. Carcinogenesis. 1988 Jun;9(6):939–945. doi: 10.1093/carcin/9.6.939. [DOI] [PubMed] [Google Scholar]
- Saeter G., Schwarze P. E., Nesland J. M., Seglen P. O. Transplantation of preneoplastic rat hepatocytes by intraportal injection. Toxicol Pathol. 1987;15(1):78–81. doi: 10.1177/019262338701500110. [DOI] [PubMed] [Google Scholar]
- Schwarze P. E., Pettersen E. O., Shoaib M. C., Seglen P. O. Emergence of a population of small, diploid hepatocytes during hepatocarcinogenesis. Carcinogenesis. 1984 Oct;5(10):1267–1275. doi: 10.1093/carcin/5.10.1267. [DOI] [PubMed] [Google Scholar]
- Schwarze P. E., Pettersen E. O., Tolleshaug H., Seglen P. O. Isolation of carcinogen-induced diploid rat hepatocytes by centrifugal elutriation. Cancer Res. 1986 Sep;46(9):4732–4737. [PubMed] [Google Scholar]
- Schwarze P. E., Seglen P. O. Reduced autophagic activity, improved protein balance and enhanced in vitro survival of hepatocytes isolated from carcinogen-treated rats. Exp Cell Res. 1985 Mar;157(1):15–28. doi: 10.1016/0014-4827(85)90148-x. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Schwarze P. E., Saeter G. Changes in cellular ploidy and autophagic responsiveness during rat liver carcinogenesis. Toxicol Pathol. 1986;14(3):342–348. doi: 10.1177/019262338601400309. [DOI] [PubMed] [Google Scholar]
- Squire R. A., Levitt M. H. Report of a workshop on classification of specific hepatocellular lesions in rats. Cancer Res. 1975 Nov;35(11 Pt 1):3214–3223. [PubMed] [Google Scholar]
- Stäubli W., Hess R., Weibel E. R. Correlated morphometric and biochemical studies on the liver cell. II. Effects of phenobarbital on rat hepatocytes. J Cell Biol. 1969 Jul;42(1):92–112. doi: 10.1083/jcb.42.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J., Nissen N. I. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry. 1983 Mar;3(5):323–327. doi: 10.1002/cyto.990030503. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Williams G. M. Enhancement of rat hepatocellular-altered foci by the liver tumor promoter phenobarbital: evidence that foci are precursors of neoplasms and that the promoter acts on carcinogen-induced lesions. J Natl Cancer Inst. 1978 Nov;61(5):1311–1314. [PubMed] [Google Scholar]
- Weiss L., Ward P. M., Holmes J. C. Liver-to-lung traffic of cancer cells. Int J Cancer. 1983 Jul 15;32(1):79–83. doi: 10.1002/ijc.2910320113. [DOI] [PubMed] [Google Scholar]
- Zawydiwski R., Duncan R. Spontaneous 51Cr release by isolated rat hepatocytes: an indicator of membrane damage. In Vitro. 1978 Aug;14(8):707–714. doi: 10.1007/BF02616167. [DOI] [PubMed] [Google Scholar]