Abstract
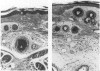
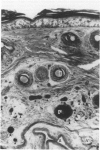
Mice were treated by an intravenous injection of 2 mg of the photosensitising drug meso-tetra (sulphonatophenyl) porphine (TPPS) and 24 h later a 2.5 cm length of their tails was exposed to visible light (photodynamic therapy, PDT). Using cross-sections from the centre of the treatment field, the absolute areas occupied by epidermis, dermis, hypodermis, tendon and bone, and also the total number and area of the blood vessels in the dermis and hypodermis, were compared between control and PDT-treated animals. There was a significant increase in the mean cross-sectional area of the epidermis, dermis and hypodermis following both 90J cm-2 (a dose expected to produce a low incidence of tail necrosis) and 180J cm-2 (expected to produce a 100% tail necrosis rate), on day 1 and day 5 following light exposure. The cross-sectional area of the vascular compartment was also significantly increased by day 5 at both dose levels. Differences were observed between the two doses when the total number of blood vessels were compared. There was a significant increase in the number of blood vessels by day 5 following 90 J cm-2 in both the dermis and hypodermis, but not following 180J cm-2. This appeared to be due to a significant increase in blood vessels with a cross-sectional area of less than 100 microns2 by day 5 at the lower dose. It is concluded that angiogenesis plays an important role in vascular recovery following PDT.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benstead K., Moore J. V. The effect of fractionation of light treatment on necrosis and vascular function of normal skin following photodynamic therapy. Br J Cancer. 1988 Sep;58(3):301–305. doi: 10.1038/bjc.1988.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benstead K., Moore J. V. Vascular function and the probability of skin necrosis after photodynamic therapy: an experimental study. Br J Cancer. 1988 May;57(5):451–454. doi: 10.1038/bjc.1988.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum M. C., Hall G. W., Hoyes A. D. Cerebral photosensitisation by haematoporphyrin derivative. Evidence for an endothelial site of action. Br J Cancer. 1986 Jan;53(1):81–89. doi: 10.1038/bjc.1986.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugelski P. J., Porter C. W., Dougherty T. J. Autoradiographic distribution of hematoporphyrin derivative in normal and tumor tissue of the mouse. Cancer Res. 1981 Nov;41(11 Pt 1):4606–4612. [PubMed] [Google Scholar]
- CASTELLANI A., PACE G. P., CONCIOLI M. Photodynamic effect of haematoporphyrin on blood microcirculation. J Pathol Bacteriol. 1963 Jul;86:99–102. doi: 10.1002/path.1700860111. [DOI] [PubMed] [Google Scholar]
- Lim H. W., Hagan M., Gigli I. Phototoxicity induced by hematoporphyrin derivative in C5-deficient, mast cell-deficient and leukopenic mice. Photochem Photobiol. 1986 Aug;44(2):175–180. doi: 10.1111/j.1751-1097.1986.tb03582.x. [DOI] [PubMed] [Google Scholar]
- Moore J. V., Keene J. P., Land E. J. Dose-response relationships for photodynamic injury to murine skin. Br J Radiol. 1986 Mar;59(699):257–261. doi: 10.1259/0007-1285-59-699-257. [DOI] [PubMed] [Google Scholar]
- Selman S. H., Kreimer-Birnbaum M., Goldblatt P. J., Anderson T. S., Keck R. W., Britton S. L. Jejunal blood flow after exposure to light in rats injected with hematoporphyrin derivative. Cancer Res. 1985 Dec;45(12 Pt 1):6425–6427. [PubMed] [Google Scholar]
- Star W. M., Marijnissen H. P., van den Berg-Blok A. E., Versteeg J. A., Franken K. A., Reinhold H. S. Destruction of rat mammary tumor and normal tissue microcirculation by hematoporphyrin derivative photoradiation observed in vivo in sandwich observation chambers. Cancer Res. 1986 May;46(5):2532–2540. [PubMed] [Google Scholar]
- Zhou C. N., Yang W. Z., Ding Z. X., Wang Y. X., Shen H., Fan X. J., Ha X. W. The biological effects of photodynamic therapy on normal skin in mice--II. An electron microscopic study. Adv Exp Med Biol. 1985;193:111–115. doi: 10.1007/978-1-4613-2165-1_13. [DOI] [PubMed] [Google Scholar]