Abstract
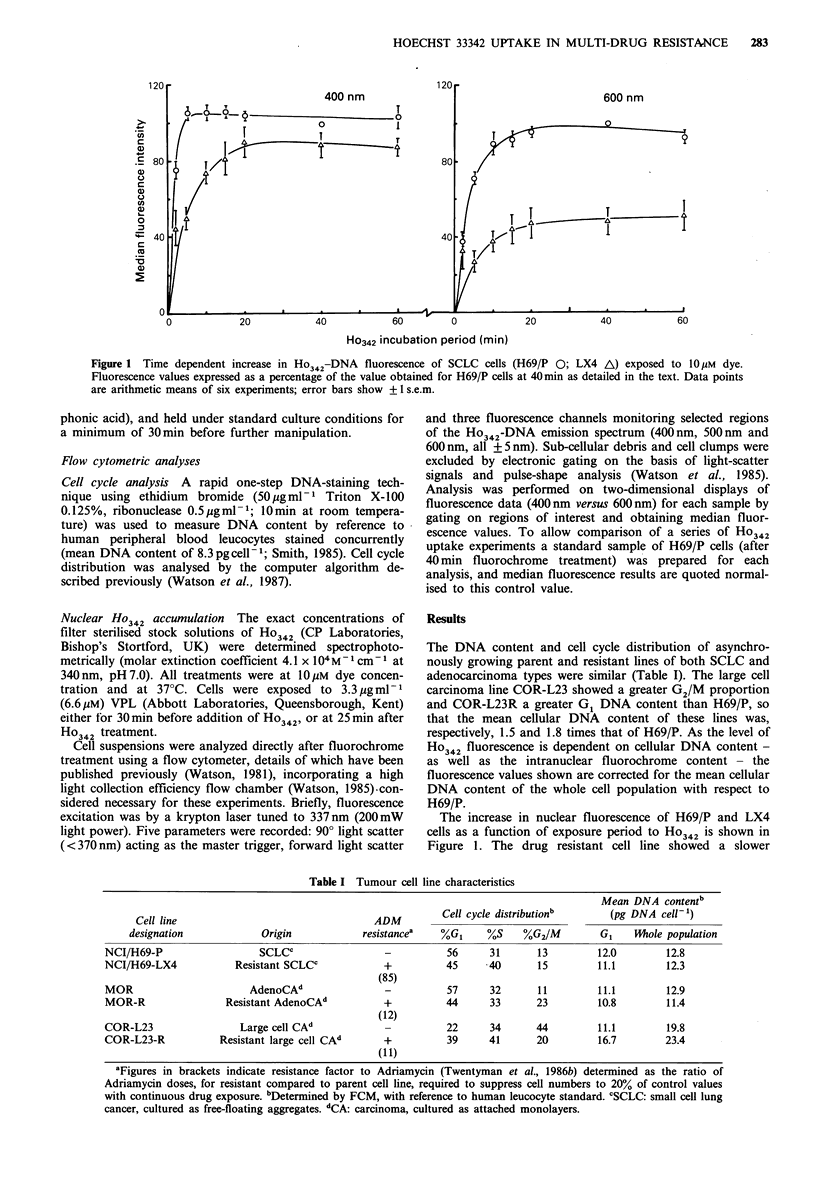
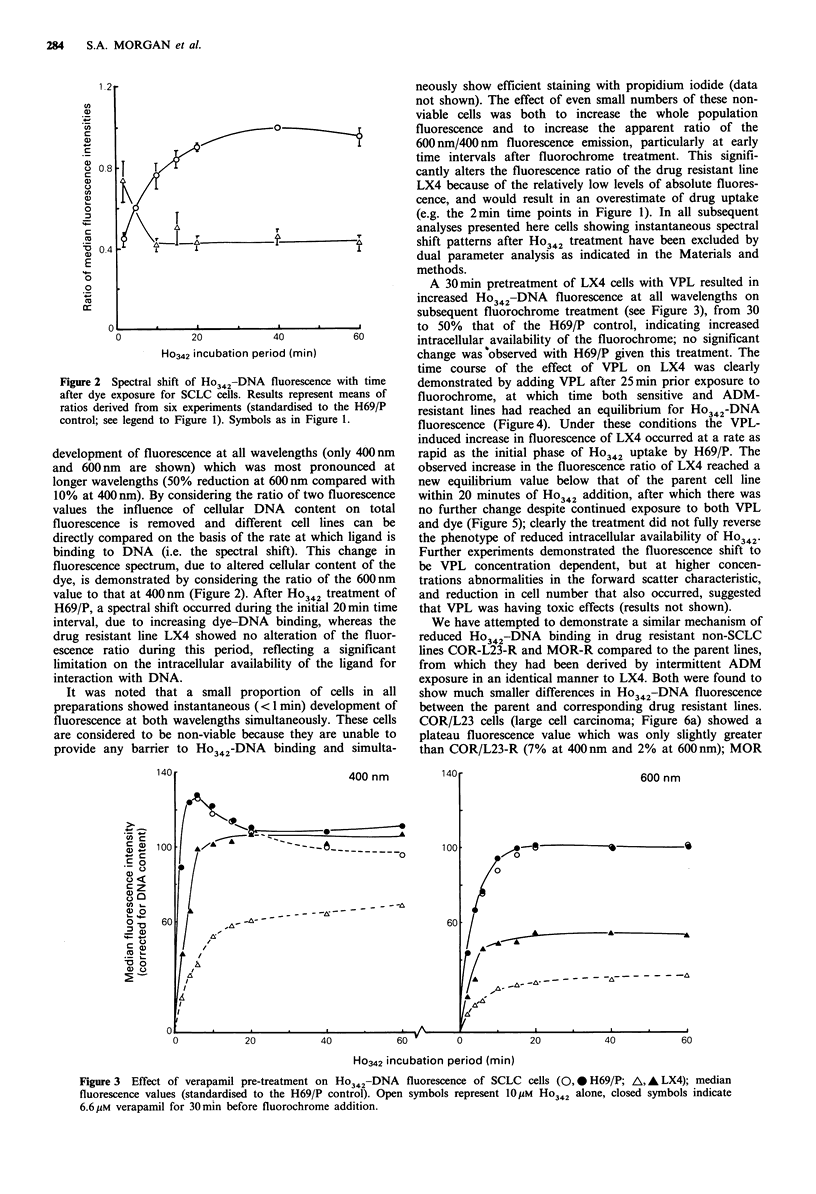
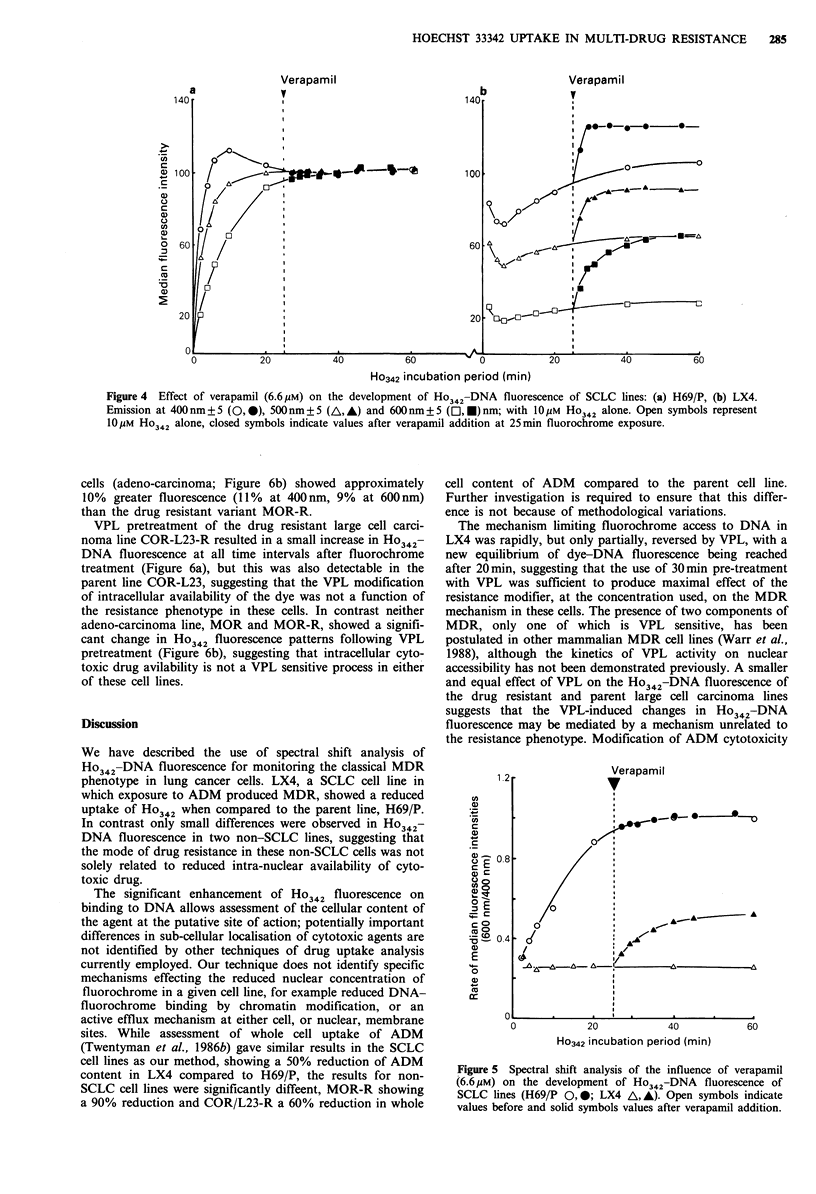
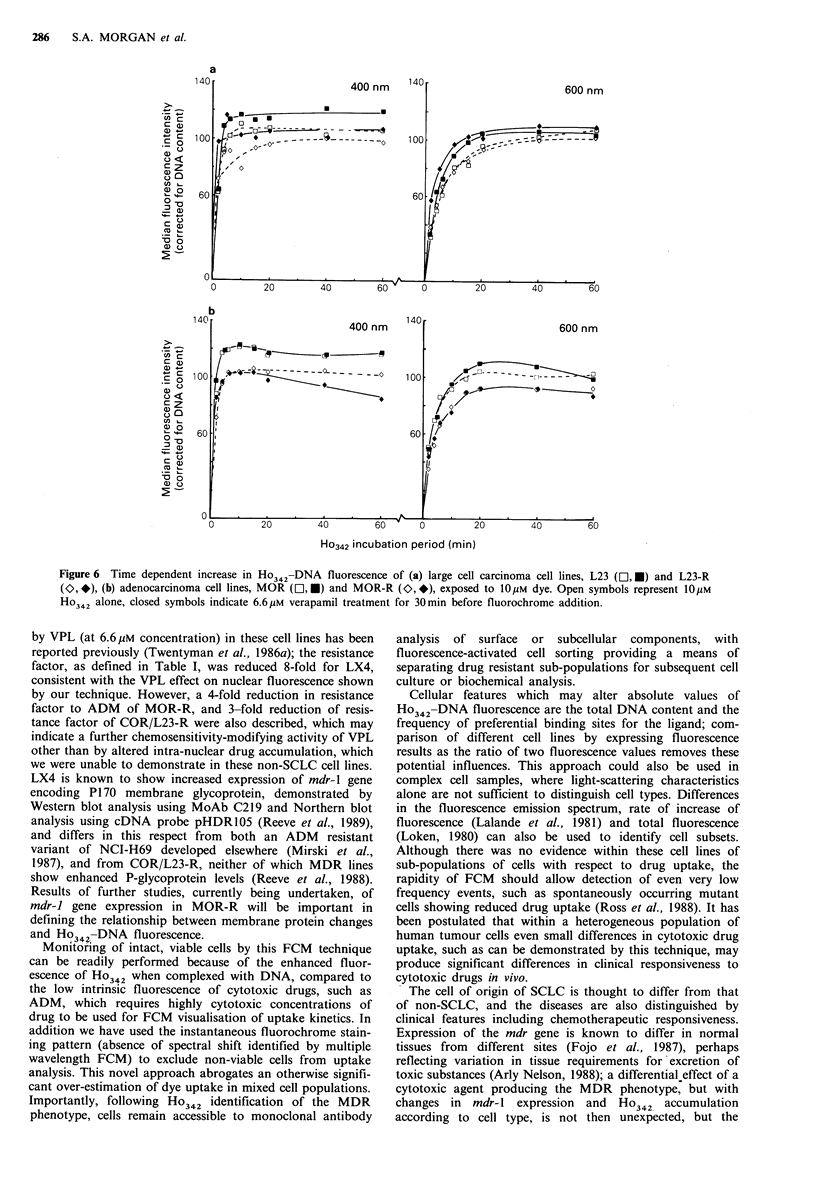
Cytotoxic drug resistance developing after chemotherapy is thought to be the main cause of treatment failure in several human tumours, including small cell lung cancer (SCLC). Cell lines showing drug resistance following prolonged exposure to a single agent frequently acquire resistance to several functionally unrelated drugs, the phenomenon of multi-drug resistance (MDR). Classical MDR is thought to arise from changes effecting a reduction in intracellular availability of cytotoxic drugs. We describe a flow cytometry (FCM) technique to monitor the MDR phenotype in drug resistant variants of SCLC and non-SCLC cell lines. The technique is based on a multiparametric analysis of the nuclear binding of a model chemotherapeutic agent, the fluorescent dye Hoechst 33342 (Ho342), which is capable of supra-vital staining of DNA in intact, viable cells. A laboratory derived drug resistant SCLC cell line, H69/LX4, showed a significant (30%) reduction in nuclear binding compared to the parental line H69/P. Exposure to verapamil (VPL) rapidly increased (within 2 min) nuclear binding of Ho342, and the new equilibrium of nuclear staining, attained within 20 min, remained lower than the level achieved in the parental cell line, suggesting some ability of H69/LX4 to limit the effect of the drug efflux blocker. A drug resistant large cell carcinoma line showed only a small reduction (10%) in nuclear binding when compared to the parent line, and this difference was not altered by VPL. A drug resistant adenocarcinoma line showed less than 10% difference in nuclear binding compared with the parental line and neither line was significantly affected by VPL treatment. Our findings suggest that different mechanisms of resistance may occur in lung tumours of different tissue types. This technique may be extended to the rapid and direct examination of biopsy specimens of human solid tumours for evidence of multi-drug resistance.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capranico G., Dasdia T., Zunino F. Comparison of doxorubicin-induced DNA damage in doxorubicin-sensitive and -resistant P388 murine leukemia cells. Int J Cancer. 1986 Feb 15;37(2):227–231. doi: 10.1002/ijc.2910370210. [DOI] [PubMed] [Google Scholar]
- Fojo A. T., Ueda K., Slamon D. J., Poplack D. G., Gottesman M. M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisson B., Gupta R., Smallwood-Kentro S., Ross W. Characterization of acquired epipodophyllotoxin resistance in a Chinese hamster ovary cell line: loss of drug-stimulated DNA cleavage activity. Cancer Res. 1986 Apr;46(4 Pt 2):1934–1938. [PubMed] [Google Scholar]
- Inaba M., Kobayashi H., Sakurai Y., Johnson R. K. Active efflux of daunorubicin and adriamycin in sensitive and resistant sublines of P388 leukemia. Cancer Res. 1979 Jun;39(6 Pt 1):2200–2203. [PubMed] [Google Scholar]
- Krishan A. Effect of drug efflux blockers on vital staining of cellular DNA with Hoechst 33342. Cytometry. 1987 Nov;8(6):642–645. doi: 10.1002/cyto.990080618. [DOI] [PubMed] [Google Scholar]
- Lalande M. E., Ling V., Miller R. G. Hoechst 33342 dye uptake as a probe of membrane permeability changes in mammalian cells. Proc Natl Acad Sci U S A. 1981 Jan;78(1):363–367. doi: 10.1073/pnas.78.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling V., Gerlach J., Kartner N. Multidrug resistance. Breast Cancer Res Treat. 1984;4(2):89–94. doi: 10.1007/BF01806390. [DOI] [PubMed] [Google Scholar]
- Loken M. R. Separation of viable T and B lymphocytes using a cytochemical stain, Hoechst 33342. J Histochem Cytochem. 1980 Jan;28(1):36–39. doi: 10.1177/28.1.6153191. [DOI] [PubMed] [Google Scholar]
- Mirski S. E., Gerlach J. H., Cole S. P. Multidrug resistance in a human small cell lung cancer cell line selected in adriamycin. Cancer Res. 1987 May 15;47(10):2594–2598. [PubMed] [Google Scholar]
- Potmesil M., Hsiang Y. H., Liu L. F., Bank B., Grossberg H., Kirschenbaum S., Forlenza T. J., Penziner A., Kanganis D., Forlenzar T. J. Resistance of human leukemic and normal lymphocytes to drug-induced DNA cleavage and low levels of DNA topoisomerase II. Cancer Res. 1988 Jun 15;48(12):3537–3543. [PubMed] [Google Scholar]
- Riordan J. R., Ling V. Genetic and biochemical characterization of multidrug resistance. Pharmacol Ther. 1985;28(1):51–75. doi: 10.1016/0163-7258(85)90082-8. [DOI] [PubMed] [Google Scholar]
- Ross D. D., Ordóez J. V., Joneckis C. C., Testa J. R., Thompson B. W. Isolation of highly multidrug-resistant P388 cells from drug-sensitive P388/S cells by flow cytometric cell sorting. Cytometry. 1988 Jul;9(4):359–367. doi: 10.1002/cyto.990090413. [DOI] [PubMed] [Google Scholar]
- Skovsgaard T., Nissen N. I. Membrane transport of anthracyclines. Pharmacol Ther. 1982;18(3):293–311. doi: 10.1016/0163-7258(82)90034-1. [DOI] [PubMed] [Google Scholar]
- Slovak M. L., Hoeltge G. A., Dalton W. S., Trent J. M. Pharmacological and biological evidence for differing mechanisms of doxorubicin resistance in two human tumor cell lines. Cancer Res. 1988 May 15;48(10):2793–2797. [PubMed] [Google Scholar]
- Smith P. J., Nakeff A., Watson J. V. Flow-cytometric detection of changes in the fluorescence emission spectrum of a vital DNA-specific dye in human tumour cells. Exp Cell Res. 1985 Jul;159(1):37–46. doi: 10.1016/s0014-4827(85)80035-5. [DOI] [PubMed] [Google Scholar]
- Supino R., Mariani M., Capranico G., Colombo A., Parmiani G. Doxorubicin cellular pharmacokinetics and DNA breakage in a multi-drug resistant B16 melanoma cell line. Br J Cancer. 1988 Feb;57(2):142–146. doi: 10.1038/bjc.1988.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supino R., Prosperi E., Formelli F., Mariani M., Parmiani G. Characterization of a doxorubicin-resistant murine melanoma line: studies on cross-resistance and its circumvention. Br J Cancer. 1986 Jul;54(1):33–42. doi: 10.1038/bjc.1986.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twentyman P. R., Fox N. E., Bleehen N. M. Drug resistance in human lung cancer cell lines: cross-resistance studies and effects of the calcium transport blocker, verapamil. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1355–1358. doi: 10.1016/0360-3016(86)90170-7. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R., Fox N. E., Wright K. A., Bleehen N. M. Derivation and preliminary characterisation of adriamycin resistant lines of human lung cancer cells. Br J Cancer. 1986 Apr;53(4):529–537. doi: 10.1038/bjc.1986.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr J. R., Anderson M., Fergusson J. Properties of verapamil-hypersensitive multidrug-resistant Chinese hamster ovary cells. Cancer Res. 1988 Aug 15;48(16):4477–4483. [PubMed] [Google Scholar]
- Watson J. V. A method for improving light collection by 600% from square cross section flow cytometry chambers. Br J Cancer. 1985 Mar;51(3):433–435. doi: 10.1038/bjc.1985.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. V., Chambers S. H., Smith P. J. A pragmatic approach to the analysis of DNA histograms with a definable G1 peak. Cytometry. 1987 Jan;8(1):1–8. doi: 10.1002/cyto.990080101. [DOI] [PubMed] [Google Scholar]
- Watson J. V. Dual laser beam focussing for flow cytometry through a single crossed cylindrical lens pair. Cytometry. 1981 Jul;2(1):14–19. doi: 10.1002/cyto.990020103. [DOI] [PubMed] [Google Scholar]
- Watson J. V., Sikora K., Evan G. I. A simultaneous flow cytometric assay for c-myc oncoprotein and DNA in nuclei from paraffin embedded material. J Immunol Methods. 1985 Oct 24;83(1):179–192. doi: 10.1016/0022-1759(85)90071-7. [DOI] [PubMed] [Google Scholar]


