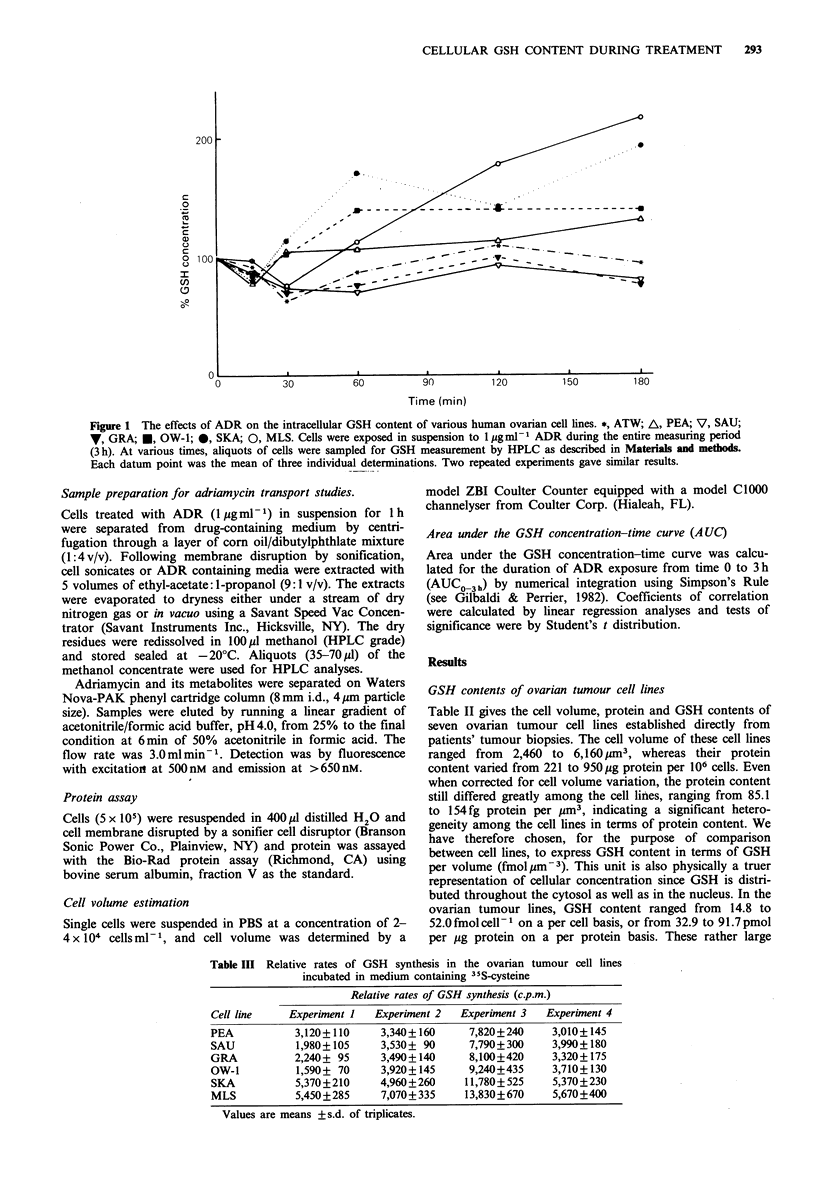
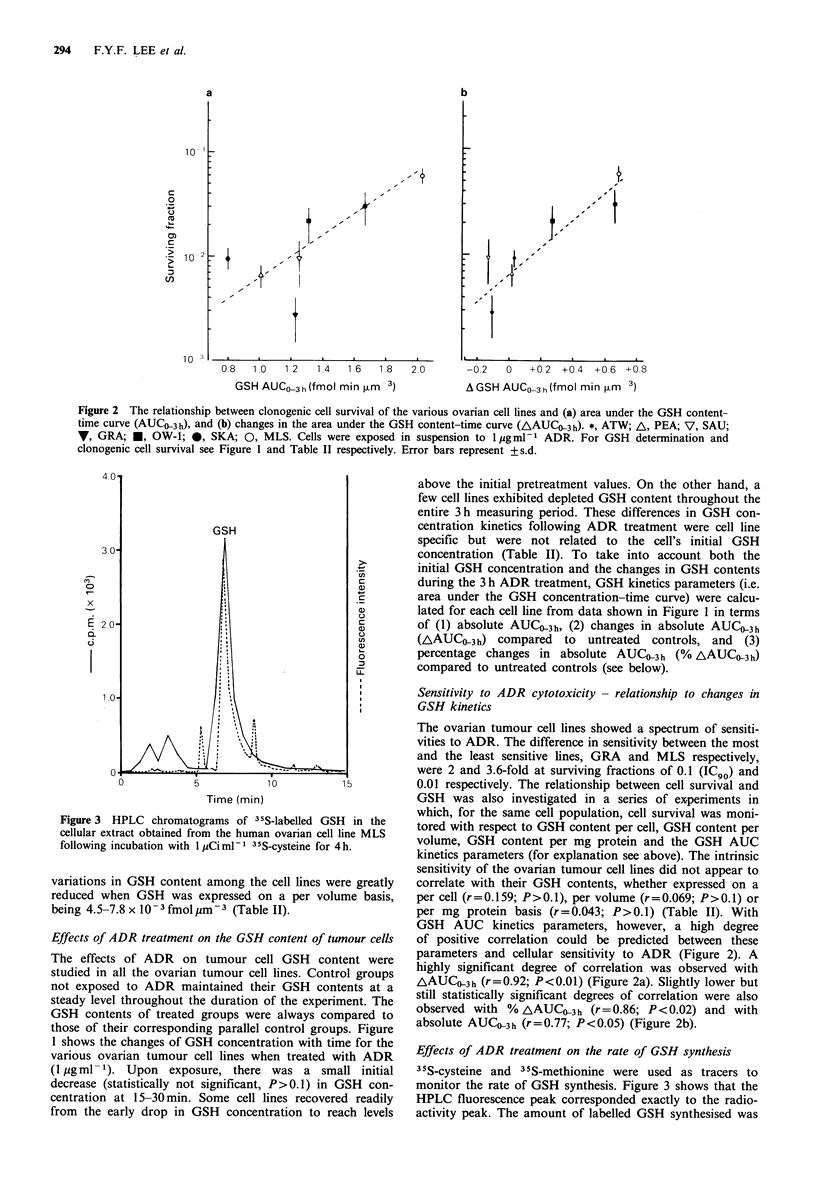
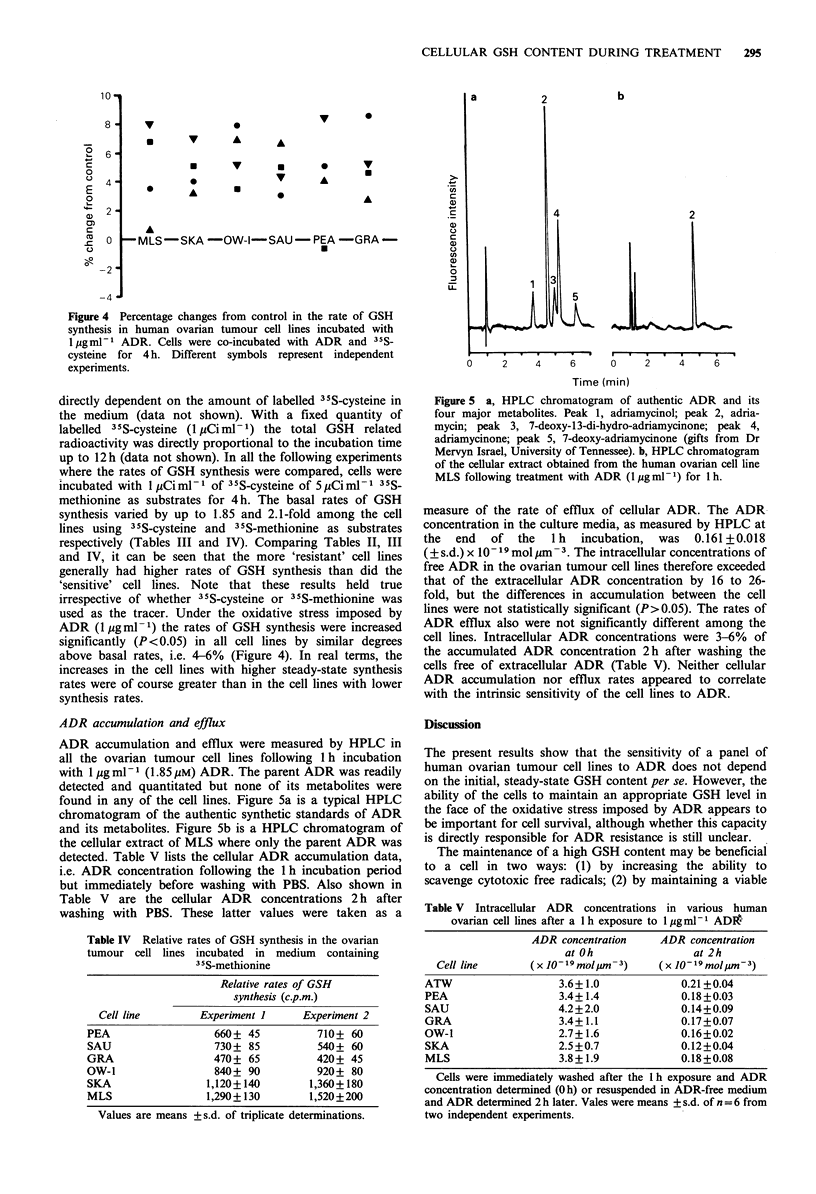
Abstract
Patients with ovarian cancer often respond well to combination chemotherapy initially but the majority eventually relapse when, with further treatment, the initially successful regimen proves ineffectual. The cause of such failures frequently has been attributed to the development of drug resistance. Although the mechanisms of acquired resistance in situ are still poorly understood, studies in vitro have shown that cells selected for resistance to one drug often exhibit cross-resistance to other seemingly unrelated agents, suggesting a somewhat generalised mechanism of resistance. We have studied the role of glutathione (GSH) and drug transport in determining the sensitivity to adriamycin (ADR) of a panel of human ovarian cell lines established directly from biopsies of patients with diverse treatment histories. These cell lines exhibited inherent differences in sensitivity to ADR by a dose factor of up to 3; a difference that was considerably less than what has been reported when cells were selected for drug resistance in vitro. The differences in drug sensitivity reported here among the various cell lines appeared to be unrelated to drug transport, in terms of both influx and efflux. Moreover, although these cell lines have a wide range of GSH content, there was only a poor correlation between drug sensitivity and cellular GSH content per se. However, when exposed to a clinically relevant dose of ADR, the GSH content of cell lines that were 'sensitive' decreased, whereas that of cell lines that were 'resistant' increased. To take these time-dependent changes in GSH into consideration, the area under the GSH content versus time curve (AUC), with and without ADR treatment, was calculated for each cell line. When this latter factor was included in the analysis, greatly improved correlations were found between GSH kinetic parameters and responses to ADR. In particular, ADR resistance was found to be closely correlated with the positive changes in absolute GSH AUC following ADR treatment (r = 0.92; P less than 0.01). Using 35S-labelled cysteine and methionine as tracers, it was found that the essential difference between the 'resistant' and 'sensitive' lines was that the 'resistant' lines had higher steady-state rates of GSH synthesis than the 'sensitive' lines. These results demonstrate that changes in cellular GSH concentration during treatment may be an important indicator of tumour cell response to ADR.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aida T., Bodell W. J. Cellular resistance to chloroethylnitrosoureas, nitrogen mustard, and cis-diamminedichloroplatinum(II) in human glial-derived cell lines. Cancer Res. 1987 Mar 1;47(5):1361–1366. [PubMed] [Google Scholar]
- Arrick B. A., Nathan C. F. Glutathione metabolism as a determinant of therapeutic efficacy: a review. Cancer Res. 1984 Oct;44(10):4224–4232. [PubMed] [Google Scholar]
- Bachur N. R., Gordon S. L., Gee M. V. A general mechanism for microsomal activation of quinone anticancer agents to free radicals. Cancer Res. 1978 Jun;38(6):1745–1750. [PubMed] [Google Scholar]
- Biaglow J. E., Varnes M. E., Clark E. P., Epp E. R. The role of thiols in cellular response to radiation and drugs. Radiat Res. 1983 Sep;95(3):437–455. [PubMed] [Google Scholar]
- Crook T. R., Souhami R. L., Whyman G. D., McLean A. E. Glutathione depletion as a determinant of sensitivity of human leukemia cells to cyclophosphamide. Cancer Res. 1986 Oct;46(10):5035–5038. [PubMed] [Google Scholar]
- Doroshow J. H. Role of hydrogen peroxide and hydroxyl radical formation in the killing of Ehrlich tumor cells by anticancer quinones. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4514–4518. doi: 10.1073/pnas.83.12.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua S. A., Moretti-Rojas I. M., Schneider S. L., McGuire W. L. P-glycoprotein expression in human breast cancer cells. Cancer Res. 1987 Apr 15;47(8):2103–2106. [PubMed] [Google Scholar]
- Ganapathi R., Grabowski D., Schmidt H. Factors governing the modulation of vinca-alkaloid resistance in doxorubicin-resistant cells by the calmodulin inhibitor trifluoperazine. Biochem Pharmacol. 1986 Feb 15;35(4):673–678. doi: 10.1016/0006-2952(86)90366-7. [DOI] [PubMed] [Google Scholar]
- Glisson B. S., Sullivan D. M., Gupta R., Ross W. E. Mediation of multi-drug resistance in a Chinese hamster ovary cell line by a mutant type II topoisomerase. NCI Monogr. 1987;(4):89–93. [PubMed] [Google Scholar]
- Goodman J., Hochstein P. Generation of free radicals and lipid peroxidation by redox cycling of adriamycin and daunomycin. Biochem Biophys Res Commun. 1977 Jul 25;77(2):797–803. doi: 10.1016/s0006-291x(77)80048-x. [DOI] [PubMed] [Google Scholar]
- Green J. A., Vistica D. T., Young R. C., Hamilton T. C., Rogan A. M., Ozols R. F. Potentiation of melphalan cytotoxicity in human ovarian cancer cell lines by glutathione depletion. Cancer Res. 1984 Nov;44(11):5427–5431. [PubMed] [Google Scholar]
- Hamilton T. C., Winker M. A., Louie K. G., Batist G., Behrens B. C., Tsuruo T., Grotzinger K. R., McKoy W. M., Young R. C., Ozols R. F. Augmentation of adriamycin, melphalan, and cisplatin cytotoxicity in drug-resistant and -sensitive human ovarian carcinoma cell lines by buthionine sulfoximine mediated glutathione depletion. Biochem Pharmacol. 1985 Jul 15;34(14):2583–2586. doi: 10.1016/0006-2952(85)90551-9. [DOI] [PubMed] [Google Scholar]
- Harker W. G., Bauer D., Etiz B. B., Newman R. A., Sikic B. I. Verapamil-mediated sensitization of doxorubicin-selected pleiotropic resistance in human sarcoma cells: selectivity for drugs which produce DNA scission. Cancer Res. 1986 May;46(5):2369–2373. [PubMed] [Google Scholar]
- Harker W. G., Sikic B. I. Multidrug (pleiotropic) resistance in doxorubicin-selected variants of the human sarcoma cell line MES-SA. Cancer Res. 1985 Sep;45(9):4091–4096. [PubMed] [Google Scholar]
- Harris E. J., Baum H. Production of thiol groups and retention of calcium ions by cardiac mitochondria. Biochem J. 1980 Mar 15;186(3):725–732. doi: 10.1042/bj1860725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. R., Timberlake N., Henson P., Schimke P., Belli J. A. Adriamycin uptake in V79 and adriamycin resistant Chinese hamster cells. Int J Radiat Oncol Biol Phys. 1979 Aug;5(8):1235–1239. doi: 10.1016/0360-3016(79)90645-x. [DOI] [PubMed] [Google Scholar]
- Inaba M., Johnson R. K. Uptake and retention of adriamycin and daunorubicin by sensitive and anthracycline-resistant sublines of P388 leukemia. Biochem Pharmacol. 1978;27(17):2123–2130. doi: 10.1016/0006-2952(78)90284-8. [DOI] [PubMed] [Google Scholar]
- Kartner N., Riordan J. R., Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983 Sep 23;221(4617):1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- Kramer R. A., Greene K., Ahmad S., Vistica D. T. Chemosensitization of L-phenylalanine mustard by the thiol-modulating agent buthionine sulfoximine. Cancer Res. 1987 Mar 15;47(6):1593–1597. [PubMed] [Google Scholar]
- Kramer R. A., Zakher J., Kim G. Role of the glutathione redox cycle in acquired and de novo multidrug resistance. Science. 1988 Aug 5;241(4866):694–697. doi: 10.1126/science.3399900. [DOI] [PubMed] [Google Scholar]
- Lee F. Y., Vessey A. R., Siemann D. W. Glutathione as a determinant of cellular response to doxorubicin. NCI Monogr. 1988;(6):211–215. [PubMed] [Google Scholar]
- Louie K. G., Hamilton T. C., Winker M. A., Behrens B. C., Tsuruo T., Klecker R. W., Jr, McKoy W. M., Grotzinger K. R., Myers C. E., Young R. C. Adriamycin accumulation and metabolism in adriamycin-sensitive and -resistant human ovarian cancer cell lines. Biochem Pharmacol. 1986 Feb 1;35(3):467–472. doi: 10.1016/0006-2952(86)90221-2. [DOI] [PubMed] [Google Scholar]
- Lötscher H. R., Winterhalter K. H., Carafoli E., Richter C. Hydroperoxides can modulate the redox state of pyridine nucleotides and the calcium balance in rat liver mitochondria. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4340–4344. doi: 10.1073/pnas.76.9.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Mirski S. E., Gerlach J. H., Cole S. P. Multidrug resistance in a human small cell lung cancer cell line selected in adriamycin. Cancer Res. 1987 May 15;47(10):2594–2598. [PubMed] [Google Scholar]
- Mustafa M. G., Tierney D. F. Biochemical and metabolic changes in the lung with oxygen, ozone, and nitrogen dioxide toxicity. Am Rev Respir Dis. 1978 Dec;118(6):1061–1090. doi: 10.1164/arrd.1978.118.6.1061. [DOI] [PubMed] [Google Scholar]
- Ono K., Shrieve D. C. Enhancement of EMT6/SF tumor cell killing by mitomycin C and cyclophosphamide following in vivo administration of buthionine sulfoximine. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1175–1178. doi: 10.1016/0360-3016(86)90252-x. [DOI] [PubMed] [Google Scholar]
- Ozols R. F., Louie K. G., Plowman J., Behrens B. C., Fine R. L., Dykes D., Hamilton T. C. Enhanced melphalan cytotoxicity in human ovarian cancer in vitro and in tumor-bearing nude mice by buthionine sulfoximine depletion of glutathione. Biochem Pharmacol. 1987 Jan 1;36(1):147–153. doi: 10.1016/0006-2952(87)90392-3. [DOI] [PubMed] [Google Scholar]
- Rice G. C., Bump E. A., Shrieve D. C., Lee W., Kovacs M. Quantitative analysis of cellular glutathione by flow cytometry utilizing monochlorobimane: some applications to radiation and drug resistance in vitro and in vivo. Cancer Res. 1986 Dec;46(12 Pt 1):6105–6110. [PubMed] [Google Scholar]
- Richert N., Akiyama S., Shen D., Gottesman M. M., Pastan I. Multiply drug-resistant human KB carcinoma cells have decreased amounts of a 75-kDa and a 72-kDa glycoprotein. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2330–2333. doi: 10.1073/pnas.82.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Révész L. The role of endogenous thiols in intrinsic radioprotection. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Apr;47(4):361–368. [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Shrieve D. C., Denekamp J., Minchinton A. I. Effects of glutathione depletion by buthionine sulfoximine on radiosensitization by oxygen and misonidazole in vitro. Radiat Res. 1985 Jun;102(3):283–294. [PubMed] [Google Scholar]
- Siegfried J. M., Tritton T. R., Sartorelli A. C. Comparison of anthracycline concentrations in S180 cell lines of varying sensitivity. Eur J Cancer Clin Oncol. 1983 Aug;19(8):1133–1141. doi: 10.1016/0277-5379(83)90039-1. [DOI] [PubMed] [Google Scholar]
- Sinha B. K., Katki A. G., Batist G., Cowan K. H., Myers C. E. Adriamycin-stimulated hydroxyl radical formation in human breast tumor cells. Biochem Pharmacol. 1987 Mar 15;36(6):793–796. doi: 10.1016/0006-2952(87)90164-x. [DOI] [PubMed] [Google Scholar]
- Suzukake K., Petro B. J., Vistica D. T. Reduction in glutathione content of L-PAM resistant L1210 Cells confers drug sensitivity. Biochem Pharmacol. 1982 Jan 1;31(1):121–124. doi: 10.1016/0006-2952(82)90249-0. [DOI] [PubMed] [Google Scholar]
- Tokes Z. A., Rogers K. E., Rembaum A. Synthesis of adriamycin-coupled polyglutaraldehyde microspheres and evaluation of their cytostatic activity. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2026–2030. doi: 10.1073/pnas.79.6.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triton T. R., Yee G. The anticancer agent adriamycin can be actively cytotoxic without entering cells. Science. 1982 Jul 16;217(4556):248–250. doi: 10.1126/science.7089561. [DOI] [PubMed] [Google Scholar]
- Tsutsui K., Komuro C., Ono K., Nishidai T., Shibamoto Y., Takahashi M., Abe M. Chemosensitization by buthionine sulfoximine in vivo. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1183–1186. doi: 10.1016/0360-3016(86)90254-3. [DOI] [PubMed] [Google Scholar]
- Whillans D. W., Rauth A. M. An experimental and analytical study of oxygen depletion in stirred cell suspensions. Radiat Res. 1980 Oct;84(1):97–114. [PubMed] [Google Scholar]
- Yoda Y., Nakazawa M., Abe T., Kawakami Z. Prevention of doxorubicin myocardial toxicity in mice by reduced glutathione. Cancer Res. 1986 May;46(5):2551–2556. [PubMed] [Google Scholar]


