Abstract
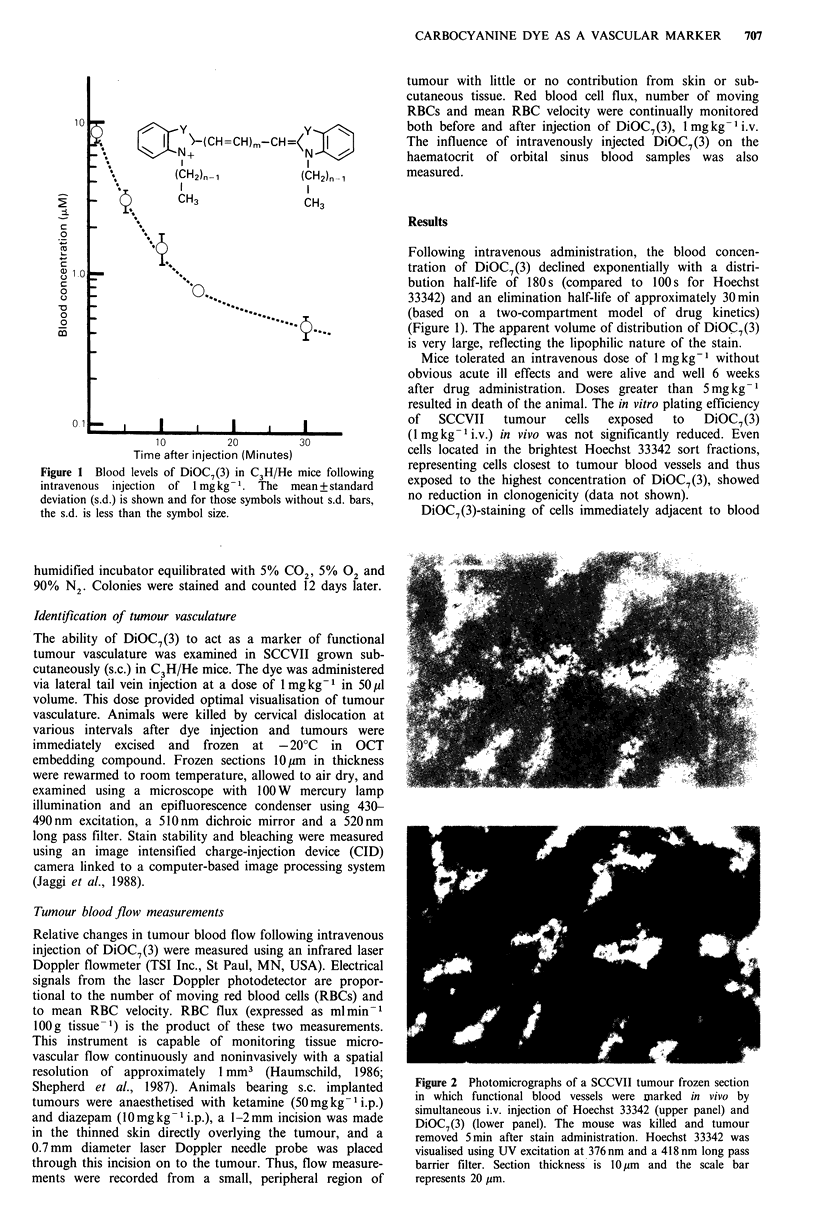
An intravenously administered fluorescent carbocyanine dye, DiOC7(3), has been evaluated for use in conjunction with Hoechst 33342 as a marker of murine tumour vasculature. DiOC7(3) stains cells immediately adjacent to blood vessels and thus, like Hoechst 33342, outlines perfused tumour vasculature. The different fluorescence excitation and emission properties of DiOC7(3) and Hoechst 33342 permit discrimination of the stains in the same tissue section. Mice tolerate a DiOC7(3) dose of 1 mg kg-1 i.v. with no ill effects. The dye has a distribution half-life in blood of 180s and staining of perivascular tumour cells is sufficiently stable to allow visualisation of vasculature for up to 30 min after DiOC7(3) injection. However, DiOC7(3) causes a 75% reduction in tumour blood flow as measured by laser Doppler techniques. Consequently, the compound appears to be most suitable as a second vascular marker, administered at some time after Hoechst 33342, to detect temporal and spatial fluctuations in tumour perfusion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. M. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br J Radiol. 1979 Aug;52(620):650–656. doi: 10.1259/0007-1285-52-620-650. [DOI] [PubMed] [Google Scholar]
- Chaplin D. J., Durand R. E., Olive P. L. Acute hypoxia in tumors: implications for modifiers of radiation effects. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1279–1282. doi: 10.1016/0360-3016(86)90153-7. [DOI] [PubMed] [Google Scholar]
- Chaplin D. J., Durand R. E., Olive P. L. Cell selection from a murine tumour using the fluorescent probe Hoechst 33342. Br J Cancer. 1985 Apr;51(4):569–572. doi: 10.1038/bjc.1985.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin D. J., Olive P. L., Durand R. E. Intermittent blood flow in a murine tumor: radiobiological effects. Cancer Res. 1987 Jan 15;47(2):597–601. [PubMed] [Google Scholar]
- GOLDACRE R. J., SYLVEN B. On the access of blood-borne dyes to various tumour regions. Br J Cancer. 1962 Jun;16:306–322. doi: 10.1038/bjc.1962.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GULLINO P. M., GRANTHAM F. H. THE VASCULAR SPACE OF GROWING TUMORS. Cancer Res. 1964 Nov;24:1727–1732. [PubMed] [Google Scholar]
- Intaglietta M., Myers R. R., Gross J. F., Reinhold H. S. Dynamics of microvascular flow in implanted mouse mammary tumours. Bibl Anat. 1977;(15 Pt 1):273–276. [PubMed] [Google Scholar]
- Jaggi B., Poon S. S., MacAulay C., Palcic B. Imaging system for morphometric assessment of absorption or fluorescence in stained cells. Cytometry. 1988 Nov;9(6):566–572. doi: 10.1002/cyto.990090609. [DOI] [PubMed] [Google Scholar]
- Olive P. L., Chaplin D. J., Durand R. E. Pharmacokinetics, binding and distribution of Hoechst 33342 in spheroids and murine tumours. Br J Cancer. 1985 Nov;52(5):739–746. doi: 10.1038/bjc.1985.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive P. L., Durand R. E. Characterization of a carbocyanine derivative as a fluorescent penetration probe. Cytometry. 1987 Nov;8(6):571–575. doi: 10.1002/cyto.990080607. [DOI] [PubMed] [Google Scholar]
- Reinhold H. S., Blachiwiecz B., Blok A. Oxygenation and reoxygenation in 'sandwich' tumours. Bibl Anat. 1977;(15 Pt 1):270–272. [PubMed] [Google Scholar]
- Reinhold H. S., Visser J. W. In vivo fluorescence of endothelial cell nuclei stained with the dye Bis-benzamide H 33342. Int J Microcirc Clin Exp. 1983;2(2):143–146. [PubMed] [Google Scholar]
- Shepherd A. P., Riedel G. L., Kiel J. W., Haumschild D. J., Maxwell L. C. Evaluation of an infrared laser-Doppler blood flowmeter. Am J Physiol. 1987 Jun;252(6 Pt 1):G832–G839. doi: 10.1152/ajpgi.1987.252.6.G832. [DOI] [PubMed] [Google Scholar]
- Sims P. J., Waggoner A. S., Wang C. H., Hoffman J. F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974 Jul 30;13(16):3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Hill S. A., Begg A. C., Denekamp J. Validation of the fluorescent dye Hoechst 33342 as a vascular space marker in tumours. Br J Cancer. 1988 Mar;57(3):247–253. doi: 10.1038/bjc.1988.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock I. F., Steel G. G. Quantitative techniques for study of the anatomy and function of small blood vessels in tumors. J Natl Cancer Inst. 1969 May;42(5):771–782. [PubMed] [Google Scholar]



