Abstract
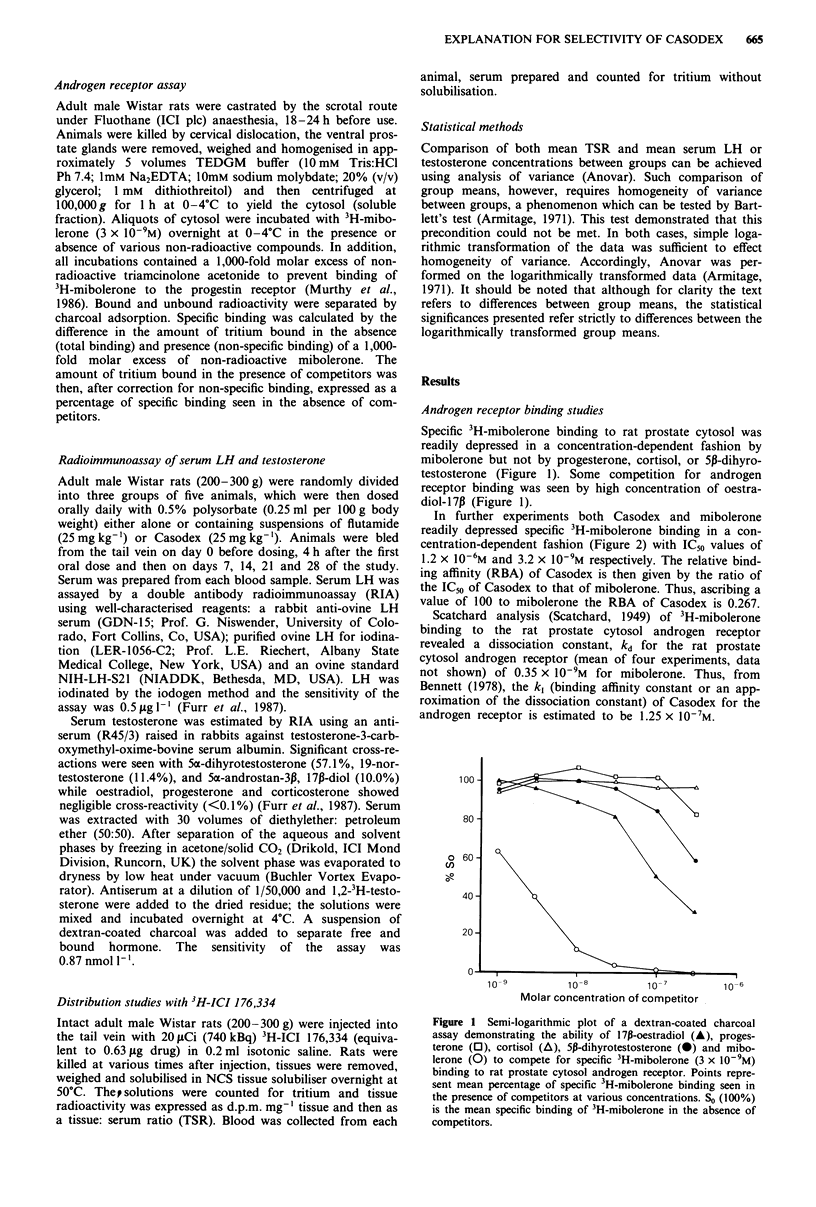
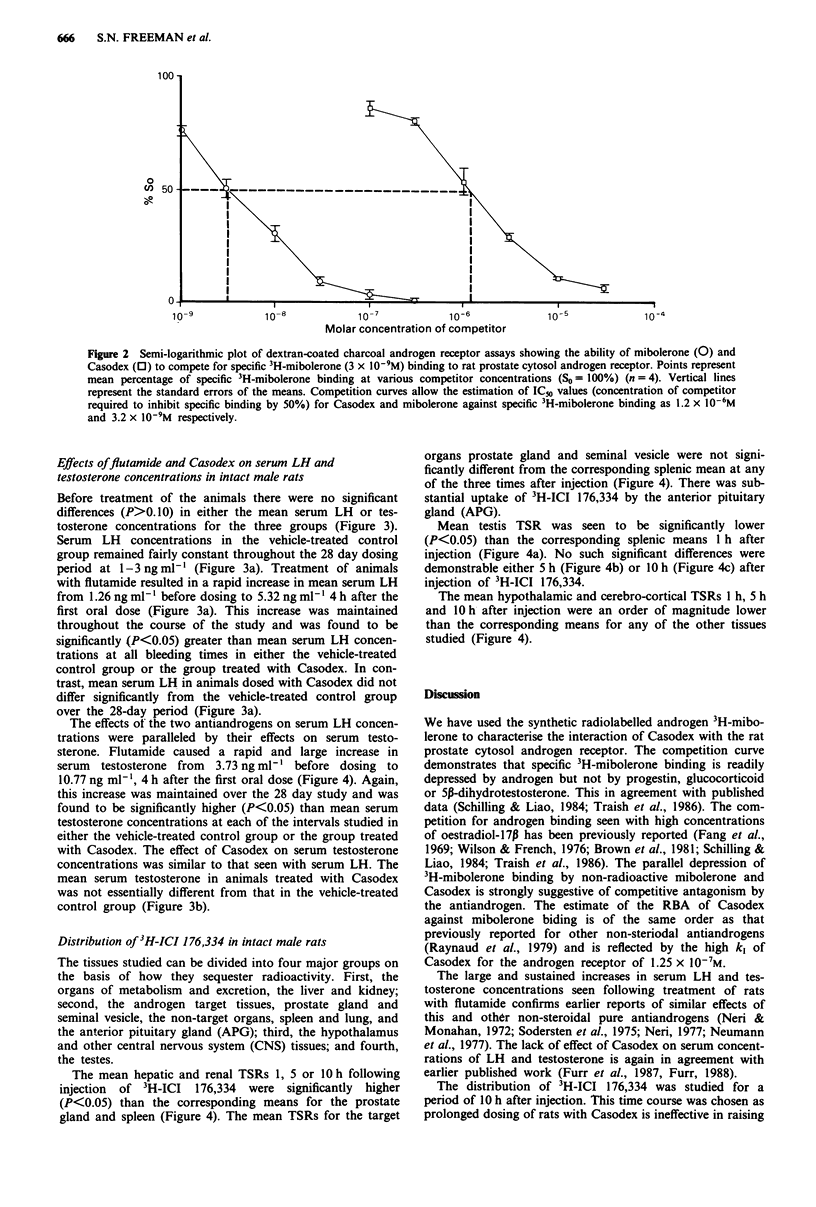
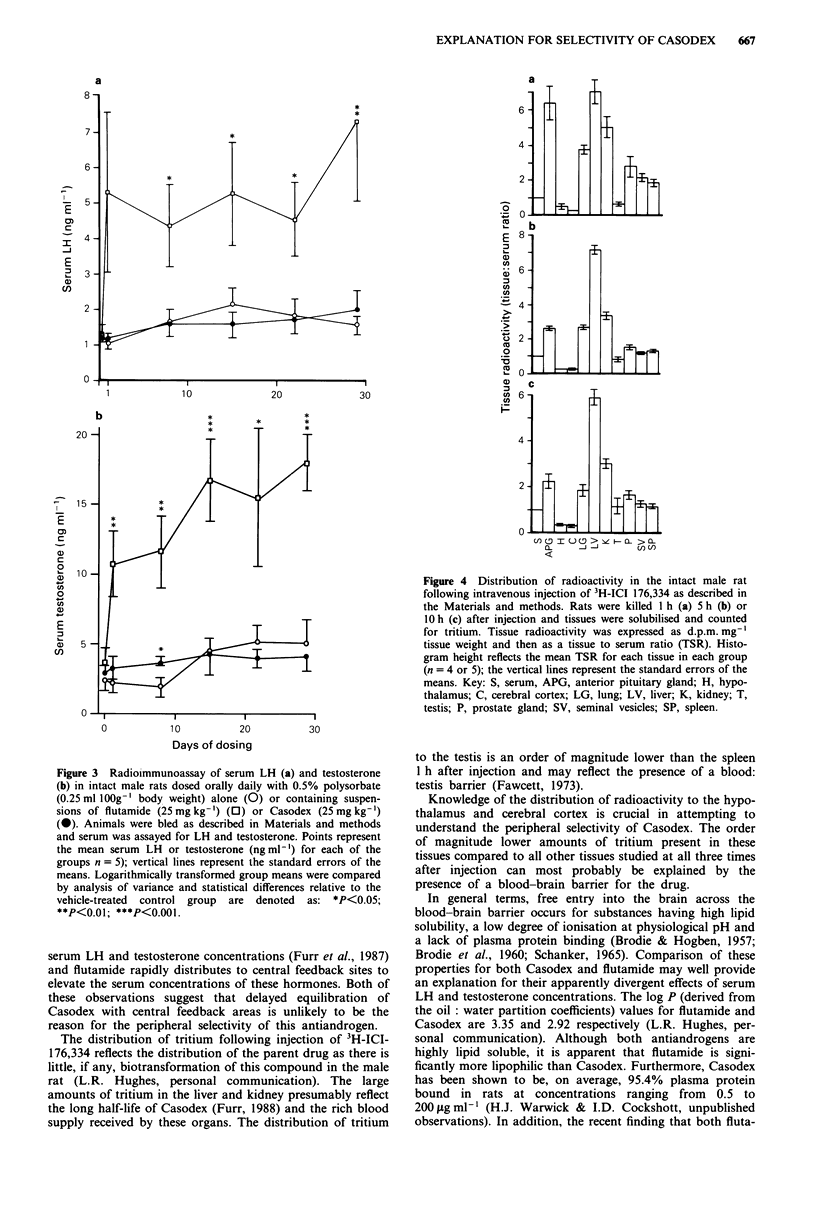
The in vivo antiandrogenicity of Casodex has been confirmed and characterised. Androgen receptor (AR) binding assays of rat ventral prostate gland cytosols revealed a relative binding affinity (RBA) for the AR of 0.267 and a k1 of 1.25 x 10(-7) M for Casodex. In addition, the peripheral selectivity of Casodex relative to other non-steroidal antiandrogens was confirmed in that daily treatment of non-castrated rats with Casodex (25 mg kg-1) did not elicit any changes in serum LH and testosterone concentrations relative to vehicle-treated controls, whereas elevated serum LH and testosterone were observed in rats treated with flutamide (25 mg kg-1). The peripheral selectivity of Casodex in the intact male rat was related to the distribution of radiolabelled antiandrogen following intravenous injection. All tissues with the exception of the hypothalamus and cerebral cortex (CC) sequestered radioactivity such that the tissue:serum ratio (TSR) for the drug was greater than unity. In the testis, the TSR was less than unity 1 h after injection but approached unity 5 h after injection and was greater than unity 10 h after injection. This may be explained by the presence of a blood-testis barrier for the drug, resulting in delayed equilibration between the blood and testis tissue. By comparison, an order of magnitude lower amounts of radioactivity in the hypothalamus and CC were maintained for the 10 h period after injection. These data, together with known physicochemical properties of Casodex suggest that a blood-brain barrier exists for the drug which results in exclusion of this antiandrogen from central sites of androgen negative feedback and that this accounts for its peripherally selective antihormonal profile.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRODIE B. B., HOGBEN C. A. Some physico-chemical factors in drug action. J Pharm Pharmacol. 1957 Jun;9(6):345–380. doi: 10.1111/j.2042-7158.1957.tb12289.x. [DOI] [PubMed] [Google Scholar]
- BRODIE B. B., KURZ H., SCHANKER L. S. The importance of dissociaton constant and lipid-solubility in influencing the passage of drugs into the cerebrospinal fluid. J Pharmacol Exp Ther. 1960 Sep;130:20–25. [PubMed] [Google Scholar]
- Bailar J. C., 3rd, Byar D. P. Estrogen treatment for cancer of the prostate. Early results with 3 doses of diethylstilbestrol and placebo. Cancer. 1970 Aug;26(2):257–261. doi: 10.1002/1097-0142(197008)26:2<257::aid-cncr2820260203>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Brown T. R., Rothwell S. W., Migeon C. J. Comparison of methyltrienolone and dihydrotestosterone binding and metabolism in human genital skin fibroblasts. J Steroid Biochem. 1981 Oct;14(10):1013–1022. doi: 10.1016/0022-4731(81)90209-0. [DOI] [PubMed] [Google Scholar]
- Fang S., Anderson K. M., Liao S. Receptor proteins for androgens. On the role of specific proteins in selective retention of 17-beta-hydroxy-5-alpha-androstan-3-one by rat ventral prostate in vivo and in vitro. J Biol Chem. 1969 Dec 25;244(24):6584–6595. [PubMed] [Google Scholar]
- Hedlund P. O., Gustafson H., Sjögren S. Cardiovascular complications to treatment of prostate cancer with estramustine phosphate (Estracyt) or conventional estrogen. A follow-up of 212 randomized patients. Scand J Urol Nephrol Suppl. 1980;55:103–105. [PubMed] [Google Scholar]
- Henriksson P., Edhag O. Orchidectomy versus oestrogen for prostatic cancer: cardiovascular effects. Br Med J (Clin Res Ed) 1986 Aug 16;293(6544):413–415. doi: 10.1136/bmj.293.6544.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi G. H., Altwein J. E., Kurth K. H., Basting R., Hohenfellner R. Treatment of advanced prostatic cancer with parenteral cyproterone acetate: a phase III randomised trial. Br J Urol. 1980 Jun;52(3):208–215. doi: 10.1111/j.1464-410x.1980.tb02961.x. [DOI] [PubMed] [Google Scholar]
- Markewitz M., Veenema R. J., Fingerhut B., Nehme-Haily D., Sommers S. C. Cyproterone acetate (SH 714) effect on histology and nucleic acid synthesis in the testes of patients with prostatic carcinoma. A preliminary report. Invest Urol. 1969 May;6(6):638–649. [PubMed] [Google Scholar]
- Murthy L. R., Johnson M. P., Rowley D. R., Young C. Y., Scardino P. T., Tindall D. J. Characterization of steroid receptors in human prostate using mibolerone. Prostate. 1986;8(3):241–253. doi: 10.1002/pros.2990080305. [DOI] [PubMed] [Google Scholar]
- Neri R. O., Monahan M. Effects of a novel nonsteroidal antiandrogen on canine prostatic hyperplasia. Invest Urol. 1972 Sep;10(2):123–130. [PubMed] [Google Scholar]
- Neri R., Florance K., Koziol P., Van Cleave S. A biological profile of a nonsteroidal antiandrogen, SCH 13521 (4'-nitro-3'trifluoromethylisobutyranilide). Endocrinology. 1972 Aug;91(2):427–437. doi: 10.1210/endo-91-2-427. [DOI] [PubMed] [Google Scholar]
- Raynaud J. P., Bonne C., Bouton M. M., Lagace L., Labrie F. Action of a non-steroid anti-androgen, RU 23908, in peripheral and central tissues. J Steroid Biochem. 1979 Jul;11(1A):93–99. doi: 10.1016/0022-4731(79)90281-4. [DOI] [PubMed] [Google Scholar]
- Raynaud J. P., Bonne C., Moguilewsky M., Lefebvre F. A., Bélanger A., Labrie F. The pure antiandrogen RU 23908 (Anandron), a candidate of choice for the combined antihormonal treatment of prostatic cancer: a review. Prostate. 1984;5(3):299–311. doi: 10.1002/pros.2990050307. [DOI] [PubMed] [Google Scholar]
- Sagalowsky A. I. Endocrine therapy for prostate cancer. Spec Top Endocrinol Metab. 1985;7:101–129. [PubMed] [Google Scholar]
- Schanker L. S. Passage of drugs into and out of the central nervous system. Antimicrob Agents Chemother (Bethesda) 1965;5:1044–1050. [PubMed] [Google Scholar]
- Schilling K., Liao S. The use of radioactive 7 alpha, 17 alpha-dimethyl-19-nortestosterone (mibolerone) in the assay of androgen receptors. Prostate. 1984;5(6):581–588. doi: 10.1002/pros.2990050603. [DOI] [PubMed] [Google Scholar]
- Smith R. B., Walsh P. C., Goodwin W. E. Cyproterone acetate in the treatment of advanced carcinoma of the prostate. J Urol. 1973 Jul;110(1):106–108. doi: 10.1016/s0022-5347(17)60128-9. [DOI] [PubMed] [Google Scholar]
- Södersten P., Gray G., Damassa D. A., Smith E. R., Davidson J. M. Effects of a non-steroidal antiandrogen on sexual behavior and pituitary-gonadal function in the male rat. Endocrinology. 1975 Dec;97(6):1468–1475. doi: 10.1210/endo-97-6-1468. [DOI] [PubMed] [Google Scholar]
- Traish A. M., Müller R. E., Wotiz H. H. Binding of 7 alpha, 17 alpha-dimethyl-19-nortestosterone (mibolerone) to androgen and progesterone receptors in human and animal tissues. Endocrinology. 1986 Apr;118(4):1327–1333. doi: 10.1210/endo-118-4-1327. [DOI] [PubMed] [Google Scholar]
- Wein A. J., Murphy J. J. Experience in the treatment of prostatic carcinoma with cyproterone acetate. J Urol. 1973 Jan;109(1):68–70. doi: 10.1016/s0022-5347(17)60352-5. [DOI] [PubMed] [Google Scholar]
- Williams G. Endocrine treatment of prostatic cancer. J R Soc Med. 1985 Oct;78(10):797–799. doi: 10.1177/014107688507801001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E. M., French F. S. Binding properties of androgen receptors. Evidence for identical receptors in rat testis, epididymis, and prostate. J Biol Chem. 1976 Sep 25;251(18):5620–5629. [PubMed] [Google Scholar]


