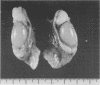
Abstract
Iron appears to play a major role in catalysing free radical production, leading to lipid peroxidation and DNA damage. We, therefore, investigated the effect of colloidal iron deposited in the peritoneum. Wistar male rats were given either ferric saccharate, ferric saccharate and nitrilotriacetic acid (NTA), NTA or saline. NTA was shown previously to 'free' iron to promote lipid peroxidation and an iron chelate of NTA is known to be carcinogenic to the kidney. Iron at a dose of 5 mg kg-1 day-1, and saline at a dose of 0.5 ml day-1 were injected i.p. for 3 months. NTA at a dose of 83.5 mg kg-1 day-1 was give i.p. for 5 months. All the rats were killed about a year later for histological examination. In nine of the 19 rats treated with ferric saccharate, mesothelial tumors were induced in the serosa of the tunica vaginalis or the length of the spermatic cord. Among rats treated with ferric saccharate and NTA, seven had localised mesotheliomas in the above locations and six had wide-spread peritoneal mesotheliomas. No mesothelial tumors developed in either NTA treated or saline treated rats. No pleural mesotheliomas were found in any group. These findings add to the evidence that iron is involved in some carcinogenic processes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aust S. D., Morehouse L. A., Thomas C. E. Role of metals in oxygen radical reactions. J Free Radic Biol Med. 1985;1(1):3–25. doi: 10.1016/0748-5514(85)90025-x. [DOI] [PubMed] [Google Scholar]
- Bates G. W., Schlabach M. R. The reaction of ferric salts with transferrin. J Biol Chem. 1973 May 10;248(9):3228–3232. [PubMed] [Google Scholar]
- Cabral J. R., Neal G. E. Testicular mesotheliomas in rats exposed to N-2-fluorenylacetamide (FAA). Tumori. 1983 Jun 30;69(3):195–199. doi: 10.1177/030089168306900304. [DOI] [PubMed] [Google Scholar]
- Churg A. M., Warnock M. L. Asbestos and other ferruginous bodies: their formation and clinical significance. Am J Pathol. 1981 Mar;102(3):447–456. [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Okada S., Hamazaki S., Ogino F., Li J. L., Midorikawa O. Nephrotoxicity and renal cell carcinoma after use of iron- and aluminum-nitrilotriacetate complexes in rats. J Natl Cancer Inst. 1986 Jan;76(1):107–113. [PubMed] [Google Scholar]
- Girotti A. W. Mechanisms of lipid peroxidation. J Free Radic Biol Med. 1985;1(2):87–95. doi: 10.1016/0748-5514(85)90011-x. [DOI] [PubMed] [Google Scholar]
- Gulumian M., Kilroe-Smith T. A. Crocidolite-induced lipid peroxidation in rat lung microsomes. I. Role of different ions. Environ Res. 1987 Jun;43(1):267–273. doi: 10.1016/s0013-9351(87)80077-4. [DOI] [PubMed] [Google Scholar]
- Gulumian M., Sardianos F., Kilroe-Smith T., Ockerse G. Lipid peroxidation in microsomes induced by crocidolite fibres. Chem Biol Interact. 1983 Apr-May;44(1-2):111–118. doi: 10.1016/0009-2797(83)90133-3. [DOI] [PubMed] [Google Scholar]
- HADDOW A., HORNING E. S. On the carcinogenicity of an iron-dextran complex. J Natl Cancer Inst. 1960 Jan;24:109–147. [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamazaki S., Okada S., Ebina Y., Fujioka M., Midorikawa O. Nephrotoxicity of ferric nitrilotriacetate. An electron-microscopic and metabolic study. Am J Pathol. 1986 May;123(2):343–350. [PMC free article] [PubMed] [Google Scholar]
- Hamazaki S., Okada S., Ebina Y., Li J. L., Midorikawa O. Effect of dietary vitamin E on ferric nitrilotriacetate-induced nephrotoxicity in rats. Toxicol Appl Pharmacol. 1988 Mar 15;92(3):500–506. doi: 10.1016/0041-008x(88)90190-1. [DOI] [PubMed] [Google Scholar]
- Hamazaki S., Okada S., Ebina Y., Midorikawa O. Acute renal failure and glucosuria induced by ferric nitrilotriacetate in rats. Toxicol Appl Pharmacol. 1985 Feb;77(2):267–274. doi: 10.1016/0041-008x(85)90326-6. [DOI] [PubMed] [Google Scholar]
- Kasai H., Nishimura S. DNA damage induced by asbestos in the presence of hydrogen peroxide. Gan. 1984 Oct;75(10):841–844. [PubMed] [Google Scholar]
- Li J. L., Okada S., Hamazaki S., Ebina Y., Midorikawa O. Subacute nephrotoxicity and induction of renal cell carcinoma in mice treated with ferric nitrilotriacetate. Cancer Res. 1987 Apr 1;47(7):1867–1869. [PubMed] [Google Scholar]
- Maekawa A., Onodera H., Tanigawa H., Furuta K., Kodama Y., Horiuchi S., Hayashi Y. Neoplastic and non-neoplastic lesions in aging Slc: Wistar rats. J Toxicol Sci. 1983 Nov;8(4):279–290. doi: 10.2131/jts.8.279. [DOI] [PubMed] [Google Scholar]
- Tanigawa H., Onodera H., Maekawa A. Spontaneous mesotheliomas in Fischer rats--a histological and electron microscopic study. Toxicol Pathol. 1987;15(2):157–163. doi: 10.1177/019262338701500205. [DOI] [PubMed] [Google Scholar]
- Weitzman S. A., Graceffa P. Asbestos catalyzes hydroxyl and superoxide radical generation from hydrogen peroxide. Arch Biochem Biophys. 1984 Jan;228(1):373–376. doi: 10.1016/0003-9861(84)90078-x. [DOI] [PubMed] [Google Scholar]
- Weitzman S. A., Weitberg A. B. Asbestos-catalysed lipid peroxidation and its inhibition by desferroxamine. Biochem J. 1985 Jan 1;225(1):259–262. doi: 10.1042/bj2250259. [DOI] [PMC free article] [PubMed] [Google Scholar]