Abstract
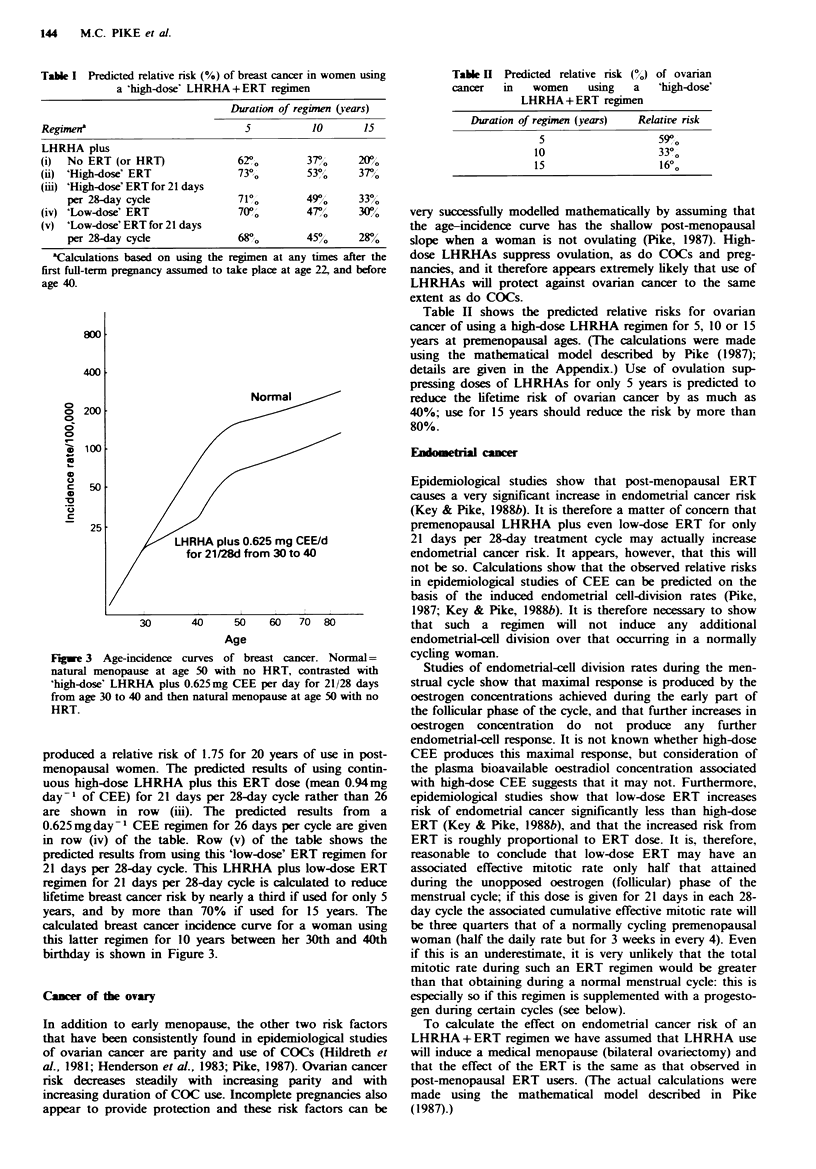
Early age at natural menopause or bilateral ovariectomy substantially reduce a woman's lifetime risk of breast cancer. Reversible 'bilateral ovariectomy' can now in effect be achieved by 'high-dose' luteinising hormone releasing hormone (LHRH) agonists (LHRHAs). The harmful effects of such medical reversible bilateral ovariectomy, in particular the increased risks of coronary heart disease and osteoporosis, can in all likelihood be obviated by 'low-dose' oestrogen replacement therapy (ERT), specifically 0.625 mg of conjugated equine oestrogens (CEE) for 21 days in each 28-day treatment cycle, and such ERT use will only negate to a relatively small extent the beneficial effect of such bilateral ovariectomy on breast cancer risk. We calculate that such an LHRHA plus low-dose ERT regimen given to a premenopausal woman for 10 years will, in addition to being a most effective contraceptive, decrease her lifetime risk of breast cancer by more than 50%. We calculate that such a 10-year regimen will also decrease her risk of ovarian cancer by two-thirds. This regimen should leave endometrial cancer risk and bone metabolism unaltered, and may reduce the risk of heart disease. The addition of a 'low-dose' progestogen to the regimen for 12 days in each 28-day treatment cycle would be beneficial to the endometrium, but it will adversely affect risk factors for heart disease and it may significantly reduce the benefit of the regimen as regards breast cancer. A satisfactory compromise may be to add a low-dose progestogen for 12 days at less frequent intervals. Another possibility may be to deliver a progestogen solely to the endometrium with an intra-uterine device; the benefits of such a regimen would be a significant reduction in the incidence of breast, ovarian and endometrial cancer.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird D. D., Tyroler H. A., Heiss G., Chambless L. E., Hames C. G. Menopausal change in serum cholesterol. Black/white differences in Evans County, Georgia. Am J Epidemiol. 1985 Dec;122(6):982–993. doi: 10.1093/oxfordjournals.aje.a114202. [DOI] [PubMed] [Google Scholar]
- Barnes R. B., Roy S., Lobo R. A. Comparison of lipid and androgen levels after conjugated estrogen or depo-medroxyprogesterone acetate treatment in postmenopausal women. Obstet Gynecol. 1985 Aug;66(2):216–219. [PubMed] [Google Scholar]
- Bernstein L., Ross R. K., Lobo R. A., Hanisch R., Krailo M. D., Henderson B. E. The effects of moderate physical activity on menstrual cycle patterns in adolescence: implications for breast cancer prevention. Br J Cancer. 1987 Jun;55(6):681–685. doi: 10.1038/bjc.1987.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen C., Christensen M. S., Transbøl I. Bone mass in postmenopausal women after withdrawal of oestrogen/gestagen replacement therapy. Lancet. 1981 Feb 28;1(8218):459–461. doi: 10.1016/s0140-6736(81)91848-1. [DOI] [PubMed] [Google Scholar]
- Elwood J. M., Cole P., Rothman K. J., Kaplan S. D. Epidemiology of endometrial cancer. J Natl Cancer Inst. 1977 Oct;59(4):1055–1060. doi: 10.1093/jnci/59.4.1055. [DOI] [PubMed] [Google Scholar]
- Ferguson D. J., Anderson T. J. Morphological evaluation of cell turnover in relation to the menstrual cycle in the "resting" human breast. Br J Cancer. 1981 Aug;44(2):177–181. doi: 10.1038/bjc.1981.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser H. M., Baird D. T. Clinical applications of LHRH analogues. Baillieres Clin Endocrinol Metab. 1987 Feb;1(1):43–70. doi: 10.1016/s0950-351x(87)80052-6. [DOI] [PubMed] [Google Scholar]
- Gudmundsson J. A., Nillius S. J., Bergquist C. Intranasal peptide contraception by inhibition of ovulation with the gonadotropin-releasing hormone superagonist nafarelin: six months' clinical results. Fertil Steril. 1986 May;45(5):617–623. doi: 10.1016/s0015-0282(16)49331-3. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt K., Landgren B. M., Edström K., Johannisson E. Biochemical and morphological changes in the human endometrium induced by the progestasert device. Contraception. 1977 Aug;16(2):183–197. doi: 10.1016/0010-7824(77)90086-5. [DOI] [PubMed] [Google Scholar]
- Henderson B. E., Ross R. K., Pike M. C., Casagrande J. T. Endogenous hormones as a major factor in human cancer. Cancer Res. 1982 Aug;42(8):3232–3239. [PubMed] [Google Scholar]
- Hildreth N. G., Kelsey J. L., LiVolsi V. A., Fischer D. B., Holford T. R., Mostow E. D., Schwartz P. E., White C. An epidemiologic study of epithelial carcinoma of the ovary. Am J Epidemiol. 1981 Sep;114(3):398–405. doi: 10.1093/oxfordjournals.aje.a113207. [DOI] [PubMed] [Google Scholar]
- Key T. J., Pike M. C. The dose-effect relationship between 'unopposed' oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. Br J Cancer. 1988 Feb;57(2):205–212. doi: 10.1038/bjc.1988.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. J., Whitehead M. I. Assessment of the potency of orally administered progestins in women. Fertil Steril. 1986 Dec;46(6):1062–1066. doi: 10.1016/s0015-0282(16)49880-8. [DOI] [PubMed] [Google Scholar]
- Lin A. L., McGill H. C., Jr, Shain S. A. Hormone receptors of the baboon cardiovascular system. Biochemical characterization of aortic and myocardial cytoplasmic progesterone receptors. Circ Res. 1982 May;50(5):610–616. doi: 10.1161/01.res.50.5.610. [DOI] [PubMed] [Google Scholar]
- Lindsay R., Hart D. M., Aitken J. M., MacDonald E. B., Anderson J. B., Clarke A. C. Long-term prevention of postmenopausal osteoporosis by oestrogen. Evidence for an increased bone mass after delayed onset of oestrogen treatment. Lancet. 1976 May 15;1(7968):1038–1041. doi: 10.1016/s0140-6736(76)92217-0. [DOI] [PubMed] [Google Scholar]
- Lindsay R., Hart D. M., Clark D. M. The minimum effective dose of estrogen for prevention of postmenopausal bone loss. Obstet Gynecol. 1984 Jun;63(6):759–763. [PubMed] [Google Scholar]
- Mann J. I., Lewis B., Shepherd J., Winder A. F., Fenster S., Rose L., Morgan B. Blood lipid concentrations and other cardiovascular risk factors: distribution, prevalence, and detection in Britain. Br Med J (Clin Res Ed) 1988 Jun 18;296(6638):1702–1706. doi: 10.1136/bmj.296.6638.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan R. I., Healy D. L., Burger H. G. Clinical aspects of LHRH analogues in gynaecology: a review. Br J Obstet Gynaecol. 1986 May;93(5):431–454. [PubMed] [Google Scholar]
- McPherson K., Drife J. O. The pill and breast cancer: why the uncertainty? Br Med J (Clin Res Ed) 1986 Sep 20;293(6549):709–710. doi: 10.1136/bmj.293.6549.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. H., Moore D. H., 2nd, Moore C. T. Breast carcinoma etiological factors. Adv Cancer Res. 1983;40:189–253. doi: 10.1016/s0065-230x(08)60681-8. [DOI] [PubMed] [Google Scholar]
- Mäkilä U. M., Wahlberg L., Vlinikka L., Ylikorkala O. Regulation of prostacyclin and thromboxane production by human umbilical vessels: the effect of estradiol and progesterone in a superfusion model. Prostaglandins Leukot Med. 1982 Feb;8(2):115–124. doi: 10.1016/s0262-1746(82)80003-6. [DOI] [PubMed] [Google Scholar]
- Ottosson U. B., Johansson B. G., von Schoultz B. Subfractions of high-density lipoprotein cholesterol during estrogen replacement therapy: a comparison between progestogens and natural progesterone. Am J Obstet Gynecol. 1985 Mar 15;151(6):746–750. doi: 10.1016/0002-9378(85)90509-5. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A., Ross R. K., Gerkins V. R., Henderson B. E., Arthur M., Mack T. M. Menopausal estrogen therapy and hip fractures. Ann Intern Med. 1981 Jul;95(1):28–31. doi: 10.7326/0003-4819-95-1-28. [DOI] [PubMed] [Google Scholar]
- Pike M. C., Krailo M. D., Henderson B. E., Casagrande J. T., Hoel D. G. 'Hormonal' risk factors, 'breast tissue age' and the age-incidence of breast cancer. Nature. 1983 Jun 30;303(5920):767–770. doi: 10.1038/303767a0. [DOI] [PubMed] [Google Scholar]
- Stadel B. V. Oral contraceptives and cardiovascular disease (second of two parts). N Engl J Med. 1981 Sep 17;305(12):672–677. doi: 10.1056/NEJM198109173051205. [DOI] [PubMed] [Google Scholar]
- Thorogood M., Carter R., Benfield L., McPherson K., Mann J. I. Plasma lipids and lipoprotein cholesterol concentrations in people with different diets in Britain. Br Med J (Clin Res Ed) 1987 Aug 8;295(6594):351–353. doi: 10.1136/bmj.295.6594.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl P., Walden C., Knopp R., Hoover J., Wallace R., Heiss G., Rifkind B. Effect of estrogen/progestin potency on lipid/lipoprotein cholesterol. N Engl J Med. 1983 Apr 14;308(15):862–867. doi: 10.1056/NEJM198304143081502. [DOI] [PubMed] [Google Scholar]


