Abstract
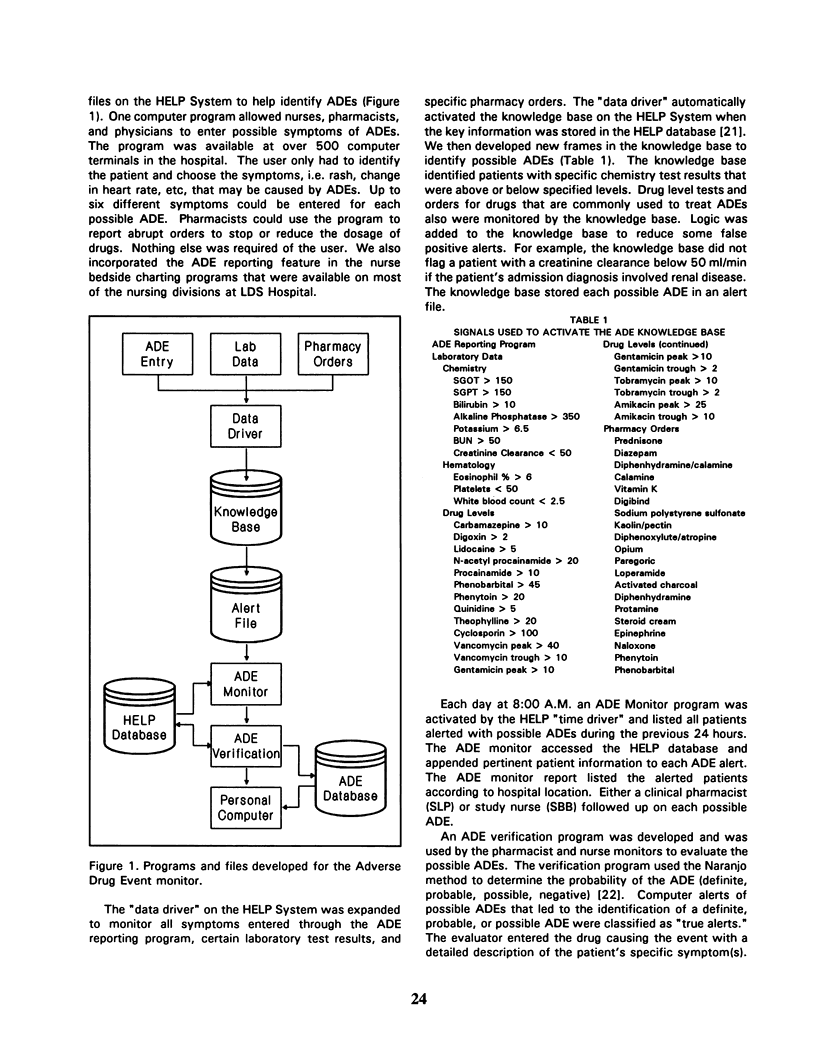
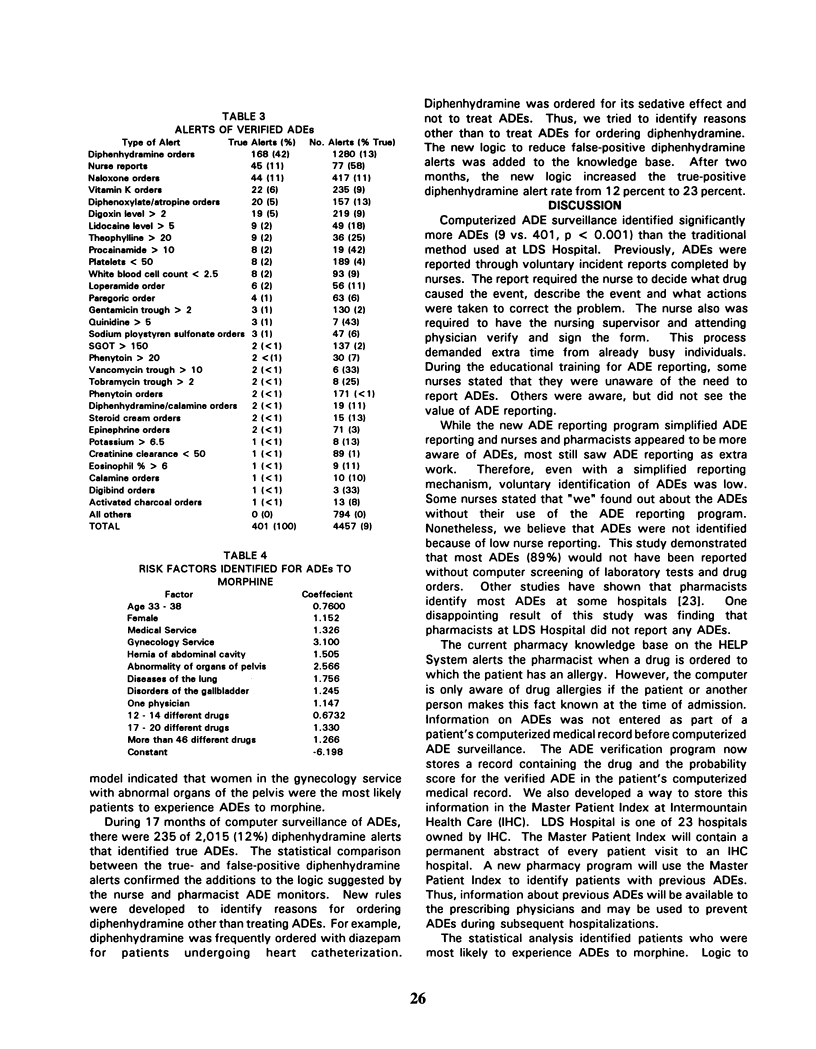
Adverse events during drug therapy are receiving renewed attention. Some adverse drug events (ADEs) are identified only after the widespread clinical use of a drug. The Food and Drug Administration advocates post-marketing surveillance systems to provide early warnings of previously undetected ADEs. The identification of ADEs by U.S. hospitals is now required by the Joint Commission on Accreditation of Healthcare Organizations. We developed a series of computer programs and data files on the HELP System to help identify ADEs. The HELP System monitors laboratory test results, drug orders, and data entered through a computerized ADE reporting program. A nurse or pharmacist verifies computer alerts of possible ADEs. The computerized system identified 401 ADEs during the first year of use compared to 9 by voluntary reporting methods during the previous year (p less than 0.001). This paper describes the development and early use of the computerized ADE surveillance system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke J. P., Tilson H. H., Platt R. Expanding roles of hospital epidemiology: pharmacoepidemiology. Infect Control Hosp Epidemiol. 1989 Jun;10(6):253–254. doi: 10.1086/646016. [DOI] [PubMed] [Google Scholar]
- Faich G. A. Adverse-drug-reaction monitoring. N Engl J Med. 1986 Jun 12;314(24):1589–1592. doi: 10.1056/NEJM198606123142427. [DOI] [PubMed] [Google Scholar]
- Faich G. A., Knapp D., Dreis M., Turner W. National adverse drug reaction surveillance: 1985. JAMA. 1987 Apr 17;257(15):2068–2070. [PubMed] [Google Scholar]
- Jick H. Drugs--remarkably nontoxic. N Engl J Med. 1974 Oct 17;291(16):824–828. doi: 10.1056/NEJM197410172911605. [DOI] [PubMed] [Google Scholar]
- Jick H., Miettinen O. S., Shapiro S., Lewis G. P., Siskind V., Slone D. Comprehensive drug surveillance. JAMA. 1970 Aug 31;213(9):1455–1460. [PubMed] [Google Scholar]
- Koch K. E. Use of standardized screening procedures to identify adverse drug reactions. Am J Hosp Pharm. 1990 Jun;47(6):1314–1320. [PubMed] [Google Scholar]
- Melmon K. L. Preventable drug reactions--causes and cures. N Engl J Med. 1971 Jun 17;284(24):1361–1368. doi: 10.1056/NEJM197106172842408. [DOI] [PubMed] [Google Scholar]
- Miwa L. J., Randall R. J. Adverse drug reaction program using pharmacist and nurse monitors. Hosp Formul. 1986 Nov;21(11):1140-3, 1146. [PubMed] [Google Scholar]
- Naranjo C. A., Busto U., Sellers E. M., Sandor P., Ruiz I., Roberts E. A., Janecek E., Domecq C., Greenblatt D. J. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981 Aug;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- Porter J., Jick H. Drug-related deaths among medical inpatients. JAMA. 1977 Feb 28;237(9):879–881. [PubMed] [Google Scholar]
- Prosser T. R., Kamysz P. L. Multidisciplinary adverse drug reaction surveillance program. Am J Hosp Pharm. 1990 Jun;47(6):1334–1339. [PubMed] [Google Scholar]
- Pryor T. A., Gardner R. M., Clayton P. D., Warner H. R. The HELP system. J Med Syst. 1983 Apr;7(2):87–102. doi: 10.1007/BF00995116. [DOI] [PubMed] [Google Scholar]
- Slone D., Gaetano L. F., Lipworth L., Shapiro S., Lewis G. P., Jick H. Computer analysis of epidemiologic data on effect of drugs on hospital patients. Public Health Rep. 1969 Jan;84(1):39–52. [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Seidl L. G., Cluff L. E. Studies on the epidemiology of adverse drug reactions. V. Clinical factors influencing susceptibility. Ann Intern Med. 1966 Oct;65(4):629–640. doi: 10.7326/0003-4819-65-4-629. [DOI] [PubMed] [Google Scholar]
- Steel K., Gertman P. M., Crescenzi C., Anderson J. Iatrogenic illness on a general medical service at a university hospital. N Engl J Med. 1981 Mar 12;304(11):638–642. doi: 10.1056/NEJM198103123041104. [DOI] [PubMed] [Google Scholar]
- Wang R. I., Terry L. C. Adverse drug reactions in a veterans administration hospital. J Clin Pharmacol New Drugs. 1971 Jan-Feb;11(1):14–18. doi: 10.1177/009127007101100102. [DOI] [PubMed] [Google Scholar]


