Abstract
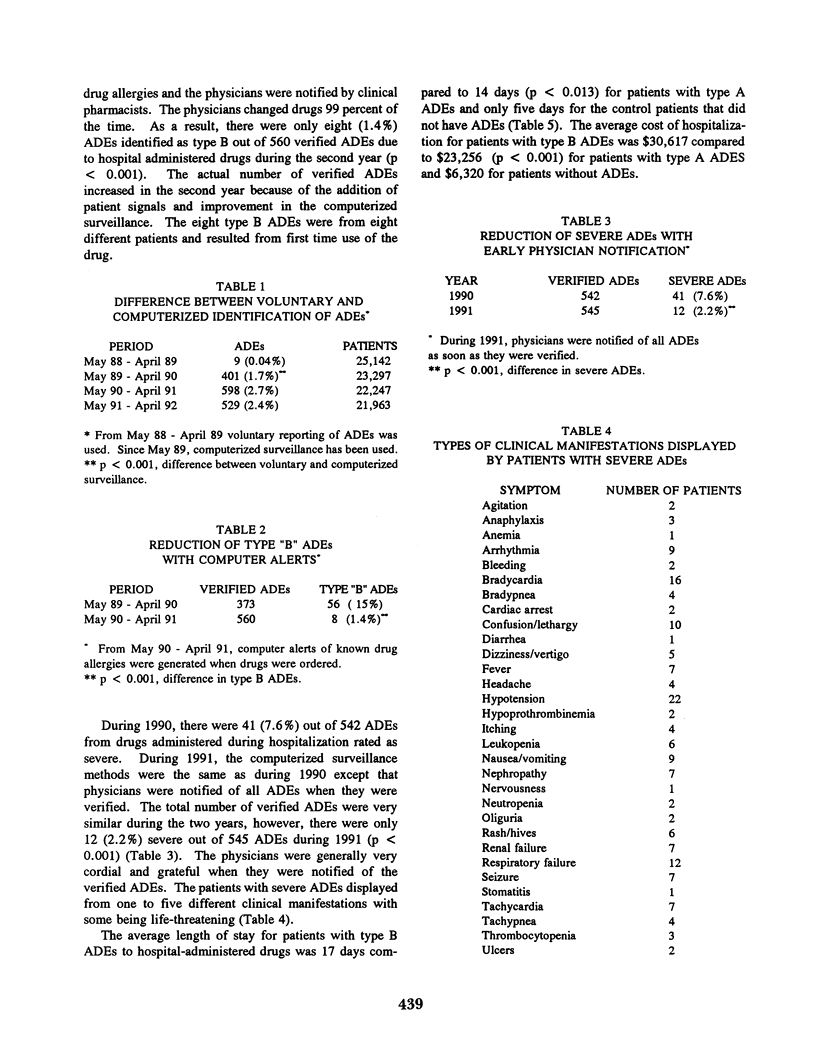
Adverse drug events (ADEs) are a serious health problem and are the leading adverse event experienced by hospitalized patients. Numerous hospitals have used different methods to improve the reporting of ADEs but few have undertaken studies aimed at the prevention of ADEs. We found that computerized ADE surveillance identified significantly more ADEs than our previous voluntary reporting method. Moreover, the computerized ADE surveillance system created a database of ADEs which allowed us to analyze the ADEs and design methods for prevention. We found that computer alerts of previously known drug allergies generated when drugs were ordered significantly reduced the number of type B ADEs, 56 vs 8 (p < 0.001). In addition, we found that the timely surveillance of ADEs combined with physician notification reduced the number of severe ADEs, 41 vs 12 (p < 0.001). Initial analysis of the ADE database has shown that on average patients with type B ADEs are hospitalized longer (17 vs 14 days) and have larger hospitalization costs ($30,617 vs $23,256) than patients with type A ADEs. Patients with severe ADEs also are hospitalized longer (20 vs 13 days) and have larger hospitalization costs ($38,007 vs $22,474) than patients with moderate ADEs. This indicates that the prevention and early treatment of ADEs can reduce the length of hospitalization and result in a considerable cost savings to the hospital.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brennan T. A., Leape L. L., Laird N. M., Hebert L., Localio A. R., Lawthers A. G., Newhouse J. P., Weiler P. C., Hiatt H. H. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991 Feb 7;324(6):370–376. doi: 10.1056/NEJM199102073240604. [DOI] [PubMed] [Google Scholar]
- Classen D. C., Pestotnik S. L., Evans R. S., Burke J. P. Computerized surveillance of adverse drug events in hospital patients. JAMA. 1991 Nov 27;266(20):2847–2851. [PubMed] [Google Scholar]
- Evans R. S., Pestotnik S. L., Classen D. C., Bass S. B., Menlove R. L., Gardner R. M., Burke J. P. Development of a computerized adverse drug event monitor. Proc Annu Symp Comput Appl Med Care. 1991:23–27. [PMC free article] [PubMed] [Google Scholar]
- Faich G. A., Knapp D., Dreis M., Turner W. National adverse drug reaction surveillance: 1985. JAMA. 1987 Apr 17;257(15):2068–2070. [PubMed] [Google Scholar]
- Hulse R. K., Clark S. J., Jackson J. C., Warner H. R., Gardner R. M. Computerized medication monitoring system. Am J Hosp Pharm. 1976 Oct;33(10):1061–1064. [PubMed] [Google Scholar]
- Jick H. Drugs--remarkably nontoxic. N Engl J Med. 1974 Oct 17;291(16):824–828. doi: 10.1056/NEJM197410172911605. [DOI] [PubMed] [Google Scholar]
- Jick H., Miettinen O. S., Shapiro S., Lewis G. P., Siskind V., Slone D. Comprehensive drug surveillance. JAMA. 1970 Aug 31;213(9):1455–1460. [PubMed] [Google Scholar]
- Melmon K. L. Preventable drug reactions--causes and cures. N Engl J Med. 1971 Jun 17;284(24):1361–1368. doi: 10.1056/NEJM197106172842408. [DOI] [PubMed] [Google Scholar]
- Porter J., Jick H. Drug-related deaths among medical inpatients. JAMA. 1977 Feb 28;237(9):879–881. [PubMed] [Google Scholar]
- Powell S. H., Schwartz P. A., Rayment C. M., Marx C. M. Pharmacy-based adverse drug reaction surveillance program. Am J Hosp Pharm. 1982 Nov;39(11):1963–1964. [PubMed] [Google Scholar]
- Rind D. M., Safran C., Phillips R. S., Slack W. V., Calkins D. R., Delbanco T. L., Bleich H. L. The effect of computer-based reminders on the management of hospitalized patients with worsening renal function. Proc Annu Symp Comput Appl Med Care. 1991:28–32. [PMC free article] [PubMed] [Google Scholar]
- Rogers A. S., Israel E., Smith C. R., Levine D., McBean A. M., Valente C., Faich G. Physician knowledge, attitudes, and behavior related to reporting adverse drug events. Arch Intern Med. 1988 Jul;148(7):1596–1600. [PubMed] [Google Scholar]
- Shapiro S., Slone D., Lewis G. P., Jick H. Fatal drug reactions among medical inpatients. JAMA. 1971 Apr 19;216(3):467–472. [PubMed] [Google Scholar]
- Slone D., Gaetano L. F., Lipworth L., Shapiro S., Lewis G. P., Jick H. Computer analysis of epidemiologic data on effect of drugs on hospital patients. Public Health Rep. 1969 Jan;84(1):39–52. [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Seidl L. G., Cluff L. E. Studies on the epidemiology of adverse drug reactions. V. Clinical factors influencing susceptibility. Ann Intern Med. 1966 Oct;65(4):629–640. doi: 10.7326/0003-4819-65-4-629. [DOI] [PubMed] [Google Scholar]
- Steel K., Gertman P. M., Crescenzi C., Anderson J. Iatrogenic illness on a general medical service at a university hospital. N Engl J Med. 1981 Mar 12;304(11):638–642. doi: 10.1056/NEJM198103123041104. [DOI] [PubMed] [Google Scholar]


