Summary
Quantal size is the postsynaptic response to the release of a single synaptic vesicle and is determined in part by the amount of transmitter within that vesicle. At glutamatergic synapses, the vesicular glutamate transporter (VGLUT) fills vesicles with glutamate. While elevated VGLUT expression increases quantal size, the minimum number of transporters required to fill a vesicle is unknown. In Drosophila DVGLUT mutants, reduced transporter levels lead to a dose-dependent reduction in the frequency of spontaneous quantal release with no change in quantal size. Quantal frequency is not limited by vesicle number or impaired exocytosis. This suggests that a single functional unit of transporter is both necessary and sufficient to fill a vesicle to completion and that vesicles without DVGLUT are empty. Consistent with the presence of empty vesicles, at dvglut mutant synapses synaptic vesicles are smaller, suggesting that vesicle filling and/or transporter level is an important determinant of vesicle size.
Introduction
The postsynaptic response to the fusion of a single synaptic vesicle is a fundamental determinant of synaptic strength that is controlled in part by the number of neurotransmitter molecules within the vesicle (Bruns et al., 2000; Colliver et al., 2000; Franks et al., 2003; Gong et al., 2003; Pothos et al., 2000). One mechanism postulated to control the transmitter content of vesicles is the number of vesicular neurotransmitter transporters on the vesicle. In many systems, increasing the level of neurotransmitter transporters can increase the amount of transmitter accumulated inside vesicles (Colliver et al., 2000; Daniels et al., 2004; Pothos et al., 2000; Song et al., 1997; Wilson et al., 2005). What is not known, however, is how many transporters are necessary to fill a vesicle to normal levels.
At glutamatergic synapses, vesicular glutamate transporters (VGLUTs) are necessary to fill vesicles—in the absence of VGLUT1, there are empty vesicles (Fremeau et al., 2004; Wojcik et al., 2004). However, these studies are complicated by the presence of multiple VGLUT isoforms in vertebrates, with many cells coexpressing two VGLUT isoforms (Boulland et al., 2004). In Drosophila, all known glutamatergic neurons express a single VGLUT gene, DVGLUT (Daniels et al., 2004). This simplified system allows us to vary the dose of the single, functional transporter at a synapse in vivo. There are two possible consequences of a reduction in DVGLUT levels. First, if multiple transporters were necessary to fill each vesicle, then a decrease in transporter dose would be expected to reduce mEJP amplitude. On the other hand, if a single transporter is sufficient to fill a vesicle, vesicles with functional transporter would be full and vesicles without transporter would be empty. Experimentally, this would be observed as a decrease in spontaneous mEJP frequency. The stoichiometry of a functional glutamate transporter is unknown, so our discussion of a single transporter encompasses as many molecules of VGLUT protein as are necessary to form an active transporter.
We have generated mutants in the Drosophila VGLUT homolog that allow us to decrease the dose of transporter at the synapse. We observe no change in mEJP amplitude, but a dose-dependent decrease in mEJP frequency. This reduction in event frequency is neither due to a lack of vesicles near the release site nor to an inability of these vesicles to fuse efficiently. These results strongly suggest that one functional unit of DVGLUT is both necessary and sufficient to fill a vesicle to equilibrium glutamate levels and that vesicles without DVGLUT are empty. At these dvglut mutant synapses, the average synaptic vesicle is smaller, consistent with the presence of empty vesicles. These results suggest that the number of transporters that reside on a synaptic vesicle must be carefully regulated.
Results and Discussion
Characterization of dvglut Mutants
To determine whether decreasing transporter levels reduce the amount of transmitter within a vesicle, we generated mutants that reduce the levels of DVGLUT protein at the synapse. Mutants were made by imprecise excision of a p-element 1.5 kb upstream of dvglut (see Experimental Procedures). In this study, we use three of these excision lines: a control line that does not disrupt the dvglut gene; the dvglut1 allele, which is a hypomorph that deletes 2.3 kb of dvglut and expresses low levels of DVGLUT protein (Figure 1A); and Df(2L)dvglut2, a larger deletion that is lethal and removes the dvglut coding region (see Experimental Procedures). To determine how these mutants affect DVGLUT abundance at synapses, we quantified anti-DVGLUT immunofluorescence at the glutamatergic larval neuromuscular junction (NMJ). Anti-DVGLUT immunofluorescence is reduced to approximately 70%, 20%, and 10% of control levels in Df(2L)dvglut2/+, dvglut1/dvglut1, and dvglut1/Df(2L)dvglut2 larvae, respectively (Figures 1B–1D, p < 0.001 for all comparisons). Hence, this series of dvglut mutant combinations allows us to assess the physiological response of the synapse to reduced doses of DVGLUT.
Figure 1. DVGLUT Protein Levels and Staining Are Reduced in dvglut Mutants.
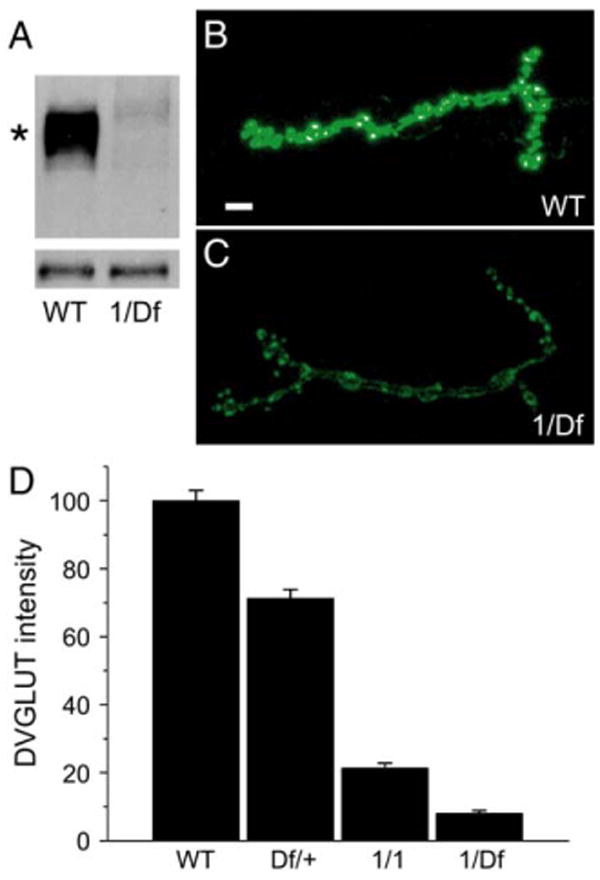
(A) Western blot of larval brain extracts from wild-type (WT) and dvglut1/Df(2L)dvglut2 (1/Df). Upper band (marked by an asterisk) is specific for DVGLUT, while lower band serves as a loading control. (B and C) Fluorescent staining for DVGLUT at muscle 4 of the third instar NMJ in control ([B], WT) and dvglut1/Df(2L)dvglut2 mutants ([C], 1/Df). Gain has been increased in both pictures to allow better visualization of the low levels of DVGLUT in the dvglut mutant. (D) α-DVGLUT staining intensity in wild-type controls (precise excision, labeled WT, n = 36), dvglut heterozygote (Df(2L)dvglut2/+, labeled Df/+, n = 13), homozygous hypomorph (dvglut1/dvglut1, labeled 1/1, n = 12), and the transheterozygote larvae (dvglut1/Df(2L)dvglut2, labeled 1/Df, n = 12). Data are shown as a percentage of wild-type staining intensity. All comparisons are significantly different with p < 0.001 (one-way ANOVA), error bars indicate SEM. Scale bar in panel (B) represents 5 μm.
Quantal Frequency Is Decreased while Quantal Size Is Unchanged in dvglut Mutants
If reducing the number of transporters on the vesicle reduced the ability of the vesicle to fill completely, we would expect a decrease in mEJP amplitude. On the other hand, if only one transporter is sufficient to fill a vesicle completely, decreasing the number of transporters should only affect the frequency, not the amplitude, of mEJPs. To test whether vesicular glutamate loading is altered in dvglut mutants, we recorded spontaneous vesicle fusion at the NMJ (Figures 2A and 2B). A dose-dependent reduction in mEJP frequency was observed without any reduction in mEJP amplitude (Figures 2C and 2D). This decrease in frequency is rescued by driving a dvglut cDNA transgene in motoneurons (Figure 2D). The dvglut1/dvglut1 hypomorph releases fewer than half as many detectable quanta as wild-type larvae, while a further reduction in DVGLUT levels in dvglut1/Df(2L)dvglut2 decreases the quantal frequency to about one-quarter of normal (Figure 2C). There is no significant difference in mEJP amplitude among the genotypes tested, except in larvae rescued by the overexpression of DVGLUT. These show a slight increase in quantal size that is consistent with the previously characterized overexpression phenotype (Figure 2C; Daniels et al., 2004).
Figure 2. mEJP Frequency but Not mEJP Amplitude Is Decreased in dvglut Mutants.
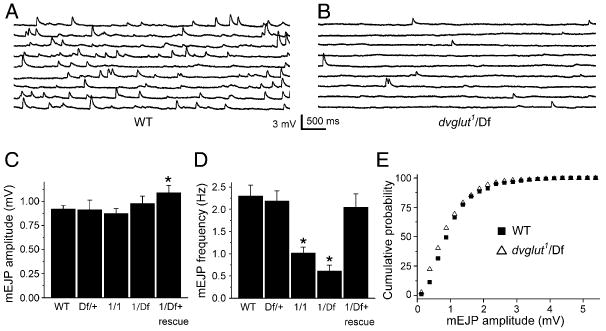
Spontaneous activity was recorded intracellularly from muscle 6 in third instar larvae. Approximately 45 s of sample voltage recordings from control ([A], WT) and dvglut mutant ([B], dvglut1/Df(2L)dvglut2, labeled dvglut1/Df) larvae. (C) Histogram of average mEJP amplitudes for wild-type (WT, n = 30), dvglut heterozygotes (Df(2L)dvglut2/+, labeled Df/+, n = 10), homozygous hypomorphs (dvglut1/dvglut1, labeled 1/1, n = 10), and the transheterozygote (dvglut1/Df(2L)dvglut2, labeled 1/Df, n = 12) and rescue larvae (BG380 Gal4/+; dvglut1/Df(2L)dvglut2; UAS-DVGLUT/+, labeled 1/Df + rescue, n = 13). There is no significant difference in mEJP amplitude except in rescue larvae, which exhibit a slight increase in mini amplitude (p < 0.05, one-way ANOVA). (D) Histogram of average mEJP frequency for the same genotypes as in (B). A dose-dependent decrease in mEJP frequency is observed in dvglut1/dvglut1 (1/1) and dvglut1/Df(2L)dvglut2 (1/Df) larvae compared to wild-type. The difference between dvglut1/dvglut1 and dvglut1/Df(2L)dvglut2 is also significant, p < 0.05). (E) Cumulative probability distribution of minis from wild-type (WT, filled squares) and dvglut1/Df(2L)dvglut2 (1/Df, open triangles). The distributions are not significantly different by the K-S test (p > 0.2). *p < 0.05 versus wild-type. Error bars indicate SEM.
The decrease in mEJP frequency with no change in their amplitude in the dvglut hypomorph is consistent with the model that a single, functional vesicular transporter is sufficient to fill a synaptic vesicle. With reduced levels of DVGLUT, many vesicles do not contain a functional DVGLUT and are empty, while those vesicles that do include a functional transporter have a normal amount of glutamate. However, alternate explanations for the data are possible. Might the persistence of normal quantal amplitude reflect postsynaptic changes in response to glutamate or presynaptic filling by a second DVGLUT gene? Is the reduction in quantal frequency due to the presence of empty vesicles, or might there be defects in vesicle biogenesis or in the ability of partially full vesicles to fuse? We address and exclude these alternate explanations in the following sections.
Normal Quantal Size Is Not Due to Postsynaptic Compensation or the Presence of a Second DVGLUT Gene
The lack of a change in mEJP amplitude in the mutants suggests that all detectable events result from the fusion of vesicles containing a normal number of transmitter molecules. Might, instead, the normal quantal size reflect a postsynaptic process that masks a decrease in transmitter content? We think this is unlikely for the following reasons. First, at this synapse, receptors are not saturated (Daniels et al., 2004; Karunanithi et al., 2002), so a decrease in transmitter should result in a reduced quantal size. Such reductions in quantal size can be detected without concomitant decreases in mini frequency (Petersen et al., 1997). Second, the distribution of mEJP amplitudes is not significantly different between wild-type and strong dvglut hypomorphs (Figure 2E, K-S test, p > 0.2), so if a postsynaptic homeostatic mechanism exists to keep quantal size constant, it would have to scale perfectly to wild-type amplitudes. Third, an increase in postsynaptic response to transmitter would result in both normal quantal size and frequency and thus is inconsistent with the data. Fourth, we detect no increase in glutamate receptor levels at the NMJ in the mutant and find that each release site is still apposed by a glutamate receptor punctum (data not shown). Fifth, we directly assayed the postsynaptic response to exogenously applied glutamate, a technique that assays both synaptic and extrasynaptic receptors at the Drosophila NMJ, and found no increase in the dvglut1/Df mutant (−41.7 ± 2.6 nA [n = 8] in wt versus −31.2 ± 1.9 nA [n = 7] in mutants). Hence, the normal quantal size in the dvglut mutants cannot be due to an increase in postsynaptic sensitivity to transmitter.
Might a second, unidentified DVGLUT protein fill vesicles in the dvglut mutant and thereby explain the normal quantal size? If so, then the release of glutamate-filled vesicles should persist in the absence of all DVGLUT protein. To test this possibility, we recorded from homozygous Df(2L)dvglut2 mutant embryos in which the entire DVGLUT gene is deleted. These animals die as paralyzed, late-stage embryos. No events were detected in the null (>30 min of recording from six embyros), while spontaneous miniature excitatory junctional currents (mEJCs) were abundant in wild-type (Figures 3A–3D). This defect is presynaptic because the response to pressure-ejected glutamate is not significantly different between wild-type and mutant muscles (Figures 3E and 3F). In addition, glutamate receptors cluster in the mutant, demonstrating that spontaneous vesicular glutamate release is not required for glutamate receptor clustering (see Figure S1 in the Supplemental Data available online). The lack of glutamate-containing vesicle fusion in the dvglut null despite normal glutamate receptor function demonstrates that DVGLUT is the only vesicular glutamate transporter at the NMJ.
Figure 3. No Spontaneous Events Are Detected in dvglut Null Mutants Despite Normal Glutamate Responses.
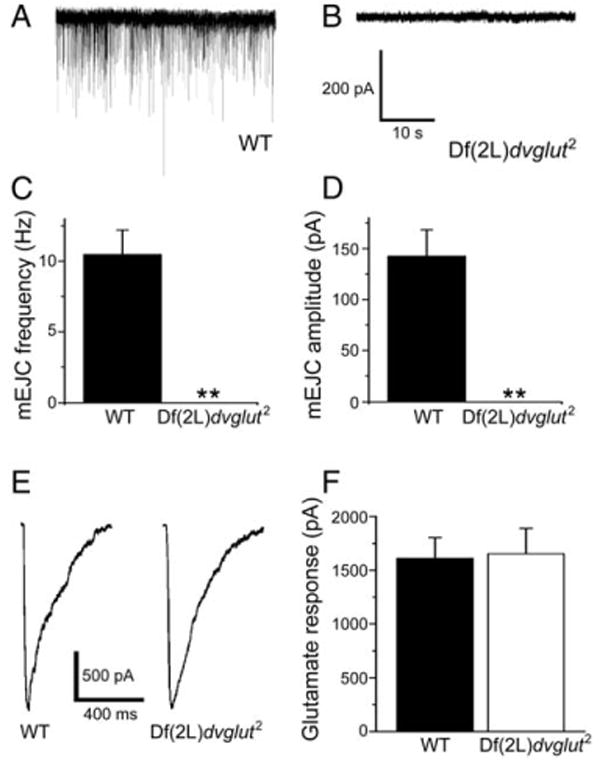
(A and B) Sample traces from wild-type (A) and dvglut null mutant embryos ([B], labeled Df(2L)dvglut2). (C and D) Average mEJC frequency (C) and amplitude (D) of spontaneous events in wild-type embryos. No events were detected in dvglut null mutants. (E and F) Glutamate receptors are present and respond equally to pressure ejection of glutamate. Sample individual current traces are shown in (E), and average response is shown in (F) (for wild-type [WT], n = 8, for Df(2L)dvglut2, n = 9, p > 0.8). Data are presented as mean ± SEM, **p < 0.01, Student's t test.
Vesicles Are Abundant but Smaller on Average in dvglut Mutants
Why is DVGLUT necessary to maintain mEJP frequency? The most obvious explanation is that it fills vesicles with glutamate, so decreasing DVGLUT levels would result in a mix of empty and full vesicles. However, two other possibilities exist: DVGLUT could be required for either vesicle biogenesis or for normal vesicle fusion. Supporting a role for VGLUT in vesicle biogenesis, mouse VGLUT1 knockout central synapses were found to have ∼50% fewer vesicles than in wild-type, although no change in the number of vesicles within 100 nm of the active zone was observed (Fremeau et al., 2004).
To examine whether DVGLUT is required for vesicle biogenesis, we used electron microscopy to image the synapse and then counted the number of vesicles adjacent to the active zone at NMJs from wild-type and dvglut1/Df(2L)dvglut2 larvae. Vesicles are abundant at both wild-type and dvglut mutant NMJs (Figures 4A and 4B). Within the first 100 nm of the active zone, the dvglut mutant displays only a 14% reduction in vesicle number (24.4 ± 1.0 for wt [n = 43 AZs] versus 21.1 ± 1.2 for dvglut1/Df(2L)dvglut2; Figure 4C, n = 39 AZs, p < 0.05). Even at 300 nm from the active zone, the reduction is less than 20% in the mutant (61.6 ± 2.6 for wt [n = 43 AZs] versus 49.6 ± 3.1 for dvglut1/Df(2L)dvglut2 [n = 39 AZs], p < 0.01, data not shown). However, this small decrease in the number of vesicles close to release sites is unlikely to account for the 4-fold reduction in mEJP frequency.
Figure 4. Synaptic Vesicles Are Abundant but Smaller on Average in dvglut Mutants.
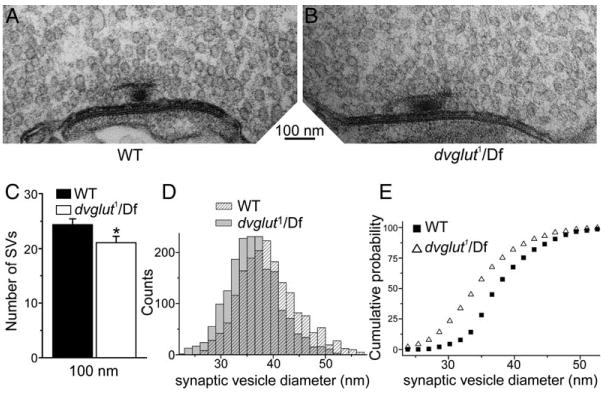
(A and B) Sample micrographs of active zones from control ([A], labeled WT) and mutant dvglut1/Df(2L)dvglut2 larvae ([B], labeled dvglut1/Df). (C) Synaptic vesicles within 300 nm of active zones were counted in 100 nm bins and the cumulative number of vesicles present within 100 nm of the active zone is shown for wild-type (filled bars, n = 43 active zones) and dvglut mutants (open bars, n = 39 active zones). Error bars indicate SEM. (D) Frequency histogram of synaptic vesicle outer diameters measured from EM sections of NMJs from wild-type (shaded bars, WT) and dvglut mutants (dvglut1/Df(2L)dvglut2, hatched bars). (E) Cumulative probability plot of the data in (D), with wild-type data (WT, black squares) and dvglut mutant data (dvglut1/Df, open triangles), n = 1840 vesicles for each genotype. The population of vesicle diameters is significantly shifted to smaller sizes in the dvglut mutant (K-S test, p < 0.05).
Because vesicles are abundant, it is likely that many are empty. We previously demonstrated that overexpressing DVGLUT increases synaptic vesicle size (Daniels et al., 2004). Based on this observation, we concluded that either vesicle filling or levels of DVGLUT protein regulate vesicle size. If this hypothesis were correct, we would predict synaptic vesicles to be smaller in the dvglut mutants. We measured the size of synaptic vesicles and found that vesicles are indeed smaller in dvglut1/Df(2L)dvglut2 synapses than in controls. Average vesicle outer diameter is 39.1 ± 0.1 nm in the wild-type and 36.3 ± 0.1 nm in the mutant (p < 0.05, Kolmogorov-Smirnov test, n = 1840 for each genotype, Figures 4D and 4E). This corresponds to a 32% decrease in average vesicle volume. Because of the broad distribution of vesicle diameters even in wild-type, we are unable to resolve individual peaks for empty and full vesicles in the mutant. However, because the physiology demonstrates that full vesicles are present at the synapse, the actual empty vesicle size must be smaller than the average we observed in the mutant. Synaptic vesicles may be smaller either due to the absence of glutamate in the vesicle lumen or the lack of transporter in the vesicle membrane. Regardless of the mechanism, our results demonstrate that DVGLUT levels can affect synaptic vesicle size.
Additional ultrastructural analysis revealed that average active zone length, percent of bouton circumference occupied by active zones (defined as sites of tight apposition of electron-dense membranes), and number of T-bars per active zone are unchanged compared to controls (data not shown). In addition, the number of synaptic boutons is not decreased in the mutant (data not shown). These results demonstrate that dvglut mutant synapses are grossly normal and that defects in active zone size and number do not explain the dramatic decrease in mEJP frequency.
Vesicles Can Be Efficiently Released at dvglut Mutant Synapses
An alternate explanation for the decrease in mEJP frequency in dvglut mutants is that DVGLUT directly influences the ability of vesicles to fuse. All vesicles could be full but not efficiently released, or partially full vesicles may exist but be selectively unable to fuse. If this were the case, then the mutant would show defects in vesicle cycling as assayed by the fluorescent dye FM 1-43. No such defect was observed (Figures 5A1 and 5A2 and 5B1 and B2). The number of vesicles labeled and subsequently destained (the cycling pool size) was not significantly different between control and mutant larvae using either high K+ depolarization (Figure 5C, wt: 100% ± 21%, n = 12; dvglut1/Df: 91% ± 23%, n = 9) or nerve-evoked stimulation (Figure 5D, wt: 100% ± 23%, n = 9; dvglut1/Df: 95% ± 19%, n = 9). This result is consistent with optical measurement of synaptic vesicle cycling performed in cultured hippocampal neurons from mice lacking VGLUT1 in which vesicles cycle normally despite lower levels of transporter (Wojcik et al., 2004). These data demonstrate that neither the fusion-competence of vesicles nor their availability limits the frequency of spontaneous events at this synapse. Optical measurements of nerve-evoked release contrast with electrical measurements of nerve-evoked release (Figures 5A3 and B3). The average nerve-evoked EJP amplitude is 27.9 ± 1.5 mV in wild-type (n = 12) compared to 10.4 ± 1.1 mV in dvglut1/Df larvae (Figure 5E, p ≪ 0.01). Because the EJP amplitude reflects the fusion of glutamate-filled vesicles while the optical measurements reflect the fusion of all vesicles, the difference indicates the release of empty vesicles. These results are consistent with the decrease in mEJP frequency observed in the dvglut mutants.
Figure 5. Cycling Vesicle Pool Is Unchanged in dvglut Hypomorphs, Despite Decreased EJP Amplitude.
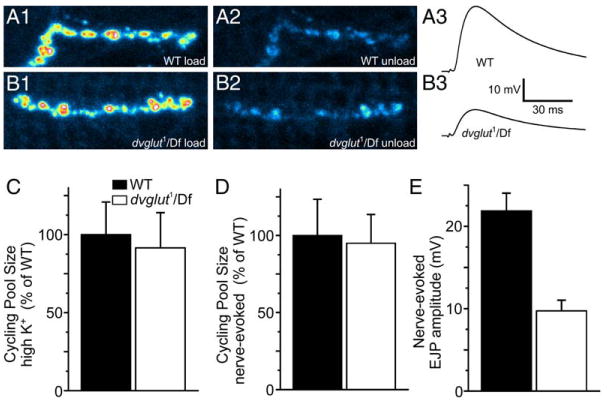
Pseudocolor image of wild-type (A1) and mutant (B1) synaptic boutons loaded with FM 1-43. (A2) and (B2) show the same region after unloading by nerve-evoked stimulation in wild-type and mutants, respectively. Intracellular recordings of nerve-evoked potentials are decreased in dvglut mutants. Sample traces from wild-type (A3) and mutant (B3) cells. (C) There is no difference in the optically determined cycling pool size after loading in 90 mM K+ saline and subsequently unloading in 90 mM K+ saline in wild-type (WT) and mutant (dvglut1/Df) larvae. (D) There is no difference in the optically determined cycling pool size after loading in 90 mM K+ saline and unloading by segmental nerve stimulation in normal saline in wild-type (WT) and mutant (dvglut1/Df) larvae. (E) Average nerve-evoked EJP amplitude is decreased in dvglut mutants (dvglut1/Df) compared to wild-type (WT) larvae. Error bars indicate SEM.
Because alternate explanations are not consistent with our data, we conclude that in dvglut mutants, vesicles are released at a similar rate as in wild-type, but a majority of these vesicles do not have functional glutamate transport and are therefore empty and electrically silent. If the reduction in DVGLUT levels results in many vesicles without any transporter, then most of the vesicles with transporter will likely have just a single functional unit. Because mEJP amplitude remains unchanged in the mutant, we conclude that a single unit of transporter is sufficient to fill a vesicle with normal levels of glutamate.
Conclusions
A vesicle contains an estimated 20 to 40 unique proteins and only ∼800 protein molecules overall, so there is a modest number of copies of each protein on a vesicle (Fernández-Chacón and Südhof, 1999). Here we have demonstrated that a single functional unit of vesicular glutamate transporter is capable of filling a vesicle to normal levels. While one transporter is sufficient, how many transporters are usually present on a vesicle? Although we cannot provide a definitive answer, the data presented here in conjunction with our previous studies of DVGLUT overexpression suggest that one is a plausible number. When we decrease DVGLUT levels, there is no change in quantal size, and empty vesicles appear. When we increase DVGLUT, levels there is an increase in quantal size (Daniels et al., 2004). These results can be reconciled by the hypothesis that there is normally a single functional transporter on a vesicle. Increasing levels of VGLUT can add a second transporter and increase quantal size, while decreasing VGLUT levels results in a mix of vesicles that either retain the normal one transporter or have no transporter and are empty. These results have important implications for vesicle biogenesis and recycling. Mechanisms that sort transporters to synaptic vesicles must act with high fidelity to ensure that empty vesicles are rare and yet still be flexible enough to accommodate more transporters when they are overexpressed. Future studies will determine how VGLUT is trafficked to vesicles, how its copy number is controlled, and whether regulation of transporter number is a mechanism for modifying synaptic strength.
Experimental Procedures
Fly Stocks
All crosses were kept on standard Drosophila media at 25°C. dvglut1 and Df(2L)dvglut2 flies were kept as stocks over In(2LR)Gla. The near-precise excision of KG06301 used as a control line was indistinguishable from wild-type in every experiment performed; some electrophysiology results using CS flies outcrossed to w- males were therefore combined with those from the near-precise excision.
Creation of dvglut Mutants
The p-element KG06301 is inserted approximately 1.5 kb upstream of the first exon of dvglut and was excised by introduction of Δ2,3 recombinase. Individual w- males were used to establish lines, and excisions were characterized molecularly by PCR. The Df(2L)dvglut2 mutation completely removes the dvglut coding region and disrupts the adjacent gene CG18641 (a pancreatic lipase homolog), resulting in embryonic lethality. The dvglut1 allele is a viable mutation that deletes parts of the promoter and the first three exons of the dvglut gene, including an exon containing a predicted start methionine. A second potential start methionine is retained in the fourth exon and would produce a protein 21 amino acids shorted than previously predicted for DVGLUT (Daniels et al., 2004). This shorter protein would include all conserved regions and predicted transmembrane domains of DVGLUT and must be functional because spontaneous mEJCs are abolished in the DVGLUT null.
Rescue Construct
The UAS-dvglut cDNA used for rescue was reported previously (Daniels et al., 2004), as was the BG380-Gal4 line (generously provided by Dr. V. Budnik).
Electrophysiology
As described previously (Daniels et al., 2004). In brief, mEJPs and evoked release were recorded from muscle 6 in HL-3 saline containing 0.47 mM Ca2+ at room temperature. Average resting potentials were (in mV) −66.8 ± 1.0 for wild-type, −67.4 ± 1.8 for Df(2L)dvglut2/+, −73.4 ± 3.2 for dvglut1/dvglut1, −70.9 ± 2.8 for dvglut1/Df(2L)dvglut2, and 68.2 ± 1.9 for BG380-Gal4;dvglut1/Df(2L)dvglut2;UAS-DVGLUT/+, none of which are significantly different from wild-type. Average input resistances were (in MOhms) 8.2 ± 0.4 for wild-type, 9.5 ± 0.9 for Df(2L)dvglut2/+, 7.8 ± 0.5 for dvglut1/dvglut1, 8.4 ± 0.6 for dvglut1/Df(2L)dvglut2, and 7.5 ± 0.6 for BG380-Gal4;dvglut1/Df(2L)dvglut2;UAS-DVGLUT/+, which are also not significantly different from wild-type. Seventy consecutive spontaneous events were measured for each cell by using MiniAnalysis (Synaptosoft, Decatur, GA), and the mean amplitude and frequency for each cell was averaged for each genotype. Glutamate iontophoresis was performed using two-electrode voltage clamp as described previously (Marrus et al., 2004) and an iontophoresis pipette with an access resistance of 6–7 Mohm. The iontophoresis pipette was positioned adjacent to synaptic boutons on muscle 6 with the use of DIC optics, and a 10 ms negative current pulse was used to apply the glutamate (100 mM, dissolved in HL-3, pH ∼9) at 0.5 Hz. Muscles were clamped at −70 mV, and records were rejected if the clamp was not stable or if voltage responses during glutamate application were >10 mV.
Whole-cell patch-clamp electrophysiology on Drosophila embryos was performed as previously described on ventral longitudinal muscle 6 (Featherstone et al., 2000). Briefly, muscle 6 was whole-cell patch clamped −60 mV) in standard Drosophila embryonic saline with the use of standard patch-clamp techniques. To assay the glutamate-gated currents, 1 mM glutamate was pressure ejected (100 ms pulse) from a small-tipped (∼5 μm opening) pipette directly onto the NMJ. Data were acquired and subsequently analyzed using a MULTICLAMP 700A amplifier and PClamp 9 (Axon Instruments, Union City, CA).
Immunohistochemistry
As described previously (Daniels et al., 2004). After fixing, samples were blocked in 5% normal goat serum in PBS + 0.1% Triton-X (PBX) for 1 hr followed by 1 hr incubation with primary antibodies coupled to fluorescent probes (1:1000 anti-DVGLUT-Alexa-488, 1:1000 anti-HRP-Cy3; Jackson Immunoresearch, West Grove, PA). The average intensity in each color channel of a maximum projection volume rendering of z-stacks though the synapse was measured with the use of MetaMorph software (Molecular Devices, Downingtown, PA). Comparisons were made between larvae stained in the same tubes as controls and imaged with identical settings. Boutons were identified and counted using a combination of anti-DVGLUT and anti-HRP immunoreactivity. Glutamate receptor clustering opposite active zones was assayed by staining with nc82 (gift from Erich Buchner) and DGluRIII and looking for close apposition (Marrus et al., 2004; Marrus and DiAntonio, 2004).
Electron Microscopy
As described previously (Daniels et al., 2004). Pictures were taken on a Hitashi H-7500 TEM at 50 kx and 100 kx. Two wild-type larvae (precise dvglut excision) and two mutant larvae (dvglut1/Df(2L)dvglut2) were sectioned. A total of 95 wild-type and 97 mutant active zones from a uniquely identified motoneuron (MN6/7 Ib) were analyzed. Data were collected from multiple MN6/7 Ib neurons for each genotype. Vesicles were measured blind to genotype, and vesicle populations were compared using the Kolmogorov-Smirnov test. For quantification of the number of vesicles near the active zone, a 600 nm × 300 nm grid was superimposed onto and centered over active zones in MetaMorph. 600 nm was chosen because it was the average active zone length in both control and mutant sections. Vesicles within 100, 200, and 300 nm of the presynaptic membrane were counted and then averaged across each genotype.
FM 1-43 Cycling
Synapses were loaded using 10 μM FM 1-43 (Invitrogen, Carlsbad, CA) dissolved in 90 mM K+ Jan's saline containing (in mM) 45 NaCl, 90 KCl, 2 MgCl2, 36 sucrose, 5 HEPES, and 2 CaCl2 (pH 7.3) (Jan and Jan, 1976). After loading for 5 min, larvae were washed by perfusion with HL-3 saline containing 0 mM Ca2+ and 0.5 mM EGTA for 12 min (∼50 mL) and then muscle 4 in segments A3 and A4 were imaged on a Nikon C1 confocal using a 40× water-immersion lens. Terminals were subsequently unloaded using either 90 mM K+ Jan's saline or by stimulation at 20 Hz in 1.5 mM Ca2+ HL-3 for 7.5 min. After unloading, larvae were washed again for 12 min and imaged using identical acquisition settings. There was no difference in total dye loaded, unloaded, or the difference between loaded and unloaded (cycling pool size) conditions between genotypes (data not shown and Figures 5C and 5D). For image analysis, confocal z-stacks were flattened and intensity measured with MetaMorph software.
Statistics
Results are presented at average ± SEM. Significance between groups was determined by a p value of less than 0.05. Statistical tests were performed with Origin 7.0 software (Origin Lab, North-hampton, MA) or Clampfit 9.2 (Molecular Devices, Sunnyvale, CA). Populations were compared using the Kolmogorov-Smirnov test, groups of means were compared using one-way ANOVA, and comparisons between two means were performed using Student's t test.
Supplementary Data
The Supplemental Data for this article can be found online at http://www.neuron.org/cgi/content/full/49/1/11/DC1/.
Acknowledgments
This work was supported by grants from the NIH (NS051453 and NS043171), Keck, and McKnight Foundations to A.D., and an NIH grant (NS045628) (to D.E.F.). R.W.D. was supported by NIH training grant T32 GM08151. We thank the Inner Ear Research Core Center at Washington University for use of their electron microscopy facility, Jaime Dant for help with EM sample preparation, Xiaolu Sun for excellent technical assistance, and members of the laboratory for helpful discussions. Present address for M.V.G. is: Department of Neurobiology, Harvard Medical School, 220 Longwood Ave., Boston, MA 02115.
References
- Boulland JL, Qureshi T, Seal RP, Rafiki A, Gunderson V, Bergersen LH, Fremeau RT, Jr, Edwards RH, Storm-Mathisen J, Chaudhry FA. Expression of the vesicular glutamate transporters during development indicates the widespread corelease of multiple neurotransmitters. J Comp Neurol. 2004;480:264–280. doi: 10.1002/cne.20354. [DOI] [PubMed] [Google Scholar]
- Bruns D, Riedel D, Klingauf J, Jahn R. Quantal release of serotonin. Neuron. 2000;28:205–220. doi: 10.1016/s0896-6273(00)00097-0. [DOI] [PubMed] [Google Scholar]
- Colliver TL, Pyott SJ, Achalabun M, Ewing AG. VMAT-mediated changes in quantal size and vesicular volume. J Neurosci. 2000;20:5276–5282. doi: 10.1523/JNEUROSCI.20-14-05276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RW, Collins CA, Gelfand MV, Dant J, Brooks ES, Krantz DE, DiAntonio A. Increased expression of the Drosophila vesicular glutamate transporter leads to excess glutamate release and a compensatory decrease in quantal content. J Neurosci. 2004;24:10466–10474. doi: 10.1523/JNEUROSCI.3001-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone DE, Rushton EM, Hilderbrand-Chae M, Phillips AM, Jackson FR, Broadie K. Presynaptic glutamic acid decarboxylase is required for induction of the postsynaptic receptor field at a glutamatergic synapse. Neuron. 2000;27:71–84. doi: 10.1016/s0896-6273(00)00010-6. [DOI] [PubMed] [Google Scholar]
- Fernández-Chacón R, Südhof TC. Genetics of synaptic vesicle function: toward the complete functional anatomy of an organelle. Annu Rev Physiol. 1999;61:753–776. doi: 10.1146/annurev.physiol.61.1.753. [DOI] [PubMed] [Google Scholar]
- Franks KM, Stevens CF, Sejnowski TJ. Independent sources of quantal variability at single glutamatergic synapses. J Neurosci. 2003;23:3186–3195. doi: 10.1523/JNEUROSCI.23-08-03186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004;304:1815–1819. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- Gong LW, Hafez I, de Toledo GA, Lindau M. Secretory vesicles membrane area is regulated in tandem with quantal size in chromaffin cells. J Neurosci. 2003;23:7917–7921. doi: 10.1523/JNEUROSCI.23-21-07917.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Properties of the larval neuromuscular junction in Drosophila melanogaster. J Physiol. 1976;262:189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunanithi S, Marin L, Wong K, Atwood HL. Quantal size and variation determined by vesicle size in normal and mutant Drosophila glutamatergic synapses. J Neurosci. 2002;22:10267–10276. doi: 10.1523/JNEUROSCI.22-23-10267.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrus SB, DiAntonio A. Preferential localization of glutamate receptors opposite sites of high presynaptic release. Curr Biol. 2004;14:924–931. doi: 10.1016/j.cub.2004.05.047. [DOI] [PubMed] [Google Scholar]
- Marrus SB, Portman SL, Allen MJ, Moffat KG, DiAntonio A. Differential localization of glutamate receptor subunits at the Drosophila neuromuscular junction. J Neurosci. 2004;24:1406–1415. doi: 10.1523/JNEUROSCI.1575-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron. 1997;19:1237–1248. doi: 10.1016/s0896-6273(00)80415-8. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Larsen KE, Krantz DE, Liu YJ, Haycock JW, Setlik W, Gershon MD, Edwards RH, Sulzer D. Synaptic vesicle transporter expression regulates vesicle phenotype and quantal size. J Neurosci. 2000;20:7297–7306. doi: 10.1523/JNEUROSCI.20-19-07297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HJ, Ming GL, Fon E, Bellocchio E, Edwards RH, Poo MM. Expression of a putative vesicular acetylcholine transporter facilitates quantal transmitter packaging. Neuron. 1997;18:815–826. doi: 10.1016/s0896-6273(00)80320-7. [DOI] [PubMed] [Google Scholar]
- Wilson NR, Kang J, Hueske EV, Leung T, Varoqui H, Murnick JG, Erickson JD, Liu G. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci. 2005;25:6221–6234. doi: 10.1523/JNEUROSCI.3003-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci USA. 2004;101:7158–7163. doi: 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Supplemental Data for this article can be found online at http://www.neuron.org/cgi/content/full/49/1/11/DC1/.


