Abstract
Glass has become the standard substrate for the preparation of DNA arrays. Typically, glass is modified using silane chemistries to provide an appropriate functional group for nucleic acid synthesis or oligonucleotide immobilization. We have found substantial issues with the stability of these surfaces as manifested in the unwanted release of oligomers from the surface when incubated in aqueous buffers at moderate temperatures. To address this issue, we have explored the use of carbon-based substrates. Here, we demonstrate in situ synthesis of oligonucleotide probes on carbon-based substrates using light-directed photolithographic phosphoramidite chemistry and evaluate the stabilities of the resultant DNA arrays compared to those fabricated on silanized glass slides. DNA arrays on carbon-based substrates are substantially more stable than arrays prepared on glass. This superior stability enables the use of high-density DNA arrays for applications involving high temperatures, basic conditions, or where serial hybridization and dehybridization is desired.
INTRODUCTION
DNA arrays have become a vital component in genomic research for high-throughput gene expression analysis (1,2), mutation detection (3,4), gene discovery and genetic mapping studies (5) and protein–DNA interaction analysis (6–9).
Several methods for fabricating DNA arrays have been described (10). These fall into two groups: in situ synthesis (11–16), or deposition and immobilization of pre-synthesized DNA sequences (17–26). Immobilization of pre-synthesized oligonucleotides offers the flexibility needed to quickly make low and medium-density arrays containing anywhere from several dozen to several hundred features per array. Although the technologies and skills needed to prepare low density arrays are readily available to a large number of researchers, there is an increasing need for high-throughput array-based analyses with high density arrays (>50 000 features per square centimeter). Both photolithographic and ink-jet array synthesis methods prepare arrays in a combinatorial manner, one nucleotide at a time. This permits the end user to create a high-density array with a greater diversity of sequences in less time and at a lower cost than spotting pre-synthesized oligonucleotides.
Glass, with its low intrinsic fluorescence, non-porosity and ease of modification using silane chemistries has become the standard surface for fabricating DNA arrays (23). A disadvantage of glass substrates, however, is the intrinsic hydrolytic instability of the siloxyl linkage employed in glass chemical modification (27,28). Glass substrates are limited to mild pH conditions and moderate-to-low temperatures, limiting the applications of arrays fabricated on this material. While this may not affect common applications such as gene expression or SNP detection, which are formulated to work with current glass-based platforms, new substrates exhibiting greater stabilities under a broader range of conditions would enable new applications such as those employing extended high temperature incubations or harsh chemical conditions.
Several non-glass substrates including silicon (29–33), gold (34,35) and polymeric materials (36,37), have been reported for deposition of pre-synthesized oligonucleotides. While these substrates can offer added benefits such as increased conductivity or flexibility over their glass counterparts, they have yet to become a widely used alternative.
The use of carbon-based substrates for the fabrication of low-density hand-spotted DNA arrays (38–40) has also been described. DNA arrays fabricated on such carbon-based surfaces are extremely robust due to both the intrinsic chemical stability of the substrate and to the carbon–carbon bonds employed for surface attachment; arrays prepared on carbon-based substrates exhibit greater stability than arrays prepared on either silicon or gold substrates (38,40).
In this study, we show the utility of carbon substrates for the in situ light-directed synthesis of DNA arrays. The stability of the resulting DNA arrays is dramatically increased compared to DNA arrays prepared on glass. This increased stability expands the utility of high-density arrays by enabling their use under higher temperature conditions and with extended reaction times and greater extremes of pH, as well as permitting their regeneration and reuse.
MATERIALS AND METHODS
All reagents were purchased from Sigma Aldrich and used without further purification unless otherwise stated.
Silanization of glass slides
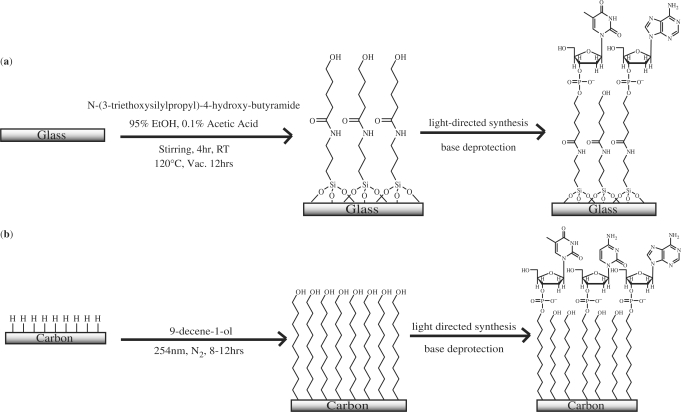
A 0.1% acetic acid in 95% ethanol stock solution was prepared. ArrayIt SMC Superclean glass slides (Telechem International, Inc., Sunnyvale, CA, USA) were stirred in 2% (v/v) N-(3-triethoxysilylpropyl)-4-hydroxy-butyramide (Gelest, Inc., Morrisville, PA, USA) in stock solution for 4 h at room temperature. The slides were then rinsed by stirring in fresh stock solution for 15 min. After being rinsed three times in diethyl ether, slides were transferred to a pre-heated (120°C) oven for a minimum of 2 h, after which time they were cured under vacuum overnight. Slides were stored desiccated until ready for use (Scheme 1a).
Scheme 1.
(a) Clean glass slides are functionalized using a 2% silane solution, rinsed and heat cured under vacuum. Functionalization of glass slides results in siloxane bonds between the linker and the surface. (b) Clean carbon substrates are functionalized with neat alcohol-alkene using 254 nm light, rinsed and stored until use. Photofunctionalization of carbon substrates results in a carbon–carbon covalent bond between the surface and the linker. Once functionalized, both glass and carbon substrates are subjected to identical light-directed DNA synthesis reactions. Crowding effects result in <100% coupling efficiency of amidites to surface hydroxyl sites (see Discussion section).
Glassy carbon material
Custom sized (2.5 cm × 4.0 cm) glassy carbon plates (Sigradur K) were purchased from Hochtemperatur-Werkstoffe GmbH, Thierhaupten, Germany. Glassy (vitreous) carbon is a hard, ceramic-like material produced by the pyrolysis of carbon-rich polymers. The resulting material is composed of graphitic ribbons of pure carbon with fullerene-like microstructures (41,42). Like graphitic carbon, it is primarily composed of sp2 carbons, although the density of glassy carbon is greater than that of its graphitic counterpart. Detailed information on this material can be found in the cited references as well as in several books on the subject of carbon materials (43,44).
Preparation of nanocrystalline diamond thin films
Nanocrystalline diamond samples were a gracious gift from Dr James Butler at Naval Research Laboratories. Thin films of 0.50–0.59 μm CVD diamond were deposited on n-type Si <100> at 850°C using 6.4 Torr methane at 2.50 Hz, 1000 W (45).
Hydrogen termination of glassy carbon and diamond substrates
Glassy carbon plates and diamond substrates were chemically cleaned prior to hydrogen termination. Each substrate was sonicated for 5 min in CHCl3 and cleaned with a series of acid treatments at 60°C for a minimum of 1 h first in 4 : 3 : 1 water : nitric acid : hydrochloric acid and then in 3 : 2 sulfuric acid : nitric acid. (Caution: these acid washes and associated fumes are highly caustic, proper protective equipment and appropriate ventilation must be employed.) In between acid treatments, the substrates were rinsed with deionized (DI) H2O. Prior to functionalization, glassy carbon and diamond surfaces were heated to ∼900°C under vacuum and treated with a 13.56 MHz inductively coupled H-plasma for 20 min at 50 Torr to generate a hydrogen terminated surface (40) (Scheme 1b).
Generation of free alcohol groups on carbon substrates
Following hydrogen termination, the carbon surfaces were photochemically functionalized by placing 30 µl of 9-decene-1-ol directly onto the surface and covering with a clean quartz coverslip. The surfaces were irradiated under N2 purge with a low-pressure mercury vapor quartz grid lamp (λ = 254 nm) for 8–12 h. After the photoreaction, the surfaces were briefly rinsed with ethanol, DI H2O and sonicated 2 × 5 min in CHCl3 before being immersed in concentrated ammonium hydroxide for 2 min. The surfaces are then stored desiccated until ready for use (Scheme 1b).
In situ oligonucleotide synthesis
Light-directed photolithographic synthesis was performed with a digital micromirror-based Biological Exposure and Synthesis System (BESS) connected to a Perseptive Biosystems Expedite Nucleic Acid Synthesis System (Framingham, MA, USA) as described previously (12,16,46). Oligonucleotide synthesis was carried out using a modified DNA synthesis procedure, where the removal of the photolabile NPPOC (3′-nitrophenylpropyloxycarbonyl)-protecting group was achieved by irradiation with 3.95 J/cm2 of 365 nm light from a 200 W Hg/Xe arc lamp (Newport, Stratford, CT, USA). The optimum dose for removal of the NPPOC protecting group was determined empirically (data not shown). The UV-irradiation time was determined by measuring the lamp power (in mW/cm2) at 365 nm and adjusting the exposure time to ensure a dose of 3.95 J/cm2.
Oligonucleotide synthesis reagents [DCI activator, acetonitrile (dry wash and amidite diluent) and oxidizer solution] were purchased from Sigma-Proligo; exposure solvent was purchased from Nimblegen Systems Inc. (Madison, WI, USA). All anhydrous reagents were kept over molecular sieves (Trap Packs, Aldrich).
All NPPOC-protected phosphoramidites [5′-NPPOC-dAdenosine(tac) 3′-β-cyanoethylphosphoramidite (dA), 5′-NPPOC-dThymidine 3′-β-Cyanoethylphosphoramidite (dT), 5′-NPPOC-dCytidine(ib) 3′-β-cyanoethylphosphoramidite (dC), 5′-NPPOC-dGuanosine(ipac) 3′-β-cyanoethylphosphoramidite (dG)] were manufactured by Proligo Biochemie GmbH (Hamburg, Germany) and purchased from Nimblegen Systems Inc.; NPPOC-phosphoramidites were diluted (1 g in 60 mL) with dry acetonitrile (amidite diluent).
Addition of a NPPOC-protected phosphoramidite proceeds as follows: (i) after condensation of the previous NPPOC-protected base to the growing DNA strand, the synthesis flow cell (volume ∼100 μl) is flushed with 500 μl of exposure solvent; (ii) a digital image (mask) representing the locations for the next base addition illuminates the surface with 3.95 J/cm2. During irradiation of the array, the exposure solvent is constantly flowed through the flow cell at a rate of 100 μl/0.5J/cm2 to maintain sufficiently basic conditions (47) to drive the photo-catalysed elimination reaction. Following irradiation, (iii) the array is washed with acetonitrile (∼400 μl) to remove residual exposure solvent, dry wash (∼300 μl) to remove trace water, and activator solution (∼100 μl). Coupling of the next base is achieved by filling the flow cell with a 1:1 solution of the desired phosphoramidite and activator. All 5′-NPPOC-protected amidites undergo a single 40s coupling step, whereas the Cy3 dye phosphoramidite [0.03 M, Glen Research, Sterling, VA, USA)] is subjected to two 300s coupling steps. After amidite coupling, the array is washed with acetonitrile (∼100 μl) and either oxidized by flushing the cell with oxidizer solution (THF, pyridine, iodine, water; ∼500 μl) or subjected to the next phosphoramidite addition. The non-acidic conditions of deprotection (47) allow for oxidation of the backbone phosphite groups only after every fourth coupling step and at the end of the synthesis, rather than at every coupling step. We have found that this modification does not negatively impact sequence quality (Richmond, K.E., Rodesch, M.J., Kaysen, J. and Cerrina, F., unpublished data) and reduces both the synthesis time and the amount of waste generated.
After synthesis is completed, the nucleoside bases are deprotected in 1:1 ethylene diamine: absolute ethanol solution at room temperature for 2–4 h.
Table 1 contains the probe sequences synthesized on all low density arrays. The 3′ end of sequences 2–4 were separated from the surface by a 10-thymidine spacer, providing a distance of ∼30 Å from the surface; this has been shown to increase hybridization efficiency (26,48). Probe 1 was terminally labeled with Cy3, while sequences complementary to probes 2–4 in Table 1 were modified at the 3′ end with a fluorescein label. For hybridization density determination, sequence 4 was used; all other data presented is only for sequence 1, in order to simplify the analysis.
Table 1.
Probe sequences used
| Probe sequence | Sequence: 3′ → 5′ |
|---|---|
| 1 | TTTTTTTTTT-Cy3 |
| 2 | (T)10TTATTGAAACGTTGTCACC |
| 3 | (T)10GTTATTGAAACGTTGTCACT |
| 4 | (T)10GGCTACTGGACGTTCTCA |
DNA hybridization and washing
Complementary oligonucleotides for probes 2–4 were purchased from IDT (Coralville, IA, USA) and the University of Wisconsin – Madison Biotechnology Center (Madison, WI, USA). All arrays were hybridized by placing 30 μl of the fluorescently-tagged complement (1 μM, 1 × SSPE [10 mM NaH2PO4, 0.15 M NaCl, 1mM EDTA, pH = 7.4], 45°C) on the surface, covering with a coverslip, and incubating for 1 hr in a humid chamber. Nonspecifically bound DNA was removed by incubating the surface in 1 × SSPE for 15 min at 37°C. Dehybridization was achieved by incubating the surfaces in 8 M urea (RT, 20 min). Unless otherwise noted, fluorescence scans were taken on a Genomic Solutions GeneTac UC 4 × 4 scanner (Ann Arbor, MI, USA). Arrays hybridized with fluorescein-labeled complementary DNA strands were scanned in 1 × SSPE.
Determination of hybridization density
Hybridization density was determined using a wash-off method as described previously (49). Arrays consisting of a single sequence (Sequence 4, Table 1) were synthesized over the entire (1 cm × 1.3 cm) synthesis area. After hybridization and washing, the array was transferred to 20 ml of wash-off buffer (40 mM KCl/132 mM KOH) in a 50 ml falcon tube and shaken vigorously for 15–20 min. Calibration solutions containing fluorescein-labeled target DNA (1 × 10−11 to 1 × 10−8 M) were prepared in 42 mM KCl, 132 mM KOH. Using a fluorescence plate reader (BIOTEK, Flx 800, 200 μl per well), the fluorescence from the calibration solutions and unknown samples were measured and the density of hybridized DNA was calculated.
Thermal stability determination
In a stirring isothermal water bath, 40 ml of 2xSSPE with 0.2% (v/v) SDS was pre-warmed to either 45°C or 60°C in 50 ml falcon tubes fitted with stir vanes. After an initial scan, arrays were incubated at one of the indicated temperatures. At pre-determined time points (0, 0.5, 1–4 h in 1h intervals, and 4–24 h in 2 h intervals) the arrays were removed from the solution, rinsed with RT 1xSSPE and hybridized before being scanned and returned to the warm 2 × SSPE–SDS solution to continue incubating.
Hybridization stability determination
Arrays were subjected to 20 hybridization cycles: hybridized as described earlier, scanned, incubated in 8 M urea at RT for 20 min, rinsed with water, 1xSSPE and another hybridization step was performed. Complete dehybridization was verified by fluorescence imaging.
Base stability determination
Arrays were incubated in a solution of NH4OH (15%, 55°C). (Caution: produces noxious fumes, ensure appropriate ventilation and open carefully as containers pressurize under elevated temperatures.) Fluorescence scans of the arrays were taken at pre-determined time points (0, 0.5, 1–12 h) by rinsing the array with DI H2O, 1xSSPE, and hybridizing as described earlier.
PCR stability determination
A master mix consisting of 0.05 U/ml PicoMaxx enzyme, 0.1mM each dNTP, in 1xPicoMaxx buffer (all Stratagene), augmented with 0.1% BSA (Promega) and 0.1% Tween-20 (Pierce) was made. Arrays were hybridized and fluorescence images captured before being fitted with a hybridization chamber (Grace Biolabs) and 250 µl of pre-warmed solution. The arrays were placed in a thermocycler (MJ Research) and subjected to a PCR protocol that has been previously reported (50). Briefly, the array was initially heated to 94°C for 9 min followed by 50 cycles of 94°C for 45 s, 65°C for 3 min and 72°C for 4 min. Following this treatment, the arrays were rinsed with water, hybridized and scanned. After scanning, the process was repeated.
Comparison of array image quality
A high-density array using a one-in-four design was employed to evaluate high-density image quality. This consists of a single pixel-sized feature (16 μm × 16 μm) containing single probe species separated by a single pixel on all sides (one-in-four). Probe sequence (3′-TTTTTCTGGTCCCACCAAGTACTACTACTG) was synthesized as described earlier with UV-deprotection dose varying from 0.7 to 12.1 J/cm2. Following synthesis and nucleic acid deprotection, the array was hybridized with a Cy3 tagged complementary DNA target for 1 h and imaged using a Nikon E800 fluorescent microscope fitted with a cooled CCD imaging system and imaging software by Metamorph (Universal Imaging Corporation).
RESULTS
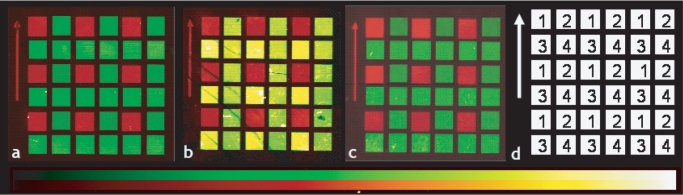
Two-color fluorescence images of identical array designs on three different substrates are shown in Figure 1. Sequence 1 is terminally labeled with Cy3 and is presented in false-color red, sequences 2–4 are hybridized with their respective fluorescein-labeled perfect match complementary sequence (false-color green). While all three substrates exhibit similar Cy3 intensities and comparably low background fluorescence, all sequences hybridized on glassy carbon (b) exhibit a greater fluorescence than those hybridized on either glass or diamond. This is likely an artifact due to optical effects from the scanner used to image the array—in a similar comparison (see Array image quality section) using a fluorescence microscope, diamond showed greater fluorescence while glass and glassy carbon were comparable. This is consistent with the hybridization density results given subsequently.
Figure 1.
Two color fluorescence images of arrays containing nine fluorescently tagged Cy-3 control features and 27 hybridizable features (nine features for each of three DNA sequences, Table 1) on glass (a), glassy carbon (b) and diamond (c). A schematic for the array design is shown in panel (d). Each feature measures 580 μm × 580 μm; Sequence 1 is terminally labeled with Cy3 and is presented in false color red, sequences 2–4 are hybridized with their respective perfect-match fluorescein-tagged complementary sequences and are shown in false-color green. The false-color intensity scales are displayed below the array images.
Hybridization density
The hybridization density of a surface is a measure of the number of probe oligomers that are accessible to bind complementary DNA. Wash-off studies in which DNA is first hybridized to and then eluted from the surface for measurement in solution are a preferred method of analysis as substrate-specific and any quenching effects are minimized or eliminated. The hybridization densities determined for all three surfaces are shown in Table 2. The density of fluorescently labeled complementary oligonucleotides hybridized to the surface was between 2 × 1012 (4 pmol/cm2) and 4 × 1012 molecules/cm2 (7 pmol/cm2). These are 12–52% lower than densities reported for oligonucleotides immobilized on gold (5 × 1012 oligonucleotides/cm2, 8 pmol/cm2) (49) and are 14–26% below the theoretical maximum oligonucleotide density of 1.7 × 1013 oligonucleotides/cm2 (17 pmol/cm2) as calculated when the dsDNA helices are assumed to be tightly packed cylinders with diameters of 2 nm each.
Table 2.
Number of probe molecules accessible to hybridization with their perfect match DNA, complement on various surfaces as measured by the number of target oligomers that can be hybridized and collected
| Substrate | Glass | Glassy carbon | Diamond |
|---|---|---|---|
| Density (×1012) molecules/cm2 | 2.69 | 2.38 | 4.40 |
| Percent deviation | ± 0.86% | ± 1.74% | ± 1.64% |
Thermal stability
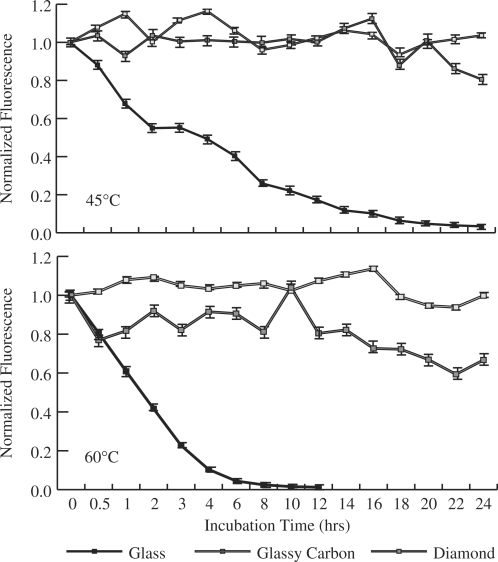
To increase the specificity of hybridization it is often desirable to incubate arrays at higher temperatures. Such temperatures increase the rate of hydrolysis and lead to problems with array stability. To investigate this issue, the surface stability of arrays prepared on glassy carbon, diamond and silanized glass was compared at both 45°C and 60°C (Figure 2).
Figure 2.
Change in fluorescence signal from arrays prepared on glass (solid square), glassy carbon (open square) and diamond (gray shaded square) upon incubation in 2 × SSPE w/0.2% SDS at 45°C (top) and 60°C (bottom). No fluorescence was detectable on glass following 12 h of incubation at 60°C.
Under all conditions, glassy carbon and diamond exhibit greater stability than glass. After 24 h of incubation at either 60°C or 45°C, glassy carbon retains 99 ± 1% of the initial fluorescence signal. Arrays prepared on diamond retain 80 ± 5% of the initial fluorescence signal at 45°C and 67 ± 5% at 60°C after 24 h of incubation. Following 20 h of incubation at 45°C glass retains <5% of initial fluorescence and a similar loss is observed after only 6 h at 60°C.
Base stability
A similar trend is seen when the substrates are immersed in 15% ammonium hydroxide at 55°C. After 12 h of incubation, the glassy carbon arrays retain 100 ± 3% of their initial signal and diamond-based arrays retain 78 ± 4% of the initial signal. Fluorescence signals from arrays on glass substrates have <5% of their initial signal after 1 h and exhibit no detectable signal after 4 h of incubation (Figure 3).
Figure 3.
Loss of signal from arrays prepared on glass (solid square), glassy carbon (open square) and diamond (gray shaded square) resulting from extended incubation in 15% ammonium hydroxide at 55°C. Following 4 h of incubation, no fluorescence was detected on the glass.
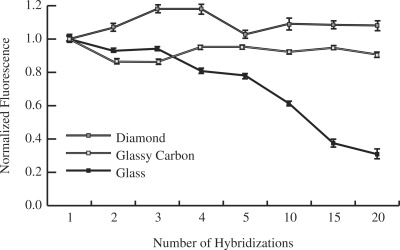
Stabilities to multiple hybridization cycles
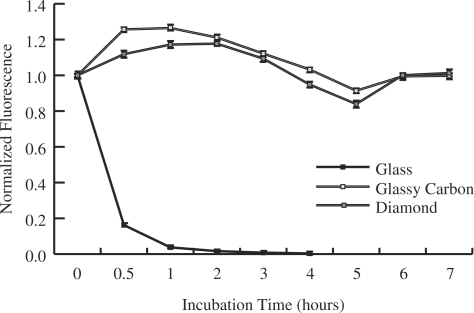
Incubation in solutions containing either high salt concentrations or helix destabilizing reagents are standard methods to achieve DNA dehybridization. Incubation in 8M urea for 20 min at room temperature is relatively mild, yet sufficient to completely dehybridize probe and target molecules (data not shown). The changes in fluorescence signal for multiple hybridization cycles separated by incubations in 8M urea solution for 20 min were monitored (Figure 4). After 20 cycles, glass exhibited 31 ± 1% and glassy carbon exhibited 91 ± 5% of the initial fluorescence, while diamond exhibited no measurable loss of fluorescence (108 ± 7%).
Figure 4.
Change in fluorescence signal of arrays prepared on glass (solid square), glassy carbon (open square) and diamond (gray shaded square) following multiple hybridization (1 × SSPE, 1 h, RT, followed by: 15 min, 37°C) and dehybridization (20 min, 8 M urea) cycles.
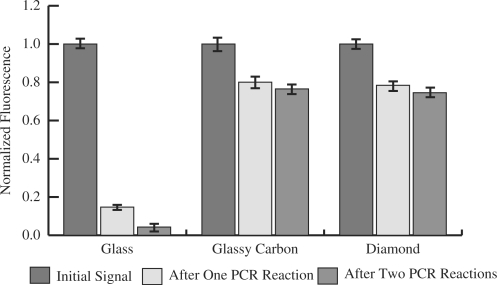
Stability to PCR cycles
Glass showed very poor stability when subjected to the conditions of a typical PCR reaction (Figure 5). Following a 50-cycle reaction, the arrays synthesized on glass retained only 15 ± 3% of the initial fluorescence. After two such reactions, <5% of the initial signal was detectable. Arrays prepared on glassy carbon and diamond exhibited greater stability. Arrays prepared on both substrates maintained ∼80% of their initial fluorescence signal following initial treatment and displayed no measurable loss following the second treatment. Glassy carbon exhibited 80 ± 6% and 77 ± 5% and diamond exhibited 78 ± 5% and 74 ± 5% of their initial fluorescence levels following the first and second treatments, respectively.
Figure 5.
Loss of fluorescence signal from arrays prepared on glass, glassy carbon and diamond following subjection to a 50-cycle PCR reaction with annealing, chain extension and melting temperatures of 65°C, 72°C and 94°C, respectively.
Comparison of array image quality
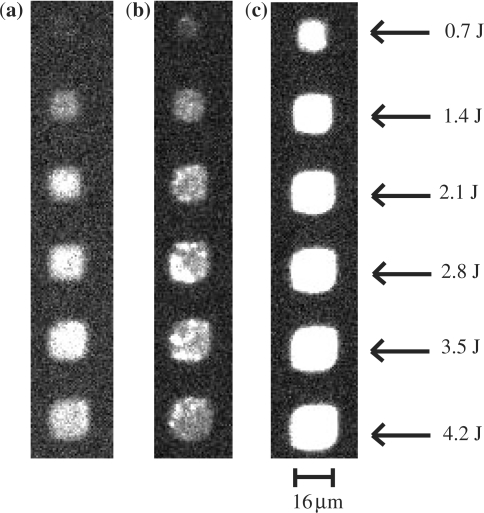
Images of one-in-four arrays (a single 16 μm × 16 μm feature separated on all sides by 16 μm) were obtained using a fluorescence microscope (Figure 6). The images clearly demonstrate that there is no crosstalk between the features on any substrate. The unpolished finish of the glassy carbon surface contributes to the non-uniformity of the features at this magnification. However, this may be easily overcome by using commercially available polished surfaces. It is interesting to note, that even at this high density and small feature size, the UV dose needed to remove the NPPOC protecting group remains unchanged. The apparent increase in feature size with larger UV-dose is characteristic of the light directed in situ growth method and can be corrected for by using inverse capping (51). The polished silicon substrate behind the diamond thin film acts as a mirror, enhancing the observed fluorescence. This phenomenon is well known and is used in spotted arrays to increase signal to noise (52).
Figure 6.
Fluorescence microscope images of a Cy3-labeled complementary DNA sequence hybridized to identical probe sequence fabricated using an increasing UV dose (measured in Joules) on glass (a), glassy carbon (b) and diamond (c). Each feature is a single pixel (16 μm × 16 μm) separated by 16 μm. The fluorescence observed from the diamond substrate is greater than that from either glass or glassy carbon. This may be the result of the polished silicon acting as a mirror behind the thin film diamond.
DISCUSSION
Because nucleic acids will not attach efficiently to untreated glass slides and because the uniformity of the slide surface is critical to the quality and reproducibility of arrays, glass slides are typically modified using silane chemistries. However, the susceptibility of siloxane linkages to hydrolysis under standard conditions and the increase in the rate of hydrolysis at elevated temperatures and at basic conditions is well known (53–55). Typically, silanization is used to introduce aldehyde, amino or poly-lysine groups to the surface.
A similar flexibility in surface functionality can be achieved on carbon substrates by using an alkene containing the desired functional group (38,39,56,57). The carbon–carbon covalent bonds within the carbon substrate and between the substrate and the linker moiety are not susceptible to hydrolysis (54). The high stability of DNA arrays fabricated on carbon substrates, compared to their glass counterparts, reflects this fact. This increased stability of DNA arrays is important for any application where it is desirable to employ higher temperatures, extended reaction times, or basic pH conditions. Such applications include solid-phase-PCR (50,58) and surface invasive cleavage reactions (57,59–61). The stability of carbon substrates also permits their use in serial hybridization–dehybridization cycles. Arrays prepared on glass exhibit a loss of fluorescence with each subsequent chemical dehybridization cycle, while the fluorescence signal obtained from carbon substrates does not follow this trend.
The hybridization densities as measured by wash-off and collection on in situ prepared DNA arrays are lower than the densities measured when pre-synthesized oligonucleotides are immobilized on gold-thiol self-assembled monolayers (SAMs). The reduced number of hybridizable oligonucleotides per square centimeter is most likely an artifact of the in situ synthesis process. Steric hindrance due to the close proximity of the growing strand to both the surface and adjacent strands reduces the efficiencies of all steps in the synthesis process resulting in truncated DNA strands (62). While the efficiencies of coupling between NPPOC phosphoramidites and the DMT-protected phosphoramidites are comparable (14,63), post-synthesis purification for removal of truncated strands is not possible when using in situ synthesis methods. As such, the absolute number of full-length probes available for hybridization will be higher for arrays spotted on alkane-thiol SAMs. The greater the percentage of proper probe sequences, the greater the number of targets that can be subsequently captured.
This work has focused on two types of carbon substrates, glassy carbon and diamond, as materials for in situ DNA arrays. The choice of glassy carbon was based upon the commercial availability of the material from a number of vendors in either a polished or an unpolished state. Diamond deposited on silicon was included in this study to permit comparisons between this work and previously reported immobilizations of biomolecules (38,40,57). The principles established are applicable to other carbon-based materials (amorphous carbon films, nanotubes, nanorods) and carbon-rich materials (silicon carbide).
We have demonstrated that in situ photolithographic synthesis of DNA arrays on non-glass substrates is readily achievable and that these arrays offer superior stability compared to their glass counterparts under a variety of conditions. This technology enables both the repeated reuse of DNA arrays, and the use of DNA arrays for applications involving high temperatures and extremes in pH not previously accessible.
ACKNOWLEDGEMENTS
The authors thank Professor Robert Hamers for useful discussions relating to carbon surfaces, and Dr James Butler for the generous gift of the diamond substrates; Dr Kathryn Richmond for numerous discussions on DNA and synthesis quality; Omar Negrete for fluorescence microscopy assistance; Kurt Heindrich for engineering assistance and DongGee Hong for software support. This work was funded by the National Institutes of Health (R01HG02298, R01HG003275, R01EB00269), MRL is supported by the UW Technology Interface Fund. Funding to pay the Open Access publication charges for this article was provided by NIH R01HG02298.
Conflict of interest statement. None declared.
REFERENCES
- 1.Blaese RM, Culver KW, Miller AD, Carter CS, Fleisher T, Clerici M, Shearer G, Chang L, Chiang YW, et al. T-Lymphocyte-directed gene-therapy for ADA(-) SCID- initial trial results after 4 years. Science. 1995;270:475–480. doi: 10.1126/science.270.5235.475. [DOI] [PubMed] [Google Scholar]
- 2.Nuwaysir EF, Huang W, Albert TJ, Singh J, Nuwaysir K, Pitas A, Richmond T, Gorski T, Berg JP, et al. Gene expression analysis using oligonucleotide arrays produced by maskless photolithography. Genome Res. 2002;12:1749–1755. doi: 10.1101/gr.362402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altshuler D, Daly M. Guilt beyond a reasonable doubt. Nat. Genet. 2007;39:813–815. doi: 10.1038/ng0707-813. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Luhm R, Lei M. SNP and mutation analysis. Adv. Exp. Med. Biol. 2007;593:105–116. doi: 10.1007/978-0-387-39978-2_11. [DOI] [PubMed] [Google Scholar]
- 5.Shai RM. Microarray tools for deciphering complex diseases. Front Biosci. 2006;11:1414–1424. doi: 10.2741/1892. [DOI] [PubMed] [Google Scholar]
- 6.Bulyk ML. DNA microarray technologies for measuring protein-DNA interactions. Curr. Opin. Biotechnol. 2006;17:422–430. doi: 10.1016/j.copbio.2006.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulyk ML, Gentalen E, Lockhart DJ, Church GM. Quantifying DNA-protein interactions by double-stranded DNA arrays. Nat. Biotechnol. 1999;17:573–577. doi: 10.1038/9878. [DOI] [PubMed] [Google Scholar]
- 8.Mintseris J, Eisen MB. Design of a combinatorial DNA microarray for protein-DNA interaction studies. BMC Bioinformatics. 2006;7:429. doi: 10.1186/1471-2105-7-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren CL, Kratochvil NCS, Hauschild KE, Foister S, Brezinski ML, Dervan PB, Phillips GNJr, Ansari AZ. Defining the sequence-recognition profile of DNA-binding molecules. Proc. Natl Acad. Sci. USA. 2006;103:867–872. doi: 10.1073/pnas.0509843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh SJ, Hong BJ, Choi KY, Park JW. Surface modification for DNA and protein microarrays. Omics. 2006;10:327–343. doi: 10.1089/omi.2006.10.327. [DOI] [PubMed] [Google Scholar]
- 11.de Gans BJ, Duineveld PC, Schubert US. Inkjet printing of polymers: state of the art and future developments. Adv. Mater. 2004;16:203–213. [Google Scholar]
- 12.Fodor SPA, Read JL, Pirrung MC, Stryer L, Lu AT, Solas D. Light-directed, spatially addressable parallel parallel chemical synthesis. Science. 1991;251:767–773. doi: 10.1126/science.1990438. [DOI] [PubMed] [Google Scholar]
- 13.Lipshutz RJ, Fodor SPA, Gingeras TR, Lockhart DJ. High density synthetic oligonucleotide arrays. Nat. Genet. 1999;21:20–24. doi: 10.1038/4447. [DOI] [PubMed] [Google Scholar]
- 14.McGall GH, Barone AD, Diggelmann M, Fodor SPA, Gentalen E, Ngo N. The efficiency of light-directed synthesis of DNA arrays on glass substrates. J. Am. Chem. Soc. 1997;119:5081–5090. [Google Scholar]
- 15.Pease AC, Solas D, Sullivan EJ, Cronin MT, Holmes CP, Fodor SPA. Light-generated oligonucleotide arrays for rapid DNA-sequence analysis. Proc. Natl Acad. Sci. USA. 1994;91:5022–5026. doi: 10.1073/pnas.91.11.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh-Gasson S, Green RD, Yue Y, Nelson C, Blattner F, Sussman MR, Cerrina F. Maskless fabrication of light-directed oligonucleotide microarrays using a digital micromirror array. Nat. Biotechnol. 1999;17:974–978. doi: 10.1038/13664. [DOI] [PubMed] [Google Scholar]
- 17.Chrisey LA, Lee GU, O’Ferrall CE. Covalent attachment of synthetic DNA to self-assembled monolayer films. Nucleic Acids Res. 1996;24:3031–3039. doi: 10.1093/nar/24.15.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Proudnikov D, Timofeev E, Mirzabekov A. Immobilization of DNA in polyacrylamide gel for the manufacture of DNA and DNA-oligonucleotide microchips. Anal. Biochem. 1998;259:34–41. doi: 10.1006/abio.1998.2620. [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen SR, Larsen MR, Rasmussen SE. Covalent immobilization of DNA onto polystyrene microwells: the molecules are only bound at the 5′ end. Anal. Biochem. 1991;198:138–142. doi: 10.1016/0003-2697(91)90518-x. [DOI] [PubMed] [Google Scholar]
- 20.Salo H, Virta P, Hakala H, Prakash TP, Kawasaki AM, Manoharan M, Lonnberg H. Aminooxy functionalized oligonucleotides: preparation, on-support derivatization, and postsynthetic attachment to polymer support. Bioconjug. Chem. 1999;10:815–823. doi: 10.1021/bc990021m. [DOI] [PubMed] [Google Scholar]
- 21.Rogers YH, Jiang-Baucom P, Huang ZJ, Bogdanov V, Anderson S, Boyce-Jacino MT. Immobilization of oligonucleotides onto a glass support via disulfide bonds: a method for preparation of DNA microarrays. Anal. Biochem. 1999;266:23–30. doi: 10.1006/abio.1998.2857. [DOI] [PubMed] [Google Scholar]
- 22.Beier M, Hoheisel JD. Versatile derivatisation of solid support media for covalent bonding on DNA-microchips. Nucleic Acids Res. 1999;27:1970–1977. doi: 10.1093/nar/27.9.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung VG, Morley M, Aguilar F, Massimi A, Kucherlapati R, Childs G. Making and reading microarrays. Nat. Genet. 1999;21:15–19. doi: 10.1038/4439. [DOI] [PubMed] [Google Scholar]
- 24.Cohen G, Deutsch J, Fineberg J, Levine A. Covalent attachment of DNA oligonucleotides to glass. Nucleic Acids Res. 1997;25:911–912. doi: 10.1093/nar/25.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morozov VN, Morozova T. Electrospray deposition as a method for mass fabrication of mono- and multicomponent microarrays of biological and biologically active substances. Anal. Chem. 1999;71:3110–3117. doi: 10.1021/ac981412h. [DOI] [PubMed] [Google Scholar]
- 26.Guo Z, Guilfoyle RA, Thiel AJ, Wang R, Smith LM. Direct fluorescence analysis of genetic polymorphisms by hybridization with oligonucleotide arrays on glass supports. Nucleic Acids Res. 1994;22:5456–5465. doi: 10.1093/nar/22.24.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aelion R, Loebel A, Eirich F. Hydrolysis of ethyl silicate. J. Am. Chem. Soc. 1950;72:5705–5712. [Google Scholar]
- 28.Keefer K. In: Silicon Based Polymer Science: A Comprehensive Resource. Zeigler J, Fearon F, editors. Washington, DC: American Chemical Society; 1990. pp. 227–270. [Google Scholar]
- 29.Edman CF, Raymond DE, Wu DJ, Tu E, Sosnowski RG, Butler WF, Nerenberg M, Heller MJ. Electric field directed nucleic acid hybridization on microchips. Nucleic Acids Res. 1997;25:4907–4914. doi: 10.1093/nar/25.24.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strother T, Cai W, Zhao XS, Hamers RJ, Smith LM. Synthesis and characterization of DNA-modified silicon (111) surfaces. J. Am. Chem. Soc. 2000;122:1205–1209. [Google Scholar]
- 31.Ulman A. An Introduction to Ultrathin Organic Films: From Langmuir-Blodgett to Self-assembly. Boston: Academic Press; 1991. [Google Scholar]
- 32.Boncheva M, Scheibler L, Lincoln P, Vogel H, Akerman B. Design of oligonucleotide arrays at interfaces. Langmuir. 1999;15:4317–4320. [Google Scholar]
- 33.Strother T, Hamers RJ, Smith LM. Covalent attachment of oligodeoxyribonucleotides to amine-modified Si (001) surfaces. Nucleic Acids Res. 2000;28:3535–3541. doi: 10.1093/nar/28.18.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brockman JM, Frutos AG, Corn RM. A multistep chemical modification procedure to create DNA arrays on gold surfaces for the study of protein-DNA interactions with surface plasmon resonance imaging. J. Am. Chem. Soc. 1999;121:8044–8051. [Google Scholar]
- 35.Thiel AJ, Frutos AG, Jordan CE, Corn RM, Smith LM. In situ surface plasmon resonance imaging detection of DNA hybridization to oligonucleotide arrays on gold surfaces. Anal. Chem. 1997;69:4948–4956. [Google Scholar]
- 36.Moorcroft MJ, Meuleman WR, Latham SG, Nicholls TJ, Egeland RD, Southern EM. In situ oligonucleotide synthesis on poly(dimethylsiloxane): a flexible substrate for microarray fabrication. Nucleic Acids Res. 2005;33:e75. doi: 10.1093/nar/gni075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fixe F, Dufva M, Telleman P, Christensen CB. Functionalization of poly(methyl methacrylate) (PMMA) as a substrate for DNA microarrays. Nucleic Acids Res. 2004;32:e9. doi: 10.1093/nar/gng157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strother T, Knickerbocker T, Russell JN, Butler JE, Smith LM, Hamers RJ. Photochemical functionalization of diamond films. Langmuir. 2002;18:968–971. [Google Scholar]
- 39.Sun B, Colavita PE, Kim H, Lockett M, Marcus MS, Smith LM, Hamers RJ. Covalent photochemical functionalization of amorphous carbon thin films for integrated real-time biosensing. Langmuir. 2006;22:9598–9605. doi: 10.1021/la061749b. [DOI] [PubMed] [Google Scholar]
- 40.Yang W, Auciello O, Butler JE, Cai W, Carlisle JA, Gerbi JE, Gruen DM, Knickerbocker T, Lasseter TL, et al. DNA-modified nanocrystalline diamond thin-films as stable, biologically active substrates. Nat. Mater. 2002;1:253–257. doi: 10.1038/nmat779. [DOI] [PubMed] [Google Scholar]
- 41.Fitzer E. Thermal-degradation of polymers to polymeric carbon - An approach to the synthesis of new materials. Angew. Chem. Int. Edit. 1980;19:375–385. doi: 10.1002/anie.198003751. [DOI] [PubMed] [Google Scholar]
- 42.Pajasova L, Soukup L, Jastrabik L, Chvostova D. Optical properties of glassy carbon. Surf. Rev. Lett. 2002;9:473–477. [Google Scholar]
- 43.Pierson HO. Handbook of Carbon, Graphite, Diamond, and Fullerenes. Park Ridge, NJ: Noyes Data Corportation; 1994. [Google Scholar]
- 44.Setton R, Bernier P, Lefrant S. Carbon Molecules and Materials. London, England: CRC Press; 2002. [Google Scholar]
- 45.Celii FG, Butler JE. Diamond chemical vapor-deposition. Annu. Rev. Phys. Chem. 1991;42:643–684. [Google Scholar]
- 46.Richmond KE, Li M-H, Rodesch MJ, Patel M, Lowe AM, Kim C, Chu LL, Venkataramaian N, Flickinger SF, et al. Amplification and assembly of chip-eluted DNA (AACED): a method for high-throughput gene synthesis. Nucleic Acids Res. 2004;32:5011–5018. doi: 10.1093/nar/gkh793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walbert S, Pfleiderer W, Steiner UE. Photolabile protecting groups for nucleosides: mechanistic studies of the 2-(2-nitrophenyl)ethyl group. Helv. Chim. Acta. 2001;84:1601–1611. [Google Scholar]
- 48.Katzhendler J, Cohen S, Rahamim E, Weisz M, Ringel I, Deutsch J. The effect of spacer, linkage and solid support on the synthesis of oligonucleotides. Tetrahedron. 1989;45:2777–2792. [Google Scholar]
- 49.Peelen D, Smith LM. Immobilization of amine-modified oligonucleotides on aldehyde-terminated alkanethiol monolayers on gold. Langmuir. 2005;21:266–271. doi: 10.1021/la048166r. [DOI] [PubMed] [Google Scholar]
- 50.Adessi C, Matton G, Ayala G, Turcatti G, Mermod JJ, Mayer P, Kawashima E. Solid phase DNA amplification: characterisation of primer attachment and amplification mechanisms. Nucleic Acids Res. 2000;28:E87. doi: 10.1093/nar/28.20.e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim C, Cerrina F. 2005. Patent USA-20070134677. [Google Scholar]
- 52.Fouque B, Schaack B, Obeid P, Combe S, Getin S, Barritault P, Chaton P, Chatelain F. Multiple wavelength fluorescence enhancement on glass substrates for biochip and cell analyses. Biosens. Bioelectron. 2005;20:2335–2340. doi: 10.1016/j.bios.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Ishida H, Koenig JL. Effect of hydrolysis and drying on the siloxane bonds of a silane coupling agent deposited on E-glass fibers. J. Polym. Sci., Part B: Polym. Phys. 1980;18:233–237. [Google Scholar]
- 54.Pawlenko S. Organosilicon Chemistry. Berlin; New York: W. de Gruyter; 1986. [Google Scholar]
- 55.Plueddemann EP. Silane Coupling Agents. 2nd. New York: Plenum Press; 1991. [Google Scholar]
- 56.Knickerbocker T, Strother T, Schwartz MP, Russell JN, Butler J, Smith LM, Hamers RJ. DNA-modified diamond surfaces. Langmuir. 2003;19:1938–1942. [Google Scholar]
- 57.Lu MC, Knickerbocker T, Cai W, Yang WS, Hamers RJ, Smith LM. Invasive cleavage reactions on DNA-modified diamond surfaces. Biopolymers. 2004;73:606–613. doi: 10.1002/bip.20007. [DOI] [PubMed] [Google Scholar]
- 58.Pemov A, Modi H, Chandler DP, Bavykin S. DNA analysis with multiplex microarray-enhanced PCR. Nucleic Acids Res. 2005;33:e11. doi: 10.1093/nar/gnh184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y, Shortreed MR, Olivier M, Smith LM. Parallel single nucleotide polymorphism genotyping by surface invasive cleavage with universal detection. Anal. Chem. 2005;77:2400–2405. doi: 10.1021/ac0483825. [DOI] [PubMed] [Google Scholar]
- 60.Lu M, Hall JG, Shortreed MR, Wang L, Berggren WT, Stevens PW, Kelso DM, Lyamichev V, Neri B, et al. Structure-specific DNA cleavage on surfaces. J. Am. Chem. Soc. 2002;124:7924–7931. doi: 10.1021/ja012082c. [DOI] [PubMed] [Google Scholar]
- 61.Lu M, Shortreed MR, Hall JG, Wang L, Berggren TW, Stevens P, Kelso DM, Lyamichev V, Neri B, et al. A surface invasive cleavage assay for highly parallel SNP analysis. Hum. Mutat. 2002;19:416–422. doi: 10.1002/humu.10071. [DOI] [PubMed] [Google Scholar]
- 62.Temsamani J, Kubert M, Agrawal S. Sequence identity of the n-1 product of a synthetic oligonucleotide. Nucleic Acids Res. 1995;23:1841–1844. doi: 10.1093/nar/23.11.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Albert TJ, Norton J, Ott M, Richmond T, Nuwaysir K, Nuwaysir EF, Stengele KP, Green RD. Light-directed 5′ → 3′ synthesis of complex oligonucleotide microarrays. Nucleic Acids Res. 2003;31:e35. doi: 10.1093/nar/gng035. [DOI] [PMC free article] [PubMed] [Google Scholar]