Abstract
The chloroplast genome of higher plants contains 20–40 C-to-U RNA editing sites, whose number and locations are diversified among plant species. Biochemical analyses using in vitro RNA editing systems with chloroplast extracts have suggested that there is one-to-one recognition between proteinous site recognition factors and their respective RNA editing sites, but their rigidness and generality are still unsettled. In this study, we addressed this question with the aid of an in vitro RNA editing system from tobacco chloroplast extracts and with UV-crosslinking experiments. We found that the ndhB-9 and ndhF-1 editing sites of tobacco chloroplast transcripts are both bound by the site-specific trans-acting factors of 95 kDa. Cross-competition experiments between ndhB-9 and ndhF-1 RNAs demonstrated that the 95 kDa proteins specifically binding to the ndhB-9 and ndhF-1 sites are the identical protein. The binding regions of the 95 kDa protein on the ndhB-9 and ndhF-1 transcripts showed 60% identity in nucleotide sequence. This is the first biochemical demonstration that a site recognition factor of chloroplast RNA editing recognizes plural sites. On the basis of this finding, we discuss how plant organellar RNA editing sites have diverged during evolution.
INTRODUCTION
RNA editing is a process in which the nucleotide sequences of transcripts are changed by insertion/deletion or conversion of nucleotides, and various types of RNA editing have been found in diverse organisms (1,2). In the organelles of vascular plants, specific C residues on the transcripts are converted to U, and U-to-C editing rarely occurs (1,3,4). Chloroplast and mitochondrial genomes of higher plants have 20–40 and 400–500 RNA editing sites, respectively, and in most cases, RNA editing restores phylogenetically conserved codons (1,3,4), including those of functional importance (5–8). Therefore, RNA editing is an indispensable process for plant organellar genomes to produce functional proteins.
An intriguing issue of plant organellar RNA editing is the mechanism by which specific C residues are recognized for editing substrates, since no consensus motif or secondary structure is found in the vicinity of the editing sites. With the aid of transplastomic plants and of in vitro RNA editing systems from chloroplast lysates, cis-sequences required for RNA editing were analyzed for several tobacco chloroplast editing sites and revealed that cis-elements are generally located within 20 nucleotides upstream of the editing sites (9–17). Proteinous trans-acting factors that specifically bind to the cis-elements were evidenced by UV-crosslinking experiments with in vitro RNA editing systems; cis-elements of tobacco psbL, psbE and petB editing sites are bound by the proteins of 25, 57 and 70 kDa, respectively (12,14,15). From the study of an Arabidopsis mutant deficient in the editing activity of the ndhD-1 site, a site-specific RNA-binding protein, CRR4, was identified (8). CRR4 is a member of the pentatricopeptide repeat (PPR) family (18) and specifically binds to the immediate upstream region of the ndhD-1 editing site (19). PPR proteins constitute an extraordinarily large family in higher plants, and many are involved in the maturation processes of organellar transcripts (20). As CRR4 does not have a catalytic domain, it is likely to recruit a catalytic subunit of unknown identity to the editing site (8,19).
The above findings suggest that sequence-specific binding of the trans-acting factors to the upstream cis-elements is a crucial process of the accurate site recognition of chloroplast RNA editing. If so, how specifically do the trans-acting factors recognize their respective cis-elements? Site recognition mechanisms of RNA editing seem analogous between chloroplasts and mitochondria (21–26). If so, how many trans-acting factors are necessary to recognize whole editing sites of plant organelles? Overexpression of the psbL editing site in tobacco chloroplasts reduced the editing efficiency of endogenous psbL mRNA, but not of the other editing sites, implying that the psbL-specific trans-acting factor is exclusively recruited to the psbL editing site (27). As mentioned above, trans-acting factors specifically binding to tobacco psbL, psbE, petB, and Arabidopsis ndhD-1 editing sites have distinct molecular masses (8,12,14,15). These genetic and biochemical observations might support a ‘one factor to one site’ hypothesis for plant organellar RNA editing. However, this hypothesis is now challenged from other viewpoints. The Arabidopsis genome encodes ca. 450 PPR proteins (20), while the total number of chloroplast and mitochondrial editing sites of this plant amounts to more than 480 (28–30). If trans-acting factors responsible for site recognition are exclusively PPR proteins, ‘one factor to plural sites’ could also be the case. Chateigner-Boutin and Hanson proposed the ‘one factor to plural sites hypothesis’ on the basis of their observations with transplastomic plants; overexpression of the rpoB-2 or ndhF-2 editing sites reduced the editing efficiencies of several sites (31). However, the ‘one factor to plural sites hypothesis’ has not been proven by biochemical investigations.
This study attempted to verify the ‘one factor to plural sites’ hypothesis on the basis of biochemical analysis. We first compared the molecular masses of trans-acting factors specifically binding to several tobacco chloroplast editing sites by UV-crosslinking and sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), and found that those of the ndhB-9 and ndhF-1 editing sites seem to be very similar. Next, we examined the identities of ndhB-9 and ndhF-1-specific trans-acting factors by cross-competition experiments in the in vitro RNA editing system of tobacco chloroplast extracts. The results unequivocally showed that the ndhB-9 and ndhF-1 editing sites are recognized by the same trans-acting factor. This is the first clear demonstration that ‘one factor to plural sites’ recognition operates in plant organellar RNA editing. Sequence identity in the trans-factor-binding regions of ndhB-9 and ndhF-1 (−15 to −1 relative to the editing site as +1) is only 60%. On the basis of this finding, we discuss how plant organellar RNA editing sites have propagated and diverged during evolution.
MATERIALS AND METHODS
Preparation of RNA substrates
The region from −120 to +21 (relative to the editing site as +1) from the gene of interest, with a 5′ extension of a 20 nt sequence complementary to the T3 primer and a 3′ extension of a 17 nt sequence complementary to the KS primer, was amplified by PCR on plasmids from a tobacco chloroplast DNA clone bank (32) using gene-specific primer pairs (Table 1, PCR forward and PCR reverse). The amplified fragments were cloned into a pGEM-T vector using the pGEM-T Vector System (Promega). From these cloned plasmids, RNA substrates for in vitro editing and UV-crosslinking were prepared as previously described (14) with slight modifications. The upstream region of the respective genes was amplified from the plasmids by PCR using gene-specific primer pairs (Table 1, PCR forward and PCR upstream reverse), and subjected to RNA synthesis with the MEGAscript T3 Kit (Ambion) with purification according to the manufacturer's instruction. The 5′ terminal two nucleotides of the PCR upstream reverse primers were ribose 2′-methoxy analogs, which hamper nontemplated nucleotide addition by T3 RNA polymerase (33). [5′-32P]-labeled downstream RNAs (20 pmol) (Table 1) were ligated to 60 pmol of the corresponding upstream RNAs (113–123 nt) with the aid of 40 pmol of a bridging DNA oligonucleotide (Table 1) and T4 DNA ligase in 30 μl reaction mixtures at 30°C overnight. The ligated mRNAs were purified by 5% PAGE containing 7 M urea. When mutations were introduced to the RNA substrates, plasmid clones containing respective chloroplast genes were mutagenized using pairs of mutagenesis primers (Table 1) and the QuickChange Site-Directed Mutagenesis Kit (Stratagene), followed by the preparation of RNA substrates as described above.
Table 1.
Oligononucleotide primers used in this study
| Name | Sequence (5′–3′) | Purpose |
|---|---|---|
| T3+ndhB-2For | AATTAACCCTCACTAAAGGGTAGAGTACATTGAATGTACA | PCR forward |
| T3+ndhB-9For | AATTAACCCTCACTAAAGGGTAATGACTGGACGAAACCAA | PCR forward |
| T3+ndhF-lFor | AATTAACCCTCACTAAAGGGACCTGTCTATTCAGCAAATA | PCR forward |
| T3+rpoA-lFor | AATTAACCCTCACTAAAGGGTATATTTACAGGACAATCAA | PCR forward |
| T3+rpoB-lFor | AATTAACCCTCACTAAAGGGCACTCACATATTCTTCTGAA | PCR forward |
| T3+rpoB-4For | AATTAACCCTCACTAAAGGGCTCGGGGTAAATGCATTAAA | PCR forward |
| T3+vectorFor | AATTAACCCTCACTAAAGGGTAAGACACGACTTATCGCCA | PCR forward |
| KS+ndhB-2Rev | TCGAGGTCGACGGTATCAGCATAAACTGAAACATTCT | PCR reverse |
| KS+ndhB-9Rev | TCGAGGTCGACGGTATCTTGGGTTCATTGATATTCCT | PCR reverse |
| KS+ndhF-lRev | TCGAGGTCGACGGTAICCAACCGTAGTGATTAATAIT | PCR reverse |
| KS+rpoA- 1Rev | TCGAGGTCGACGGTATCAGATCCTGGAAGGCAATTCT | PCR reverse |
| KS+rpoB-lRev | TCGAGGTCGACGGTATCTATATATTCCATTGACTATA | PCR reverse |
| KS+rpoB-4Rev | TCGAGGTCGACGGTATCTAATAAGTACTGCATCTTCA | PCR reverse |
| vectorRev | GGTAACTGGCTTCAGCAGAG | PCR reverse |
| ndhB-9Ml | GAGTATGATTGTATGTGTGAATCGTTCTACTATACCAGGAATATC | PCR mutagenesis |
| ndhB-9MlC | GATATTCCTGGTATAGTAGAACGATTCACACATACAATCATACTC | PCR mutagenesis |
| ndhB-9M2 | TGATTGTATGTGTGATAGCAAGAIGTATACCAGGAATATCAAIGA | PCR mutagenesis |
| ndhB-9M2C | TCATTGATATTCCTGGTATACATCTTGCTATCACACATACAATCA | PCR mutagenesis |
| ndhB-9M3 | GTATGTGTGATAGCATCTACATATGCAGGAATATCAATGAACCCA | PCR mutagenesis |
| ndhB-9M3C | TGGGTTCATTGATATTCCTGCATATGTAGATGCTATCACACATAC | PCR mutagenesis |
| ndhF-lMl | CGGATACTTGATCGACCCACAATGATCTATTATGTCAATATTAAT | PCR mutagenesis |
| ndhF-lM1C | ATTAATATTGACATAATAGATCATTGTGGGTCGATCAAGTATCCG | PCR mutagenesis |
| ndhF-lM2 | ACTTGATCGACCCACTTACTAGATATATGTCAATATTAATCACTA | PCR mutagenesis |
| ndhF-lM2C | TAGTGATTAATATTGACATATATCTAGTAAGTGGGTCGATCAAGT | PCR mutagenesis |
| ndhF-lM3 | ATCGACCCACITACTTCTATATACACAATATTAATCACTACGGIT | PCR mutagenesis |
| ndhF-lM3C | AACCGTAGTGATTAATATTGTGTATATAGAAGTAAGTGGGTCGAT | PCR mutagenesis |
| ndhB-2(+l) | AGCATAAACTGAAACATTCTGGGGCTACAAAGATAGTTATT | Bridge DNA |
| ndhB-9(+l) | TTGGGTTCATTGATATTCCTGGTATAGTAGATGCTATCACA | Bridge DNA |
| ndhB-9(−10) | GATATTCCTGGTATAGTAGATGCTATCACACATACAATCA | Bridge DNA |
| ndhF-l(+l) | CAACCGTAGTGATTAATATTGACATAATAGAAGTAAGTGGG | Bridge DNA |
| ndhF-l(−l0) | ATTAATATTGACATAATAGAAGTAAGTGGGTCGATCAAGT | Bridge DNA |
| rpoA-l(+l) | AGATCCTGGAAGGCAATTCTGATTGGTCAATAAAAATCGAT | Bridge DNA |
| rpoB-l(+l) | TATATATTCCATTGACTATAGAAGTTCCCAGGGAATTCATT | Bridge DNA |
| rpoB-4(+l) | TAATAAGTACTGCATCTTCAGAATTGTAACCCTCCCACGGC | Bridge DNA |
| ndhB-2(+l)Rev | GGGCTACAAAGATAGTTATTAA | PCR upstream reverse |
| ndhB-9(+l)Rev | GUATAGTAGATGCTATCACACA | PCR upstream reverse |
| ndhB-9(−10)Rev | UGCTATCACACATACAATCATA | PCR upstream reverse |
| ndhF-l(+l)Rev | ACATAATAGAAGTAAGTGGGTC | PCR upstream reverse |
| ndhF-l(−10)Rev | AGTAAGTGGGTCGATCAAGTAT | PCR upstream reverse |
| rpoA-l(+l)Rev | AUTGGTCAATAAAAATCGATTT | PCR upstream reverse |
| rpoB-l(+l)Rev | AAGTTCCCAGGGAATTCATTAG | PCR upstream reverse |
| rpoB-4(+l)Rev | AATTGTAACCCTCCCACGGCAT | PCR upstream reverse |
| ndhB-2(+l) | CAGAAUGUUUCAGUUUAUGCUGAUACCGUCGACCUCGA | Downstream RNA |
| ndhB-9(+l) | CAGGAAUAUCAAUGAACCCAAGAUACCGUCGACCUCGA | Downstream RNA |
| ndhB-9(−10) | UCUACUAUACCAGGAAUAUC | Downstream RNA |
| ndhF-l(+l) | CAAUAUUAAUCACUACGGUUGGAUACCGUCGACCUCGA | Downstream RNA |
| ndhF-l(−l0) | UCUAUUAUGUCAAUAUUAAU | Downstream RNA |
| rpoA-l(+l) | CAGAAUUGCCUUCCAGGAUCUGAUACCGUCGACCUCGA | Downstream RNA |
| rpoB-l(+l) | CUAUAGUCAAUGGAAUAUAUAGAUACCGUCGACCUCGA | Downstream RNA |
| rpoB-4(+l) | CUGAAGAUGCAGUACUUAUUAGAUACCGUCGACCUCGA | Downstream RNA |
Underlines indicate ribose 2′-methoxy analogs.
Preparation of chloroplast extracts
Chloroplast extracts were prepared from tobacco leaves as previously described (34), and utilized for RNA editing reactions and UV-crosslinking.
In vitro RNA editing and UV-crosslinking
RNA editing and UV-crosslinking assays were carried out essentially as previously described (15), with slight modifications. Both reaction mixtures contained 4 μl of chloroplast extract (∼50 μg protein) and 10 fmol of mRNA substrate. For RNA editing assays, an mRNA substrate was incubated at 28°C for 1 h. RNA was isolated and digested into 5′ mononucleotides with 1 U of nuclease P1 (Wako) and 120 U of S1 nuclease (TaKaRa) in the presence of 50 mM ammonium acetate (pH 4.8) at 37°C for 3 h. Mononucleotides were separated on cellulose TLC plates (FC-2020, Funakoshi) using isopropanol:HCl:water (70:15:15). For UV-crosslinking assays, an mRNA substrate was incubated at 28°C for 1 h in the editing mixture. Reaction mixtures were irradiated with UV light (254 nm, 1.0 J/cm2) at approximately 10 cm distance using a Funacrosslinker (Funakoshi), then subjected to RNA digestion by 100 ng of RNase A at 37°C for 1 h. Protein samples were separated by 7.5% PAGE containing 0.1% SDS. 32P-labeled mononucleotides on TLC and 32P-crosslinked proteins on PAGE were visualized by STORM (GE Healthcare).
Nomenclature for RNA editing sites
The chloroplast genome of tobacco (Nicotiana tabacum) is known to have 38 RNA editing sites (34–37). In this study, the editing sites of tobacco are simply denoted as, for example, ndhB-9 that means the ninth editing site of tobacco ndhB mRNA counted from the 5′ end. However, ndhF-1 site in this nomenclature corresponds to ndhF2 of the previous reports (31,35) that name was given based on the comparison between tobacco and maize. For circumventing the confusion, the editing sites examined in this study are more precisely defined in Table 2, following the universal nomenclature used by Heyes et al. (16); NTndhB C141 means the editing site at the 141st C of the ndhB mRNA of Nicotiana tabacum.
Table 2.
trans-acting factors for cloroplast RNA editing, listed in the decreasing order of apparent molecular mass
| Editing site | Molecular mass | Detection | References |
|---|---|---|---|
| NTndhB C1481 | 95 kDa | UV crosslink | This study (ndhB-9) |
| NTndhF C290 | 95 kDa | UV crosslink | This study (ndhF-1) |
| NTrpoA C680 | 93 kDa | UV crosslink | This study (rpoA-1) |
| NTrpoB C338 | 91 kDa | UV crosslink | This study (rpoB-1) |
| NTrpoB C2000 | 76 kDa | UV crosslink | This study (rpoB-4) |
| NTpetB C611 | 70 kDa | UV crosslink | (14,15) |
| PSpetB C611 | 70 kDa | UV crosslink | (14) |
| ATndhD C2 | 68 kDa | CRR4 gene | (8) |
| NTndhB C467 | 59 kDa | UV crosslink | This study (ndhB-2) |
| NTpsbE C214 | 56 kDa | UV crosslink | (14,15) |
| NTpsbL C2 | 25 kDa | UV crosslink | (31) |
NT, Nitcotiana tabcum; PS, Pisum sativum; AT, Arabidopsis thaliana. Names of the editing sites are according to Heyes et al. (16).
RESULTS
Both ndhB-9 and ndh-F1 editing sites are crosslinked with 95 kDa protein
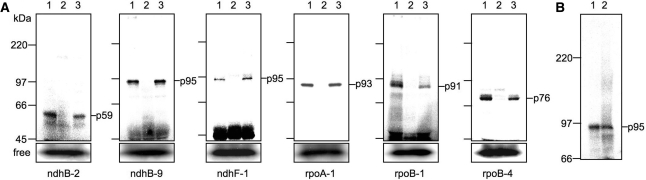
The tobacco chloroplast genome is known to have 38 RNA editing sites (34–37). We previously showed for tobacco psbE-1 and petB-1 RNA editing sites that the proteinous site-specific factors are UV-crosslinked with their respective editing sites (C at +1), as well as the cognate upstream cis-elements (15). If this is also the case for the rest of the RNA editing sites, we could detect and compare the molecular sizes of the respective site-specific factors by specific labeling of the C at +1 and subsequent UV-crosslinking. Therefore, we first examined the RNA editing efficiencies of 36 tobacco editing sites in our in vitro RNA editing system (Kobayashi et al. will be submitted elsewhere), and picked up six editing sites that exhibited relatively high editing efficiency. Next, we carried out UV-crosslinking experiments for these six editing sites using the RNA substrates (from −120 to +21) labeled at +1 with 32P, and with tobacco chloroplast extracts that favor in vitro editing reactions. After crosslinking, RNA molecules that were not crosslinked with the proteins were digested by RNase, followed by SDS–PAGE. Figure 1 shows autoradiograms of the proteins that were crosslinked with the editing sites (+1). To discriminate specific binding proteins from nonspecific binding proteins, we added either a 100-fold molar excess of the same RNA (lanes 2 in each panel in Figure 1A) or the exogenous control RNA (lanes 3 in Figure 1A) as competitors. This treatment revealed the proteins that specifically bind to the editing sites, and Figure 1A denotes their apparent molecular masses (e.g. the 59 kDa protein is denoted as p59); these factors are summarized in Table 2 in comparison with those of previously reported factors. Among them, proteins specifically binding to the ndhB-9 and ndhF-1 editing sites especially attracted our attention, because their molecular masses appeared to be both 95 kDa. As such, we compared their electrophoretic mobilities on SDS–PAGE once again in adjoining lanes and after a long distance run. However, we could not find any difference between them (Figure 1B). This hints at the possibility that p95s of ndhB-9 and ndhF-1 may be either the same or structurally similar proteins. In the following study, we investigate this possibility by examining the biochemical properties of these proteins.
Figure 1.
Trans-acting factors specifically binding to the editing sites in the extracts of tobacco chloroplasts. (A) UV-crosslinking was performed with a respective RNA probe that was labeled with 32P at +1 (C to be edited). Lanes 1, without competitor RNA; lanes 2, a 100-fold molar excess of unlabeled probe RNA was added as a competitor; lanes 3, a 100-fold molar excess of control RNA that was a 161 nt transcript of a pGEM-T vector was added as a competitor. Free indicates the bands of a free probe that migrated in front of the protein bands on SDS–PAGE. (B) Comparison of the electrophoretic mobilities of p95s binding to ndhB-9 (lane 1) and ndhF-1 (lane 2).
The 95 kDa proteins are trans-acting factors for RNA editing
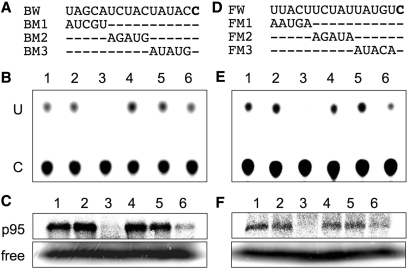
To ensure that the p95s are involved in RNA editing, we examined the correlation between the RNA editing activity and the binding of p95s to their respective RNA editing sites. Here, we introduced 5 nt scanning mutations to the −15 to −1 regions of the ndhB-9 and ndhF-1 RNA substrates (−120 to +21) as shown in Figure 2A and D, respectively, and then supplemented them to an in vitro RNA editing system as competitors.
Figure 2.
In vitro analysis of the RNA editing and the binding of p95s in the presence of mutated competitors. (A) and (D) represent the competitors derived from the ndhB-9 and ndhF-1 sequences, respectively. (B) and (E) represent RNA editing profiles of the ndhB-9 and ndhF-1 substrates, respectively, in the presence of various competitors. (C) and (F) represent binding profiles of p95s to the ndhB-9 and ndhF-1 probes, respectively. In (B) and (C): lanes 1, without competitor; lanes 2, with control competitor as was used in Figure 1; lanes 3–6, BW, BM1, BM2, and BM3 were added as competitors, respectively. In (E) and (F): lanes 1, without competitor; lanes 2, with control competitor as in Figure 1; lanes 3–6, FW, FM1, FM2, and FM3 were added as competitors, respectively.
When wild-type substrates (BW and FW in Figure 2) were added as competitors in a 100-fold molar excess, they trapped the respective trans-acting factors, causing the disappearance of C-to-U RNA editing (lanes 3 in Figure 2B and E) as well as the UV-crosslinking signals (lanes 3 in Figure 2C and F). Under the same conditions, mutations spanning −15 to −11 (BM1 and FM1) and –10 to –6 (BM2 and FM2) canceled competition, resulting in the appearance of radiolabeled signals for C-to-U RNA editing (lanes 4 and 5 in Figure 2B and E) and UV-crosslinking (lanes 4 and 5 in Figure 2C and F). These indicate that the upstream sequences from −15 to −6 are essential for recruiting trans-acting factors that ensure RNA editing reactions, as well as the contact of p95s to the C at +1. Interestingly, mutations introduced into the region from −5 to −1 (BM3 and FM3 in Figure 2) did not cancel the competition. Rather, they weakened it, resulting in weak detections of both RNA editing and p95s’ binding signals (lanes 6 in Figure 2). Therefore, RNA editing activity and the binding of p95s to the editing sites were well correlated over the mutations scanning from –15 to –1 for both the ndhB-9 and ndhF-1 editing sites. These correlations strongly suggest that p95s are trans-acting factors indispensable for RNA editing.
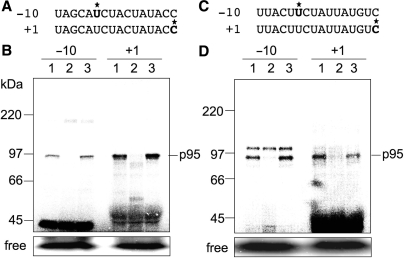
We previously reported for tobacco psbE-1 and petB-1 RNA editing sites that the site-specific trans-acting factors are recruited to the upstream cis-elements and then interact with the C residue (+1) to be edited (15). In order to test whether p95s are trans-acting factors with similar properties, we examined if p95s bind to the upstream cis-elements. As described above, cis-elements of the ndhB-9 and ndhF-1 editing sites that recruit trans-acting factors are located in the region from −15 to −6 (Figure 2). Thus, we introduced radiolabels at −10, in the midst of the respective cis-elements (Figure 3A and C, asterisk), and the resultant RNA probes were subjected to UV-crosslinking experiments. Figure 3B represents SDS–PAGE profiles of the proteins bound to –10 and +1 of the ndhB-9 editing site, and Figure 3D represents those of the ndhF-1 site. For both editing sites, p95s appeared to be specifically bound with upstream cis-elements (−10) as well as to the editing sites (+1). As for −10 of the ndhF-1 site, another crosslinked signal was detected on SDS–PAGE with an apparent molecular mass slightly larger than that of p95 (Figure 3D). However, this binding signal appeared to be nonspecific, because it did not disappear when a homologous competitor was added in excess (Figure 3D, lane 2).
Figure 3.
Detection of trans-acting factors by UV-crosslinking with ndhB-9 and ndhF-1 RNAs labeled with 32P at −10 and +1 relative to the editing site. (A) and (C) represent RNA probes of ndhB-9 and ndhF-1, respectively. Asterisks indicate labeled nucleotides. (B) and (D) represent UV-crosslinking profiles for ndhB-9 and ndhF-1, respectively. Lanes 1, without competitor; lanes 2, a 100-fold molar excess of unlabeled probe RNA was added as a competitor; lanes 3, a 100-fold molar excess of control RNA as in Figure 1 was added as a competitor.
Taken together with these results, we conclude that the p95s are site-specific trans-acting factors for the ndhB-9 and ndhF-1 RNA editing sites, and that they are recruited by the upstream cis-elements (from −15 to −6) and then interact with the editing site (+1) similarly to p56, the trans-acting factor of the psbE-1 editing site (15). However, the relationship between these two p95s that specifically bind to ndhB-9 or ndhF-1 is still unknown.
P95 recognizes both ndhB-9 and ndh-F1 editing sites
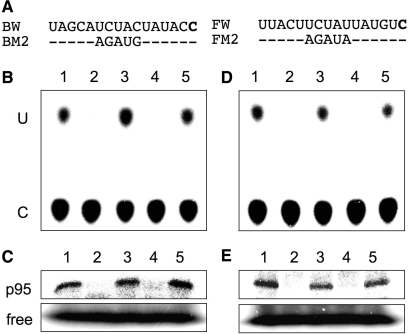
To clarify whether a given p95 can specifically bind to either or both of the ndhB-9 and ndh-F1 editing sites, we tested the binding specificity of the p95s to these sites by a cross-competition experiment (Figure 4).
Figure 4.
Cross-competition experiments between ndhB-9 and ndhF-1 RNAs. (A) Competitor sequences. (B) and (D) represent RNA editing profiles of the ndhB-9 and ndhF-1 substrates, respectively, in the presence of various competitors. (C) and (E) represent the binding profiles of p95s to the ndhB-9 and ndhF-1 RNAs, respectively. Lanes 1, without competitor; lanes 2–5, BW, BM2, FW, and FM2 were added as competitors, respectively.
First, we tested whether the p95 that specifically binds to the ndhB-9 site could also recognize the ndhF-1 site with the aid of ndhB-9 RNA (−120 to +21) radiolabeled at +1 as a probe. As was expected from Figure 2, RNA editing activity (Figure 4B) and the binding of p95 (Figure 4C) to the ndhB-9 site were both inhibited by the addition of the same RNA (BW) as a competitor (lanes 2), but not by BM2, which was mutated on the p95 binding site (lanes 3). Surprisingly, similar results were obtained when FW (−120 to +21 of the ndhF-1 site) and FM2 (the same as FW, but the cis-element was mutated) were added as competitors: FW inhibited both RNA editing and the binding of p95 to the ndhB-9 site (lanes 4), but FM2 did not (lanes 5). This result indicates that the p95 that specifically binds to the ndhB-9 site can also bind to ndhF-1 in a sequence-specific manner.
Next, we examined the reverse case, using the ndhF-1 RNA (−120 to +21) as a probe. The obtained results are shown in Figure 4D and E, indicating that the p95 that specifically binds to the ndhF-1site can also recognize the ndhB-9 site.
These complementary results let us conclude that the ndhB-9 and ndhF-1 RNA editing sites are corecognized by the identical trans-acting factor, p95.
DISCUSSION
This study demonstrated that the C residues at six RNA editing sites of tobacco chloroplast RNAs are bound by their respective site-specific proteins (Figure 1). Table 2 summarizes their apparent molecular masses in comparison with those previously reported for other editing sites (8,12,14,15). The molecular masses distribute from 25 to 95 kDa, implying that the site-specific factors have molecular diversity. CRR4, which specifically binds to the immediate upstream region of the ndhD-1 site in Arabidopsis thaliana, is a member of the PPR protein family (18,20), and CRR4 contains 10 PPR motifs (8,19). If site-specific factors detected by UV-crosslinking are all PPR proteins, they might differ in the number of PPR motifs in conjunction with their specificity to the binding sequences (38).
Competition experiments for ndhB-9 and ndhF-1 RNA editing sites (Figure 2) showed that site-specific trans-acting factors are recruited by their respective upstream cis-elements located from −15 to −6 in a sequence-specific manner, and that a close proximity (−5 to −1) has a weak effect on these interactions. These results are in accordance with previously proposed models for the site recognition of chloroplast RNA editing (9,10,12,14,15,39).
The most notable finding in this study is that the ndhB-9 and ndhF-1 RNA editing sites are recognized by the same trans-acting factor. This conclusion was obtained from cross-competition experiments between ndhB-9 and ndhF-1 RNAs in the in vitro RNA editing and p95-binding reactions (Figure 4). As shown in Figure 5A, the upstream regions (−15 to −1) of ndhB-9 and ndhF-1 represent a 60% identity in nucleotide sequence. This implies that a given trans-acting factor of chloroplast RNA editing could recognize groups of cis-elements that share moderate sequence identity. From this view, we reexamined the 38 RNA editing sites of the tobacco chloroplast genome to search for possible candidates recognized by common trans-acting factors. As shown in Figure 5B, six pairs of editing sites were found to have 60% or higher identity in their upstream sequences (−15 to −1) in addition to ndhB-9 and ndhF-1. If these pairs are really recognized by respective common factors, only 31 site-specific factors could be enough to account for 38 editing sites.
Figure 5.
Comparison of the nucleotide sequences in the upstream regions (−15 to +1) of tobacco chloroplast editing sites. (A) Comparison between ndhB-9 and ndhF-1. (B) Pairs of the editing sites that exhibit 60% or higher sequence identity.
In transplastomic tobacco plants, overexpression of the ndhF-1 editing site caused a decrease in editing efficiency in endogenous ndhF-1, ndhB-3 and ndhD-1 sites, but not in the other sites (31; ndhF-1 site was mentioned as ndhF-2 in this reference; see Materials and Methods). This appears to imply that ndhF-1 editing site shares trans-acting factor(s) with ndhB-3 and ndhD-1 sites but not with ndhB-9 site. However, the present study demonstrated that ndhF-1 and ndhB-9 share the site-recognition factor of 95 kDa. Why was cosuppression not observed for ndhF-1 and ndhB-9 in the transplastomic plants? The putative cis-acting regions of ndhF-1, ndhB-3 and ndhD-1 do not share sequence identity until gaps are introduced. In plant organellar RNA editing, spacing between upstream cis-elements and editing sites was shown to be critical, with only one base insertion/deletion in such a region causing the complete loss of editing activity (17,22,39). Therefore, one possibility might be that the above cosuppression phenotype in transplastomic plants was caused by competition for some unknown factors other than site recognition protein. In accordance with this speculation, an Arabidopsis mutant deficient in CRR4, the site recognition protein of ndhD-1, cannot edit the ndhD-1 site but still possesses normal editing activity for the ndhF-1 and ndhB-3 sites (8). We should also point out that the cosuppression phenotype of RNA editing by overexpression of given editing sites could vary according to many parameters, including the relative abundance of each transcript, its affinity to trans-factors, and possible overlapping of cis-trans network. Suggestions from cosuppression phenotypes are important information, but require further confirmation.
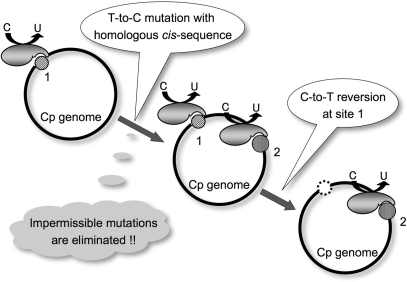
In higher plant organelles, editing sites are poorly conserved among plant species. Tobacco and Arabidopsis chloroplast genomes have 38 and 28 editing sites, respectively, but there are only 17 overlapping sites (28,29,34–37). How has this variance occurred? This question is equal to asking how the gain and loss of editing sites has occurred during plant evolution. The apparent function of plant organellar RNA editing is to compensate for genetic mutations that alter protein-coding sequences and to restore evolutionary conserved codons at the mRNA level. Therefore, the loss of the editing sites could be easily explained by the subsequent occurrence of natural mutations that restore conserved codons (40). However, the gain of new editing sites is not easy to elucidate unless we postulate that the binding sequence of the site recognition factors have some potential latitude, as was predicted by Covello and Gray (41). Figure 6 illustrates a hypothetical scheme for the diversification process of plant organellar editing sites. On the condition that preexisting editing sites are recognized by their respective trans-acting factors, new T-to-C transitions in the organellar genome are neutral only when their upstream cis-sequences are recognized by preexisting trans-acting factors, allowing the mutated C to be converted to U at the mRNA level. This study demonstrated that, in the case of ndhB-9 and ndhF-1, 60% sequence identity in the cis-region between −15 and −1 is enough for such corecognition to occur. Once such corecognition occurs between preexisting and newborn editing sites, those T-to-C mutations could be stochastically fixed in the organellar genome.
Figure 6.
Putative diversification process of chloroplast RNA editing sites during plant evolution. T-to-C transitions are permissible when their upstream sequences are similar to those of preexisting editing sites.
The above speculative model for the proliferation and sliding of the editing sites (Figure 6) predicts that RNA editing machineries of plant organelles have some potential to cope with exogenously introduced editing sites. We know that such examples exist. The spinach ndhA site I (ndhA-189) was edited when introduced into the chloroplasts of Nicotiana tabacum or Nicotiana sylvestris, although these plants do not have ndhA-189 editing sites (42,43). As the immediate upstream regions (from −15 to −1) of spinach ndhA-189 and tobacco ndhF-1 share 60% sequence identity, ndhA-189 may also be recognized by p95, similarly to ndhB-9 and ndhF-1. This possibility remains to be examined.
In conclusion, this study clearly demonstrated that two RNA editing sites with cis-acting elements of moderate sequence identity are recognized by the same trans-acting factor in tobacco chloroplasts. This finding extends our knowledge that distinct proteins recognize each editing site (8,12,14,15), and suggests that more complex cis-trans recognition networks might be operating in plant organelles.
ACKNOWLEDGEMENTS
We thank M. Sugiura, T. Miyamoto, T. Sasaki, T. Hirose, T. Ideue, T. Nakamura and M. Yukawa for useful discussions and encouragements. This work was supported in part by grants from the Ministry of Education, Science, Sports and Culture of Japan, from the DAIKO Foundation (No.9106), and from the NOVARTIS Foundation (Japan) for the Promotion of Science (No.18-103). Funding to pay the Open Access publication charges for this article was provided by the grants from the Ministry of Education, Science, Sports and Culture of Japan.
Conflict of interest statement. None declared.
REFERENCES
- 1.Smith HC, Gott JM, Hanson MR. A guide to RNA editing. RNA. 1997;3:1105–1123. [PMC free article] [PubMed] [Google Scholar]
- 2.Bass BL, editor. Oxford University Press: Oxford; 2000. RNA Editing. [Google Scholar]
- 3.Bock R. RNA editing in plant mitochondria and chloroplasts. In: Bass BL, editor. RNA Editing. Oxford: Oxford University Press; 2001. pp. 38–60. [Google Scholar]
- 4.Shikanai T. RNA editing in plant organelles: machinery, physiological function and evolution. Cell Mol. Life Sci. 2006;63:698–708. doi: 10.1007/s00018-005-5449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bock R, Kössel H, Maliga P. Introduction of a heterologous editing site into the tobacco plastid genome: the lack of RNA editing leads to a mutant phenotype. EMBO J. 1994;13:4623–4628. doi: 10.1002/j.1460-2075.1994.tb06784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasaki Y, Kozaki A, Ohmori A, Iguchi H, Nagano Y. Chloroplast RNA editing required for functional acetyl-CoA carboxylase in plants. J. Biol. Chem. 2001;276:3937–3940. doi: 10.1074/jbc.M008166200. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz-Linneweber C, Kushnir S, Babiychuk E, Poltnigg P, Herrmann RG, Maier RM. Pigment deficiency in nightshade/tobacco cybrids is caused by the failure to edit the plastid ATPase alpha-subunit mRNA. Plant Cell. 2005;17:1815–1828. doi: 10.1105/tpc.105.032474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotera E, Tasaka M, Shikanai T. A pentatricopetide repeat protein is essential for RNA editing in chloroplasts. Nature. 2005;433:326–330. doi: 10.1038/nature03229. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhuri S, Maliga P. Sequences directing C to U editing of the plastid psbL mRNA are located within a 22 nucleotide segment spanning the editing site. EMBO J. 1996;15:5958–5964. [PMC free article] [PubMed] [Google Scholar]
- 10.Bock R, Hermann M, Kössel H. In vivo dissection of cis-acting determinants for plastid RNA editing. EMBO J. 1996;15:5052–5059. [PMC free article] [PubMed] [Google Scholar]
- 11.Bock R, Hermann M, Fuchs M. Identification of critical nucleotide positions for plastid RNA editing site recognition. RNA. 1997;3:1194–1200. [PMC free article] [PubMed] [Google Scholar]
- 12.Hirose T, Sugiura M. Involvement of a site-specific trans-acting factor and a common RNA-binding protein in the editing of chloroplast mRNAs: development of a chloroplast in vitro RNA editing system. EMBO J. 2001;20:1144–1152. doi: 10.1093/emboj/20.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reed ML, Peeters NM, Hanson MR. A single alteration 20 nt 5′ to an editing target inhibits chloroplast RNA editing in vivo. Nucleic Acids Res. 2001;29:1507–1513. doi: 10.1093/nar/29.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyamoto T, Obokata J, Sugiura M. Recognition of RNA editing sites is directed by unique proteins in chloroplasts: biochemical identification of cis-acting elements and trans-acting factors involved in RNA editing in tobacco and pea chloroplasts. Mol. Cell. Biol. 2002;22:6726–6734. doi: 10.1128/MCB.22.19.6726-6734.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyamoto T, Obokata J, Sugiura M. A site-specific factor interacts directly with its cognate RNA editing site in chloroplast transcripts. Proc. Natl. Acad. Sci. USA. 2004;101:48–52. doi: 10.1073/pnas.0307163101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayes ML, Reed ML, Hegeman CE, Hanson MR. Sequence elements critical for efficient RNA editing of a tobacco chloroplast transcript in vivo and in vitro. Nucleic Acids Res. 2006;34:3750–3762. doi: 10.1093/nar/gkl490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes ML, Hanson MR. Identification of a sequence motif critical for editing of a tobacco chloroplast transcript. RNA. 2007;13:1–8. doi: 10.1261/rna.295607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Small ID, Peeters N. The PPR motif – a TPR-related motif prevalent in plant organellar proteins. Trends Biochem. Sci. 2000;25:46–47. doi: 10.1016/s0968-0004(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 19.Okuda K, Nakamura T, Sugita M, Shimizu T, Shikanai T. A pentatricopeptide repeat protein is a site recognition factor in chloroplast RNA editing. J. Biol. Chem. 2006;281:37661–37667. doi: 10.1074/jbc.M608184200. [DOI] [PubMed] [Google Scholar]
- 20.Lurin C, Andrés C, Aubourg S, Bellaoui M, Bitton F, Bruyère C, Caboche M, Debast C, Gualberto J, et al. Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell. 2004;16:2089–2103. doi: 10.1105/tpc.104.022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verbitskiy D, Takenaka M, Neuwirt J, van der Merwe JA, Brennicke A. Partially edited RNAs are intermediates of RNA editing in plant mitochondria. Plant J. 2006;47:408–416. doi: 10.1111/j.1365-313X.2006.02794.x. [DOI] [PubMed] [Google Scholar]
- 22.Neuwirt J, Takenaka M, van der Merwe JA, Brennicke A. An in vitro RNA editing system from cauliflower mitochondria: editing site recognition parameters can vary in different plant species. RNA. 2005;11:1563–1570. doi: 10.1261/rna.2740905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takenaka M, Neuwirt J, Brennicke A. Complex cis-elements determine an RNA editing site in pea mitochondria. Nucleic Acids Res. 2004;32:4137–4144. doi: 10.1093/nar/gkh763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Merwe JA, Takenaka M, Neuwirt J, Verbitskiy D, Brennicke A. RNA editing sites in plant mitochondria can share cis-elements. FEBS Lett. 2006;580:268–272. doi: 10.1016/j.febslet.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Choury D, Farre JC, Jordana X, Araya A. Different patterns in the recognition of editing sites in plant mitochondria. Nucleic Acids Res. 2004;32:6397–6406. doi: 10.1093/nar/gkh969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farré JC, Leon G, Jordana X, Araya A. cis recognition elements in plant mitochondrion RNA editing. Mol. Cell. Biol. 2001;21:6731–6737. doi: 10.1128/MCB.21.20.6731-6737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaudhuri S, Carrer H, Maliga P. Site-specific factor involved in the editing of the psbL mRNA in tobacco plastids. EMBO J. 1995;14:2951–2957. doi: 10.1002/j.1460-2075.1995.tb07295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutz KA, Maliga P. Lack of conservation of editing sites in mRNAs that encode subunits of NAD(P)H dehydrogenase complex in plastids and mitochondria of Arabidopsis thaliana. Curr. Genet. 2001;40:214–219. doi: 10.1007/s002940100242. [DOI] [PubMed] [Google Scholar]
- 29.Tillich M, Funk HT, Schmitz-Linneweber C, Poltnigg P, Sabater B, Martin M, Maier RM. Editing of plastid RNA in Arabidopsis thaliana ecotypes. Plant J. 2005;43:708–715. doi: 10.1111/j.1365-313X.2005.02484.x. [DOI] [PubMed] [Google Scholar]
- 30.Giegé P, Brennicke A. RNA editing in Arabidopsis mitochondria effects 441 C to U changes in ORFs. Proc. Natl. Acad. Sci. USA. 1999;96:15324–15329. doi: 10.1073/pnas.96.26.15324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chateigner-Boutin AL, Hanson MR. Cross-competition in transgenic chloroplasts expressing single editing sites reveals shared cis-elements. Mol. Cell. Biol. 2002;22:8448–8456. doi: 10.1128/MCB.22.24.8448-8456.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugiura M, Shinozaki K, Zaita N, Kusuda M, Kumano M. Clone bank of the tobacco (Nicotiana tabacum) chloroplast genome as a set of overlapping restriction endonuclease fragments: mapping of eleven ribosomal protein genes. Plant Sci. 1986;44:211–216. [Google Scholar]
- 33.Kao C, Zheng M, Rüdisser S. A simple and efficient method to reduce nontemplated nucleotide addition at the 3′ terminus of RNAs transcribed by T7 RNA polymerase. RNA. 1999;5:1268–1272. doi: 10.1017/s1355838299991033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasaki T, Yukawa Y, Wakasugi T, Yamada K, Sugiura M. A simple in vitro RNA editing assay for chloroplast transcripts using fluorescent dideoxynucleotides: distinct types of sequence elements required for editing of ndh transcripts. Plant J. 2006;47:802–810. doi: 10.1111/j.1365-313X.2006.02825.x. [DOI] [PubMed] [Google Scholar]
- 35.Hirose T, Kusumegi T, Tsudzuki T, Sugiura M. RNA editing sites in tobacco chloroplast transcripts: editing as a possible regulator of chloroplast RNA polymerase activity. Mol. Gen. Genet. 1999;262:462–467. doi: 10.1007/s004380051106. [DOI] [PubMed] [Google Scholar]
- 36.Kahlau S, Aspinall S, Gray JC, Bock R. Sequence of the tomato chloroplast DNA end evolutionary comparison of solanaceous plastid genome. J. Mol. Evol. 2006;63:194–207. doi: 10.1007/s00239-005-0254-5. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki T, Yukawa Y, Miyamoto T, Obokata J, Sugiura M. Identification of RNA editing sites in chloroplast transcripts from the maternal and paternal progenitors of tobacco (Nicotiana tabacum): comparative analysis shows the involvement of distinct trans-factors for ndhB editing. Mol. Biol. Evol. 2003;20:1028–1035. doi: 10.1093/molbev/msg098. [DOI] [PubMed] [Google Scholar]
- 38.Rivals E, Bruyere C, Toffano-Nioche C, Lecharny A. Formation of the Arabidopsis pentatricopeptide repeat family. Plant Physiol. 2006;141:825–839. doi: 10.1104/pp.106.077826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hermann M, Bock R. Transfer of plastid RNA-editing activity to novel sites suggests a critical role for spacing in editing-site recognition. Proc. Natl. Acad. Sci. USA. 1999;96:4856–4861. doi: 10.1073/pnas.96.9.4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tillichi M, Lehwark P, Morton BR, Maier UG. The evolution of chloroplast RNA editing. Mol. Biol. Evol. 2006;23:1912–1921. doi: 10.1093/molbev/msl054. [DOI] [PubMed] [Google Scholar]
- 41.Covello PS, Gray MW. On the evolution of RNA editing. Trends Genet. 1993;9:265–268. doi: 10.1016/0168-9525(93)90011-6. [DOI] [PubMed] [Google Scholar]
- 42.Schmitz-Linneweber C, Tillich M, Herrmann RG, Maier RM. Heterologous, splicing-dependent RNA editing in chloroplasts: allotetraploidy provides trans-factors. EMBO J. 2001;20:4874–4883. doi: 10.1093/emboj/20.17.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tillich M, Poltnigg P, Kushnir S, Schmitz-Linneweber C. Maintenance of plastid RNA editing activities independently of their target sites. EMBO Rep. 2005;7:308–313. doi: 10.1038/sj.embor.7400619. [DOI] [PMC free article] [PubMed] [Google Scholar]