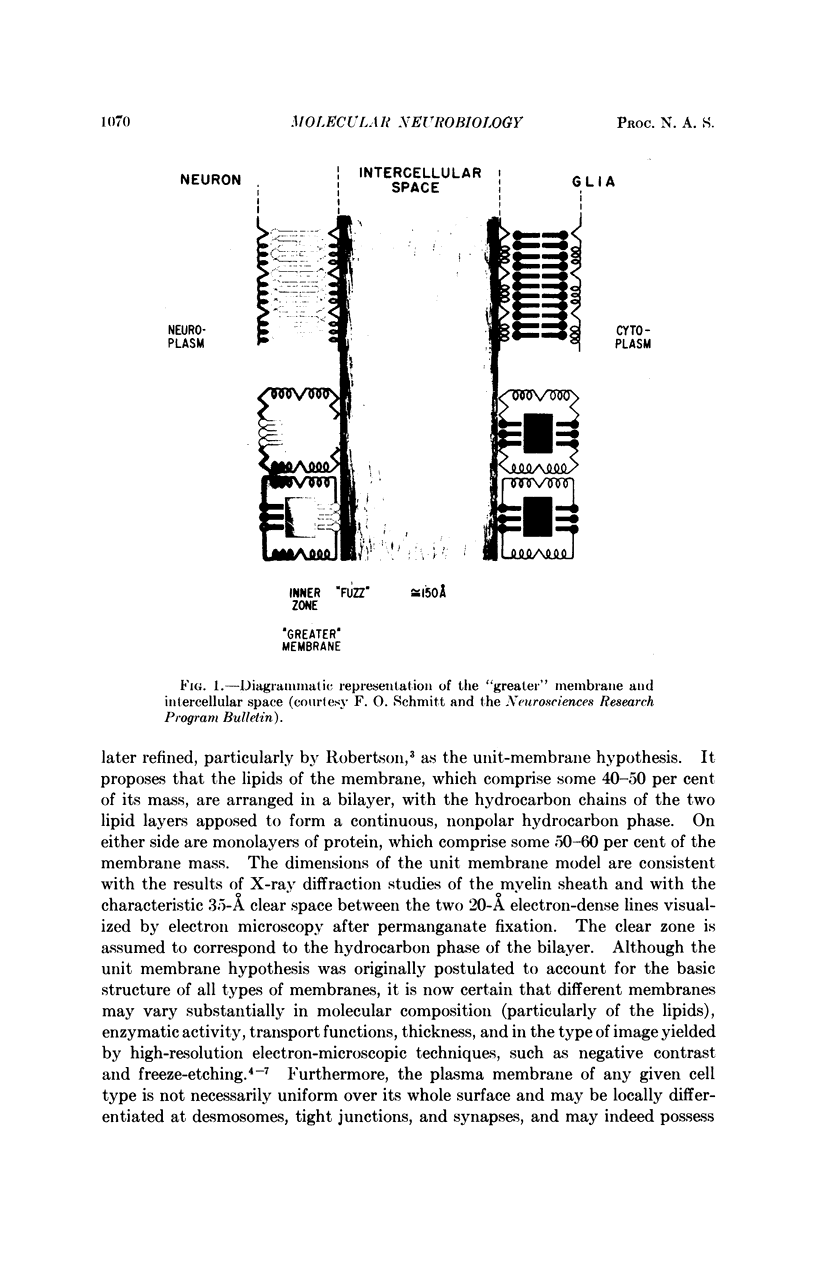
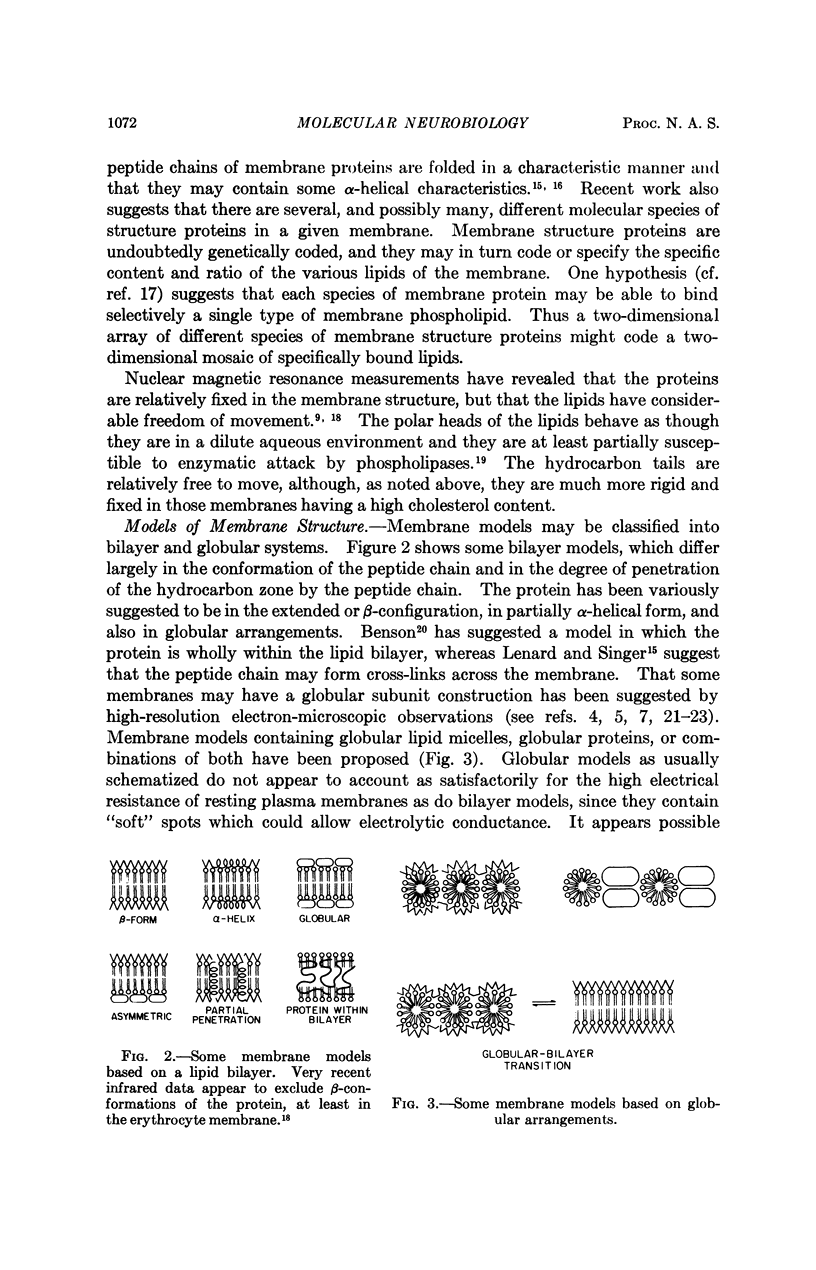
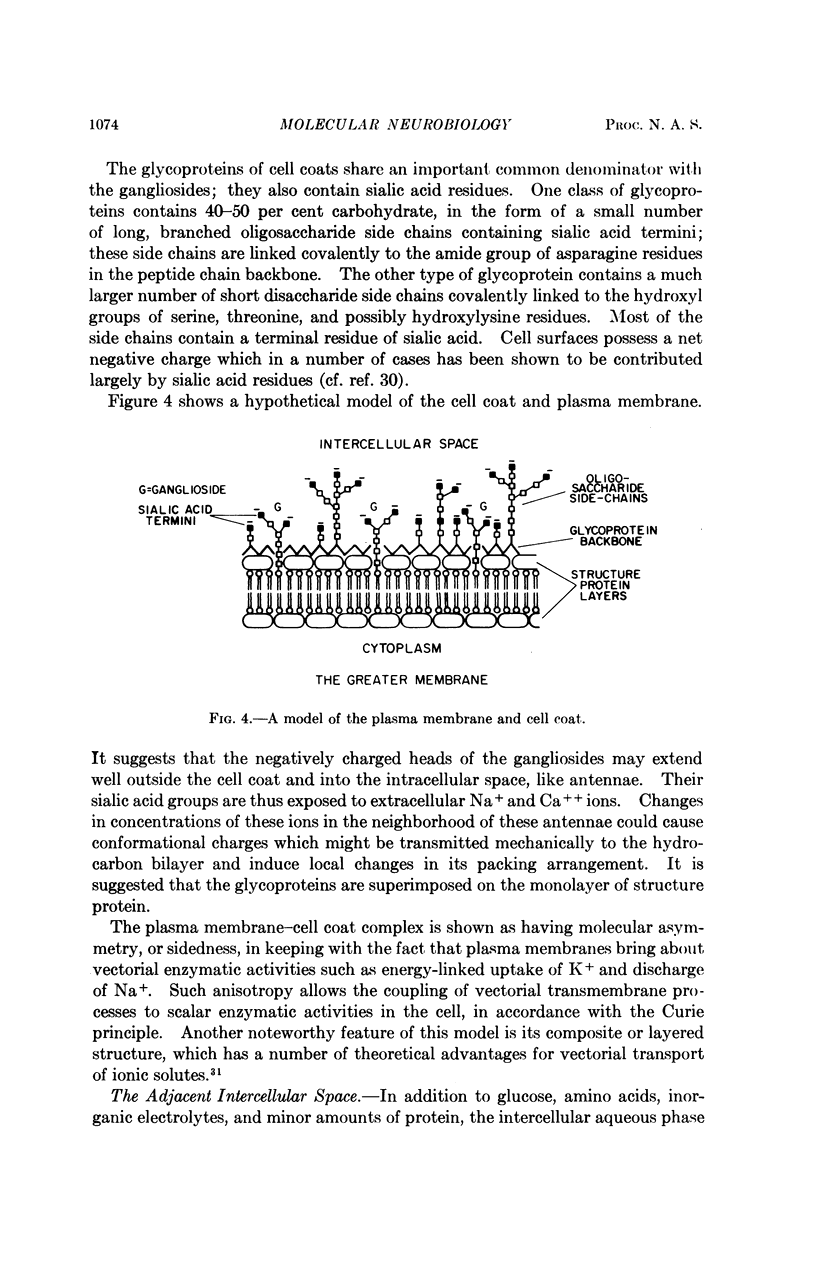
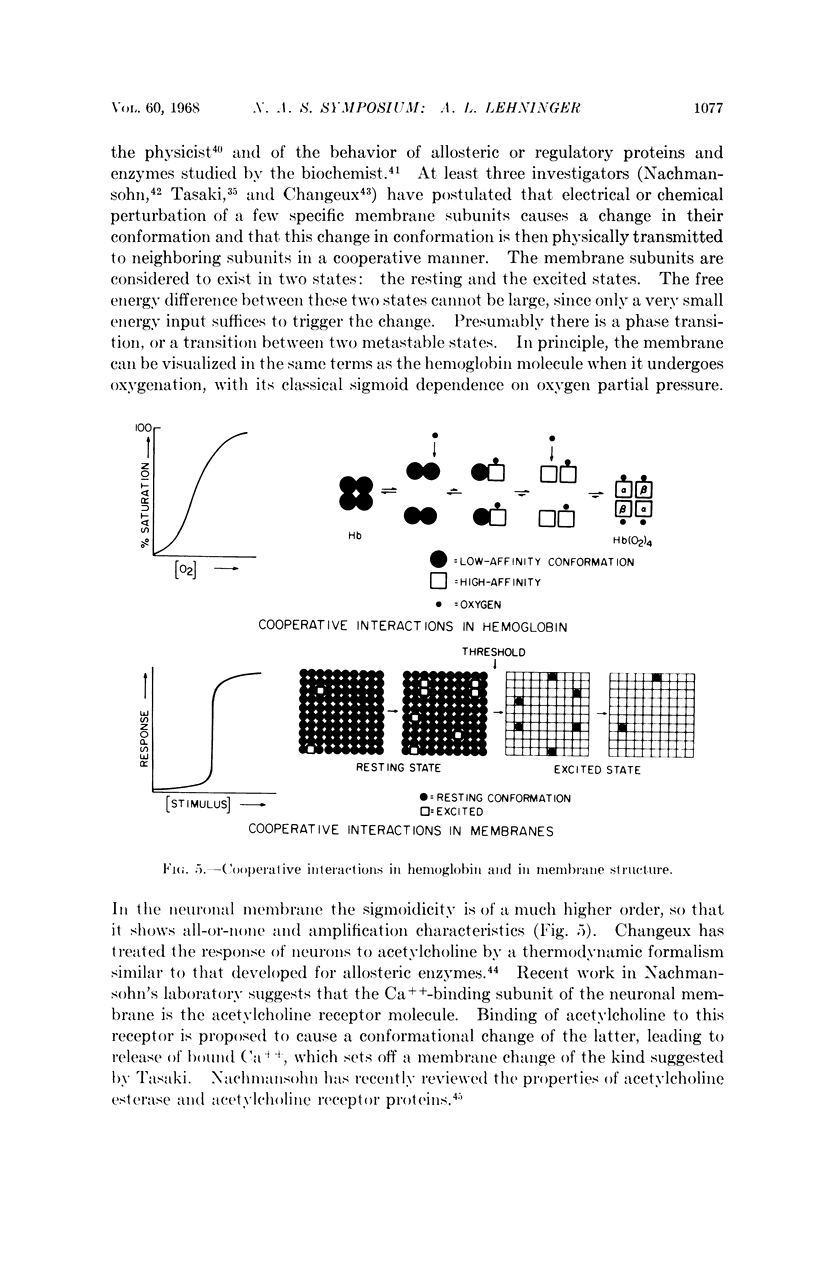
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barondes S. H. Incorporation of radioactive glucosamine into macromolecules at nerve endings. J Neurochem. 1968 Jul;15(7):699–706. doi: 10.1111/j.1471-4159.1968.tb08970.x. [DOI] [PubMed] [Google Scholar]
- Benson A. A. On the orientation of lipids in chloroplast and cell membranes. J Am Oil Chem Soc. 1966 May;43(5):265–270. doi: 10.1007/BF02609671. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Podleski T. R. On the excitability and cooperativity of the electroplax membrane. Proc Natl Acad Sci U S A. 1968 Mar;59(3):944–950. doi: 10.1073/pnas.59.3.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P. Responses of acetylcholinesterase from Torpedo marmorata to salts and curarizing drugs. Mol Pharmacol. 1966 Sep;2(5):369–392. [PubMed] [Google Scholar]
- Changeux J. P., Thiéry J., Tung Y., Kittel C. On the cooperativity of biological membranes. Proc Natl Acad Sci U S A. 1967 Feb;57(2):335–341. doi: 10.1073/pnas.57.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman D., Penkett S. A. Nuclear magnetic resonance spectroscopic studies of the interaction of phospholipids with cholesterol. Nature. 1966 Sep 17;211(5055):1304–1305. doi: 10.1038/2111304a0. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D., Hille B. Light scattering and birefringence changes during nerve activity. Nature. 1968 May 4;218(5140):438–441. doi: 10.1038/218438a0. [DOI] [PubMed] [Google Scholar]
- Derry D. M., Wolfe L. S. Gangliosides in isolated neurons and glial cells. Science. 1967 Dec 15;158(3807):1450–1452. doi: 10.1126/science.158.3807.1450. [DOI] [PubMed] [Google Scholar]
- Finean J. B. The molecular organization of cell membranes. Prog Biophys Mol Biol. 1966;16:143–170. doi: 10.1016/0079-6107(66)90005-8. [DOI] [PubMed] [Google Scholar]
- Hopfer U., Lehninger A. L., Thompson T. E. Protonic conductance across phospholipid bilayer membranes induced by uncoupling agents for oxidative phosphorylation. Proc Natl Acad Sci U S A. 1968 Feb;59(2):484–490. doi: 10.1073/pnas.59.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourn B. T., Dunitz J. D., Pioda L. A., Simon W. Structure of the K+ complex with nonactin, a macrotetrolide antibiotic possessing highly specific K+ transport properties. J Mol Biol. 1967 Dec 28;30(3):559–563. doi: 10.1016/0022-2836(67)90370-1. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Structure of biological membranes. Science. 1966 Sep 23;153(3743):1491–1498. doi: 10.1126/science.153.3743.1491. [DOI] [PubMed] [Google Scholar]
- LOWDEN J. A., WOLFE L. S. STUDIES ON BRAIN GANGLIOSIDES. IV. THE EFFECT OF HYPERCAPNIA ON GANGLIOSIDES IN VIVO. Can J Biochem. 1964 Dec;42:1703–1710. doi: 10.1139/o64-181. [DOI] [PubMed] [Google Scholar]
- Lardy H. A., Graven S. N., Estrada S. Specific induction and inhibition of cation and anion transport in mitochondria. Fed Proc. 1967 Sep;26(5):1355–1360. [PubMed] [Google Scholar]
- Laurent T. C. In vitro studies on the transport of macromolecules through the connective tissue. Fed Proc. 1966 May-Jun;25(3):1128–1134. [PubMed] [Google Scholar]
- Lehninger A. L., Carafoli E., Rossi C. S. Energy-linked ion movements in mitochondrial systems. Adv Enzymol Relat Areas Mol Biol. 1967;29:259–320. doi: 10.1002/9780470122747.ch6. [DOI] [PubMed] [Google Scholar]
- Lenard J., Singer S. J. Alteration of the conformation of proteins in red blood cell membranes and in solution by fixatives used in electron microscopy. J Cell Biol. 1968 Apr;37(1):117–121. doi: 10.1083/jcb.37.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., CHANGEUX J. P., JACOB F. Allosteric proteins and cellular control systems. J Mol Biol. 1963 Apr;6:306–329. doi: 10.1016/s0022-2836(63)80091-1. [DOI] [PubMed] [Google Scholar]
- Maddy A. H., Huang C., Thompson T. E. Studies on lipid bilayer membranes: a model for the plasma membrane. Fed Proc. 1966 May-Jun;25(3):933–936. [PubMed] [Google Scholar]
- Moore J. W., Narahashi T., Shaw T. I. An upper limit to the number of sodium channels in nerve membrane? J Physiol. 1967 Jan;188(1):99–105. doi: 10.1113/jphysiol.1967.sp008126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W., Narahashi T. Tetrodotoxin's highly selective blockage of an ionic channel. Fed Proc. 1967 Nov-Dec;26(6):1655–1663. [PubMed] [Google Scholar]
- Mueller P., Rudin D. O. Action potentials induced in biomolecular lipid membranes. Nature. 1968 Feb 24;217(5130):713–719. doi: 10.1038/217713a0. [DOI] [PubMed] [Google Scholar]
- Nachmansohn D. Chemical control of the permeability cycle in excitable membranes during electrical activity. Ann N Y Acad Sci. 1966 Jul 14;137(2):877–900. doi: 10.1111/j.1749-6632.1966.tb50207.x. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Induced active transport of ions in mitochondria. Proc Natl Acad Sci U S A. 1965 May;53(5):1076–1083. doi: 10.1073/pnas.53.5.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezbindowski K. S., Ruzicka F. J., Sun F. F., Crane F. L. A double layer of protein in mitochondrial cristae. Biochem Biophys Res Commun. 1968 Apr 19;31(2):164–169. doi: 10.1016/0006-291x(68)90724-9. [DOI] [PubMed] [Google Scholar]
- RICHARDSON S. H., HULTIN H. O., FLEISCHER S. INTERACTIONS OF MITOCHONDRIAL STRUCTURAL PROTEIN WITH PHOSPHOLIPIDS. Arch Biochem Biophys. 1964 May;105:254–260. doi: 10.1016/0003-9861(64)90006-2. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Luzzati V. X-ray diffraction study in water of lipids extracted from human erythrocytes: the position of cholesterol in the lipid lamellae. Biophys J. 1968 Jan;8(1):125–137. doi: 10.1016/S0006-3495(68)86479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. D. Granulo-fibrillar and globular substructure in unit membranes. Ann N Y Acad Sci. 1966 Jul 14;137(2):421–440. doi: 10.1111/j.1749-6632.1966.tb50174.x. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. THE DISTRIBUTION OF LIPIDS IN THE HUMAN NERVOUS SYSTEM. I. ANALYTICAL PROCEDURE. LIPIDS OF FOETAL AND NEWBORN BRAIN. J Neurochem. 1964 Dec;11:839–853. doi: 10.1111/j.1471-4159.1964.tb06735.x. [DOI] [PubMed] [Google Scholar]
- Wallach D. F., Kamat V. B. The contribution of sialic acid to the surface change of fragments of plasma membrane and endoplasmic reticulum. J Cell Biol. 1966 Sep;30(3):660–663. doi: 10.1083/jcb.30.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A., Tasaki I., Lerman L. Bi-ionic action potentials in squid giant axons internally perfused with sodium saltssalts. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2246–2252. doi: 10.1073/pnas.58.6.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A., Tasaki I., Singer I., Lerman L. Effects of tetrodotoxin on excitability of squid giant axons in sodium-free media. Science. 1967 Jan 6;155(3758):95–97. doi: 10.1126/science.155.3758.95. [DOI] [PubMed] [Google Scholar]



