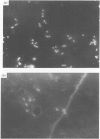
Abstract
Rabbit polyclonal hyperimmune antibodies to Yersinia pestis, and a mouse monoclonal antibody against the capsular antigen fraction 1 (F1) were compared in immunofluorescence (IF) tests. Fluorescent antibody conjugates were prepared from polyclonal antisera to four F1 positive Y. pestis strains; the conjugated antibody to strain A1122 gave the strongest IF staining of F1 positive and F1 negative Y. pestis strains. Indirect assays were rejected in favour of direct assays utilizing polyclonal and monoclonal reagents because the increased background staining reduced the effective contrast of bacterial visualisation. Polyclonal conjugates gave fairly homogeneous staining of Y. pestis bacterial populations, but in monoclonal assays a skew distribution of fluorescence intensity was observed, the majority of bacteria being poorly stained. The proportion of cells stained well by the monoclonal sufficed for easy identification of Y. pestis of the F1 positive phenotype however, and staining was not affected by washing the bacteria or treating them with formaldehyde. Y. pestis strains of the F1 positive genotype reacted with the monoclonal if bacteria were grown at 37 degrees C but not if the growth temperature was reduced to 25 degrees C thus preventing capsule production. The polyclonal conjugate reacted with bacteria of these strains that had been grown at either temperature. Strains of F1 negative genotype grown at either temperature reacted with the polyclonal conjugate but not with the monoclonal. Cross reactions between the polyclonal reagents and Y. enterocolitica biovar 2, serovar O 8 could not be removed by selective absorption; however, the monoclonal antibody gave no cross reaction. The F1 phenotypic status of bacterial preparations was verified by ELISA measurement of the fraction 1 antigen concentration. Antigen levels for F1 positive and F1 negative phenotypes differed by about three logs for suspensions of Y. pestis harvested from solid media. The polyclonal and monoclonal direct IF tests applied to spleen and blood smears of laboratory mice infected with Y. pestis were able to differentiate between lethal infection with an F1 positive strain carrying all four classical virulence determinants, an F1 positive vaccine strain, and an F1 negative strain.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER E. E., SOMMER H., FOSTER L. E., MEYER E., MEYER K. F. Studies on immunization against plague. I. The isolation and characterization of the soluble antigen of Pasteurella pestis. J Immunol. 1952 Feb;68(2):131–145. [PubMed] [Google Scholar]
- Bennett L. G., Tornabene T. G. Characterization of the antigenic subunits of the envelope protein of Yersinia pestis. J Bacteriol. 1974 Jan;117(1):48–55. doi: 10.1128/jb.117.1.48-55.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Portnoy D. A., Wolf-Watz H. Expression of the temperature-inducible outer membrane proteins of yersiniae. Infect Immun. 1985 Apr;48(1):234–240. doi: 10.1128/iai.48.1.234-240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carniel E., Mazigh D., Mollaret H. H. Expression of iron-regulated proteins in Yersinia species and their relation to virulence. Infect Immun. 1987 Jan;55(1):277–280. doi: 10.1128/iai.55.1.277-280.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Zahorchak R. J., Brubaker R. R. Plague virulence antigens from Yersinia enterocolitica. Infect Immun. 1980 May;28(2):638–640. doi: 10.1128/iai.28.2.638-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX E. N., HIGUCHI K. Synthesis of the fraction I antigenic protein by Pasteurella pestis. J Bacteriol. 1958 Feb;75(2):209–216. doi: 10.1128/jb.75.2.209-216.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B. W., Goldenberg M. I., McCluskie J. D., Larson H. E., McGuire C. D., Barnes A. M., Poland J. D. Serological and bacteriological investigations of an outbreak of plague in an urban tree squirrel population. Am J Trop Med Hyg. 1971 Mar;20(2):255–263. doi: 10.4269/ajtmh.1971.20.255. [DOI] [PubMed] [Google Scholar]
- Hudson B. W., Kartman L., Prince F. M. Pasteurella pestis detection in Fleas by fluorescent antibody staining. Bull World Health Organ. 1966;34(5):709–714. [PMC free article] [PubMed] [Google Scholar]
- MOODY M. D., WINTER C. C. Rapid identification of Pasteurella pestis with fluorescent antibody. III. Staining Pasteurella pestis in tissue impression smears. J Infect Dis. 1959 May-Jun;104(3):288–294. doi: 10.1093/infdis/104.3.288. [DOI] [PubMed] [Google Scholar]
- Mazza G., Karu A. E., Kingsbury D. T. Immune response to plasmid- and chromosome-encoded Yersinia antigens. Infect Immun. 1985 Jun;48(3):676–685. doi: 10.1128/iai.48.3.676-685.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A. P. An alternative to PPD for reducing the bleaching of fluorescein, evaluated in bacterial immunofluorescence assays. J Immunol Methods. 1984 Jan 20;66(1):189–190. doi: 10.1016/0022-1759(84)90262-x. [DOI] [PubMed] [Google Scholar]
- Phillips A. P., Martin K. L. Assessment of immunofluorescence measurements of individual bacteria in direct and indirect assays for Bacillus anthracis and Bacillus cereus spores. J Appl Bacteriol. 1982 Oct;53(2):223–231. doi: 10.1111/j.1365-2672.1982.tb04681.x. [DOI] [PubMed] [Google Scholar]
- Phillips A. P., Martin K. L., Capey A. J. Direct and indirect immunofluorescence analysis of bacterial populations by flow cytometry. J Immunol Methods. 1987 Aug 3;101(2):219–228. doi: 10.1016/0022-1759(87)90153-0. [DOI] [PubMed] [Google Scholar]
- Phillips A. P., Martin K. L. Dual-parameter scatter-flow immunofluorescence analysis of Bacillus spores. Cytometry. 1985 Mar;6(2):124–129. doi: 10.1002/cyto.990060207. [DOI] [PubMed] [Google Scholar]
- Phillips A. P., Martin K. L. Evaluation of a microfluorometer in immunofluorescence assays of individual spores of Bacillus anthracis and Bacillus cereus. J Immunol Methods. 1982 Mar 26;49(3):271–282. doi: 10.1016/0022-1759(82)90127-2. [DOI] [PubMed] [Google Scholar]
- QUAN S. F., KNAPP W., GOLDENBERG M. I., HUDSON B. W., LAWTON W. D., CHEN T. H., KARTMAN L. ISOLATION OF A STRAIN OF PASTEURELLA PSEUDOTUBERCULOSIS FROM ALASKA IDENTIFIED AS PASTEURELLA PESTIS: AN IMMUNOFLUORESCENT FALSE POSITIVE. Am J Trop Med Hyg. 1965 May;14:424–432. doi: 10.4269/ajtmh.1965.14.424. [DOI] [PubMed] [Google Scholar]
- Shepherd A. J., Hummitzsch D. E., Leman P. A., Swanepoel R., Searle L. A. Comparative tests for detection of plague antigen and antibody in experimentally infected wild rodents. J Clin Microbiol. 1986 Dec;24(6):1075–1078. doi: 10.1128/jcm.24.6.1075-1078.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER R. V. Studies on the immune response of guinea pigs to the envelope substance of Pasteurella pestis. I. Immunogenicity and persistence of large doses of fraction I in guinea pigs observed with fluorescent antibody. J Immunol. 1962 Feb;88:153–163. [PubMed] [Google Scholar]
- WINTER C. C., CHERRY W. B., MOODY M. D. An unusual strain of Pasteurella pestis isolated from a fatal human case of plague. Bull World Health Organ. 1960;23:408–409. [PMC free article] [PubMed] [Google Scholar]
- WINTER C. C., MOODY M. D. Rapid identification of Pasteurella pestis with fluorescent antibody. II. Specific identification of Pasteurella pestis in dried smears. J Infect Dis. 1959 May-Jun;104(3):281–287. doi: 10.1093/infdis/104.3.281. [DOI] [PubMed] [Google Scholar]
- Williams J. E., Arntzen L., Tyndal G. L., Isaäcson M. Application of enzyme immunoassays for the confirmation of clinically suspect plague in Namibia, 1982. Bull World Health Organ. 1986;64(5):745–752. [PMC free article] [PubMed] [Google Scholar]
- Williams J. E., Cavanaugh D. C. Chronic infections in laboratory rodents from inoculation of nonencapsulated plague bacilli (Yersinia pestis). Experientia. 1983 Apr 15;39(4):408–409. doi: 10.1007/BF01963151. [DOI] [PubMed] [Google Scholar]
- Williams J. E., Cavanaugh D. C. Potential for rat plague from nonencapsulated variants of the plague bacillus (Yersinia pestis). Experientia. 1984 Jul 15;40(7):739–740. doi: 10.1007/BF01949752. [DOI] [PubMed] [Google Scholar]
- Williams J. E., Gentry M. K., Braden C. A., Leister F., Yolken R. H. Use of an enzyme-linked immunosorbent assay to measure antigenaemia during acute plague. Bull World Health Organ. 1984;62(3):463–466. [PMC free article] [PubMed] [Google Scholar]