Abstract
Entry of retroviruses into host cells requires the fusion between the viral and cellular membranes. It is unclear how receptor binding induces conformational changes within the surface envelope protein (SU) that activate the fusion machinery residing in the transmembrane envelope protein (TM). In this report, we have isolated a point mutation, Q252R, within the proline-rich region of the 4070A murine leukemia virus SU that altered the virus-cell binding characteristics and induced cell-cell fusion. Q252R displays a SU shedding-sensitive phenotype. Cell-cell fusion is receptor dependent and is observed only in the presence of MuLV Gag-Pol. Both cellular binding and fusion by Q252R are greatly enhanced in conjunction of G100R, a mutation within the SU variable region A which increases viral binding through an independent mechanism. Deletion of a conserved histidine (His36) at the SU N terminus abolished cell-cell fusion by G100R/Q252R Env without compromising virus-cell binding. Although G100R/Q252R virus has no detectable titer, replacement of the N-terminal nine 4070A SU amino acids with the equivalent ecotropic MuLV sequence restored viral infectivity. These studies provide insights into the functional cooperation between multiple elements of SU required to signal receptor binding and activate the fusion machinery.
Entry of membraned viruses into host cells is initiated by attachment of the virus to specific receptors on the cell surface, followed by fusion of the viral and target cell membranes. For retroviruses, binding and fusion are mediated by the viral envelope protein (Env), consisting of two polypeptides: the surface (SU) and the transmembrane (TM) proteins. SU and TM are synthesized as a single precursor protein and proteolytically processed by cellular machinery (34). The TM cytoplasmic tail is further processed by the viral protease, releasing a 16-amino-acid peptide, termed the R-peptide (18). An Env protein complex on the viral surface consists of a trimer of SU/TM heterodimers (33).
Murine leukemia virus (MuLV) isolates are classified into four subgroups: ecotropic, amphotropic, polytropic, and xenotropic, based on their receptor usage (40). Among these subgroups, the Env protein sequence varies to utilize different receptors. Receptor specificity and binding of Env are mapped to the N-terminal half of the SU protein (receptor binding domain [RBD]) through the close interaction of two highly variable regions, VRA and VRB (3, 6, 14, 20). Interaction with TM through both covalent and noncovalent interactions has been mapped to the C-terminal half of SU (17, 39). A proline-rich linker region (PRR) joins the N- and C-terminal domains of the SU protein (38). The TM protein contains the minimal membrane fusion machinery (11, 15, 51).
The retroviral entry process requires the complex orchestration of multiple interactions between the SU and TM proteins through metastable conformations. Although there is considerable information describing the fusion-active conformation of the TM, the post-receptor binding configuration of SU is poorly understood. Conformational changes within the SU protein following receptor binding were shown in avian sarcoma/leukosis virus by limited proteolysis (10) and in human immunodeficiency virus (HIV) by antibody epitope mapping (46). Upon receptor binding, the MuLV SU transforms from a fusion-inhibitory conformation into a fusion-active conformation (4). For MuLV, several domains within SU have been proposed to play a role in transmitting the receptor-binding signal to trigger fusion. Elements that regulate fusion have been found to reside at the very N-terminal regions (NTR) and within the PRR of SU (2, 27, 48). Second-site mutations within the PRR were identified which restored the fusion defect of an NTR His mutation (50). Recent studies with either chimeric MuLV Env proteins or transcomplementation using soluble RBDs suggest the interaction between the RBD and the C terminus of SU is require for fusion activation (5, 28, 32). All these studies support a model in which multiple elements within SU regulate the structural transitions of the Env protein. However, an ordered mechanism of these transitional states and a direct link between these functional motifs and the receptor-binding region have not been identified.
Reverse transcription of the retroviral RNA genome generates a large pool of variants. These quasispecies can evolve based on viral fitness and selective pressure. Replication of chimeric MuLV Env variants is more restricted in D17 cells than in NIH 3T3 cells (36) and facilitates the isolation of gain-of-function mutations within Env (31). Passage of virus containing diverse chimeric MuLV Envs in these cells result in the acquisition of defined second-site mutations that are clustered at limited positions within the env gene (35).
In this report, we analyze a point mutation, Q252R, isolated by passage of a chimeric amphotropic MuLV bearing the NTR of ecotropic M-MuLV (IIA) (32). Q252R displays the combined phenotypes of altered cellular binding, increased susceptibility to SU shedding, and up-regulation of cell-cell fusion. The combinatorial effects of Q252R with additional mutations, including G100R (VRA), IIA, and ΔH36 (NTR), are examined in the context of viral entry.
MATERIALS AND METHODS
DNA manipulation.
Amplification of the env gene from Hirt extracted DNA (22) using primers 3807 and 6320 was previously described (32). The mutation Q252R was isolated by sequencing the population of PCR products using AmpliCycle (Applied Biosystems) and subcloned in the proviral vector pNCAC-Am (36) by replacing the 1.2-kb EcoRI-ClaI fragment. This was subsequently moved into the 4070A Env-expressing plasmid pHIT456 (44) by a 1.4-kb XhoI-NheI fragment exchange. Generation of chimera IIA was previously described (32). The His36 deletion was generated by overlapping PCR using primers ΔH5 (5′AGCCCCCAGGTC-TTTAATGTAACC) and ΔH3 (5′TACATTAAAGACCTGGGGGCTCTC). Primers 9312 (5′GGTCTAGAGGCCGACA-CCCAGAGTG) with an XbaI site (underlined) and 6320 served as outside primers. The PCR products were subcloned in the Env expression plasmid pHIT456 by 2-kb XbaI-NheI fragment exchange. All constructs were confirmed by DNA sequencing.
Cell lines and transfection.
Unless specified, all cell lines used were maintained in Dulbecco's modified Eagle medium supplemented with 7.5% fetal bovine serum (Gibco). 293T and D17 gag-pol cell lines were maintained in 0.4 mg of G418/ml, and TELCeB6 cell medium was supplemented with 6 μg of blasticidin S/ml. D17 gag-pol/ψGIP was constructed by stable transfection of D17 gag-pol cells with a plasmid expressing green fluorescent protein (GFP), pGIP (ψ-GFP-IRES-Pur), encoding the MuLV packaging signal flanking the MuLV long terminal repeats (9) and maintained in 1.5 μg of puromycin/ml. D17/GFP cells were generated by transfection of pGIP and selection with puromycin. D17/GFP cells were also used to establish the D17/GFP/4070A cell line, by infection of the D17/GFP cells with 4070A virus from an NIH 3T3 virus producer cell line. Challenge with 4070A virus on D17/GFP/4070A cells did not yield a detectable titer, indicating complete blockage of the receptor.
For passage of viable virus, 0.5 μg of MuLV proviral DNA with mutations of interest was introduced into 2 × 105 cells using the DEAE-dextran method (35). GFP-transducing virus was produced by introducing 10 μg of Env-expressing plasmids into 2 × 105 D17 gag-pol/ψGIP cells using Lipofectamine (Gibco), induced with 10 mM sodium butyrate for 10 h, and virus was collected 48 h posttransfection in fresh medium lacking puromycin or G418.
Cell-cell fusion (syncytium formation) analysis.
Syncytia in D17 gag-pol cells were directly observed 40 h after transfection of env constructs. Receptor dependence was performed as follows. Twenty-four hours after D17 gag-pol cells were transfected, cells were split at 105 cells per plate and cocultured with the equivalent numbers of either D17/GFP cells or D17/GFP/4070A cells. Syncytia were detected after an additional 8 h.
Virus titer, Western blot analysis of virus-associated proteins, and virus binding assays.
Forty-eight hours posttransfection of the env plasmid into D17 gag-pol cells, viral supernatant was collected and passed through a 0.45-μm-pore-size filter. Titer of virus was expressed as infectious units (IU) of GFP/ml of supernatant, scored 48 h after infection in the presence of 8 μg of Polybrene/ml. For detection of virus-associated proteins, virus was concentrated by pelleting through a 1/5 volume of 20% sucrose-TNE (10 mM Tris-HCl [pH 7.5], 50 mM NaCl, 1 mM EDTA) at 13,250 × g (13,000 rpm in an Eppendorf centrifuge) for 45 min. Alternatively, virus was pelleted without sucrose for 857 × g for 5 h (3,000 rpm in a Beckman Allegra 21R centrifuge). Viral pellets were resuspended and analyzed by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and then transferred onto polyvinylidene difluoride membranes. Western blots were performed with anti-MuLV SU 79S-842 and anti-MuLV CA 75S-287 (NCI-BCB Repository; distributed by Microbiological Associates, Inc.). The virus-binding assay was performed as previously described (32), using antibody m83A25 (13).
RESULTS
Isolation of the Q252R mutation.
Of key importance for gene delivery protocols is the titer of the infecting virus. Using retroviral vector systems, two chimeric MuLV Env proteins (IA and IIA), which exchanged the 4070A SU N terminus with that of ecotropic M-MuLV, were identified with a 7- to-10-fold-higher titer than virus bearing the wild-type 4070A Env (32). IIA replaced the 40 N-terminal 4070A SU amino acids with the corresponding 45 amino acids of the M-MuLV (ecotropic) sequence (Fig. 1A). This region encompasses a conserved histidine (H36 of 4070A; H41 of Moloney) found to regulate fusion in both ecotropic and amphotropic MuLVs (2). The increase in titer observed for IIA occurred without an increase in Env incorporation or receptor binding, implying that a postbinding process regulated by the His residue may be altered. In contrast to viral vectors, Env proteins expressed within replication-competent virus are capable of evolution through the pool of quasispecies generated during the reverse transcription process. Using this system, mutations that modify the properties of IIA can evolve during viral passage. The IIA Env was thus expressed in a replication-competent MuLV backbone and passaged in canine D17 cells to examine the stability of the IIA env gene.
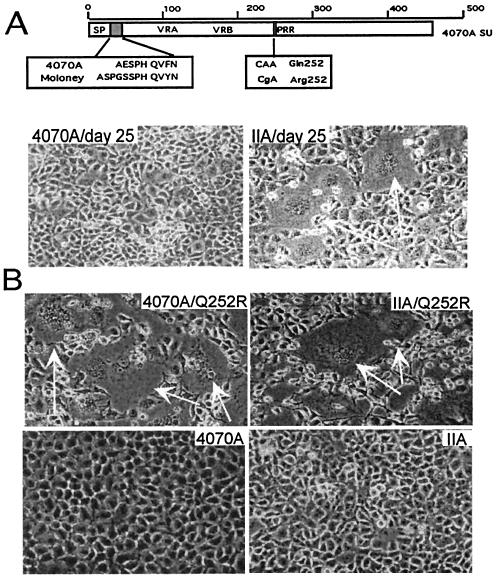
FIG. 1.
Isolation of the Q252R mutation. (A) Identification of Q252R: a schematic diagram of IIA SU is shown in the top panel. The open box region represents the 4070A sequence of SU, with general features denoted. The shaded box indicates the M-MuLV sequence replacing the 4070A N terminus of the mature protein. Sequence comparison between Moloney and 4070A MuLV Env sequences up to junction II is shown. Q252R was found at the PRR of Env in the virus cDNA population, as a result of single point mutation from CAA to CgA. The lower panel shows 4070A-bearing (left) and IIA-bearing (right) viruses at day 25 of passage. White arrows indicate syncytia observed in IIA. (B) Passage of Q252R/IIA and Q252R/4070A virus in D17 cells resulted in cell-cell fusion at the onset of active replication, day 5. The lower photos are cells carrying their parental virus at the same date.
Replication of viral MuLV bearing IIA env was not delayed in D17 cells. However, an altered phenotype was observed after 25 days' passage in tissue culture. Unlike the case with D17 cells carrying wild-type 4070A virus, massive cell-cell fusion (syncytium) was observed for D17 cells carrying IIA virus (Fig. 1A). Viral cDNA at the time of syncytium was isolated and sequenced, revealing two mutations: G100R and Q252R. The G100R mutation was previously identified after passage of 4070A virus in D17 cells without causing syncytium (31, 35) and therefore was not directly responsible for this phenotype.
The Q252R mutation was subcloned into MuLV proviruses encoding either the IIA or the 4070A Env. At the first detection of virus replication (day 5 by reverse transcriptase assay) in D17 cells, massive syncytia were observed with both Q252R/4070A and Q252R/IIA viruses (Fig. 1B) but not with their parental controls. Sequence analysis of viral DNA after passage of Q252R/4070A and Q252R/IIA in D17 cells revealed only the addition of G100R, establishing a direct correlation between the presence of Q252R and the up-regulated fusion of MuLV Env, the syncytium-inducing phenotype.
Q252R Env enhances both receptor binding and cellular fusion.
Env proteins bearing the Q252R mutation were introduced into the pHIT456 amphotropic Env expression vector (44). The direct effect of Q252R on viral binding was examined. Virus was produced by expressing the Env vector in D17 gag-pol cells and assayed for binding to NIH 3T3 target cells using a fluorescence-activated cell sorter (FACS)-based assay (24). The same panel of virus was also tested on D17 cells and yielded similar results with proportionally lower fluorescent intensity, characteristic of these cells (31). Binding was analyzed at 4°C to impede subsequent membrane fusion and internalization of virus-receptor complex. The level of bound virus was determined using a fluorescence-conjugated antibody against the SU protein (83A25) and analyzed by FACS (Fig. 2A). As previously observed, viruses bearing Env with the G100R mutation within the receptor-determining VRA region resulted in an increase in viral binding to target cells (31). Although it does not reside within the receptor-interacting VRA, interestingly, Q252R showed a strong increase in fluorescence intensity. Env with both G100R and Q252R showed a further enhancement in binding. The additive nature of G100R and Q252R suggests their independent mechanisms of action. TM protein from viruses was detected at the same level between G100R, Q252R, and the wild-type Env (data not shown), suggesting that the increased binding detected in G100R and Q252R is not due to an increase in Env synthesis and incorporation.
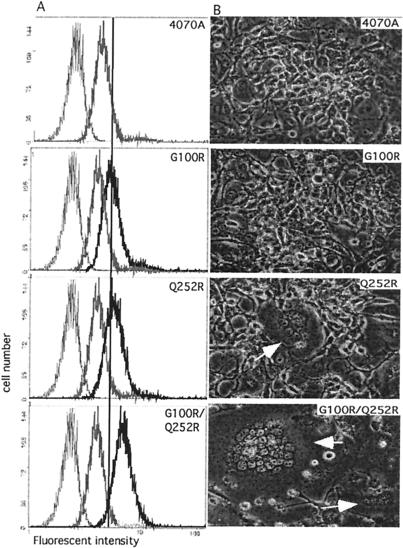
FIG. 2.
(A) Virus binding assays. Viruses with the Envs indicated at the upper right corner of each panel were used to bind NIH 3T3 cells, followed by antibody detection of conjugated viruses. Basal level, shown as a thin gray line in each panel, was assayed using mock-transfected D17 gag-pol cell supernatant. Binding level of 4070A is indicated by a thick gray line. Binding of each Env construct is shown as a thick black line in each panel. The straight reference line aligns the panels at the peak of G100R. (B) Syncytium formation in D17 gag-pol cells. env vectors indicated at the right upper corner of each panel were expressed in D17 gag-pol cells. Images were taken 48 h after the env constructs were introduced. Syncytia are indicated by white arrows. Magnification, ×320.
Distinct effects of cell-cell fusion were observed with the viral producer cells expressing these mutant Env proteins (Fig. 2B). At the time when virus was collected for analysis, small syncytia (5 to 10 nuclei) were observed on D17 gag-pol cells that express Q252R Env. Massive syncytium formation (more than 20 nuclei) was observed with cells that expressed the 4070A Env G100R/Q252R double mutant. In contrast, D17 gag-pol cells expressing either wild-type 4070A or G100R Env did not yield detectable syncytium. This fusion phenotype further distinguishes the roles of G100R and Q252R. G100R only affects binding without triggering fusion and is thus likely to involve an early event in viral entry. In contrast, Q252R is linked with fusion and, thus, the transition of Env function between a native, receptor-binding protein to a fusion-active state.
Q252R-mediated cell-cell fusion is dependent on expression of Gag-Pol and the cognate receptor.
Productive transmission of viral content requires the precise temporal regulation of the virion assembly, Env maturation, and content delivery to target cells. Steps known to regulate these processes include the maturation of the Env protein by the viral protease and binding to the cognate receptor. The requirements for cell-cell fusion with respect to Gag-Pol expression and receptor usage were therefore examined.
Cells in which syncytium formation was observed expressed MuLV Gag-Pol. To dissect the requirements for cell-cell fusion formation in canine D17 virus-producing cells, the env gene expression was uncoupled from the gag-pol genes. Syncytium formation induced by Env expression was compared in D17 cells and D17 gag-pol cells. In the absence of the gag-pol gene products, no syncytium was detected in D17 cells with the wild-type 4070A Env or Env proteins encoding either G100R, Q252R, or Q252R/G100R (Table 1).
TABLE 1.
Cell-cell fusion by Q252R Env
| Env | Cell-cell fusiona
|
|
|---|---|---|
| D17 | D17 gag-pol | |
| 4070A | − | − |
| G100R | − | − |
| Q252R | − | + |
| G100R/Q252R | − | +++ |
−, no syncytia was observed. +, <2 syncytia/field; +++, >10 syncytia/field. Magnification, ×40.
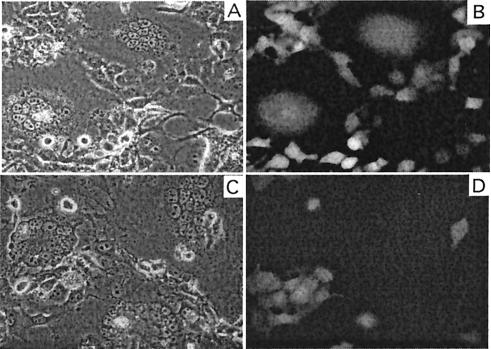
The requirement for the cognate 4070A receptor to establish cell-cell fusion was also tested. The experiment utilized viral interference, whereby the endogenous expression of a viral Env binds and blocks the accessibility of the receptor to binding of exogenous virus. D17 indicator cells were generated which expressed GFP in the presence or absence of 4070A virus (D17/GFP cells and D17/GFP/4070A cells); the latter express the 4070A virus that blocks the viral receptor. GFP-expressing cells capable of fusing with cells expressing fusogenic Env would form green fluorescent syncytia. G100R/Q252R Env was expressed in D17 gag-pol cells and cocultured with the GFP-expressing indicator cells. Green fluorescent syncytium was observed only in cocultivation of G100R/Q252R Env-expressing cells with D17/GFP cells (Fig. 3A and B) but not with D17/GFP/4070A cells (Fig. 3C and D). These results indicate that cell-cell fusion by G100R/Q252R Env in the D17 cells requires the 4070A receptor and maintains the stringent controls which are required for virus-cell fusion.
FIG. 3.
Receptor dependence of syncytium formation. D17 gag-pol cells transfected with G100R/Q252R Env expression plasmids are cocultivated with D17-GFP (A and B) and D17-GFP-4070A (C and D) cells. Photos are taken within 8 h of cocultivation. Panels B and D show fluorescence images under dark field of panels A and C.
The N terminus of the SU regulates fusion.
Q252R is selected in IIA virus, implying that fusion regulation by the ecotropic N terminus is not optimal for 4070A Env. The effect of the SU N-terminal region on G100R/Q252R Env was therefore examined. In addition to IIA, the conserved histidine residue at position 36 was deleted (ΔH) in the fusogenic G100R/Q252R Env. These constructs were introduced into D17 gag-pol cells to test their effects on binding and fusion.
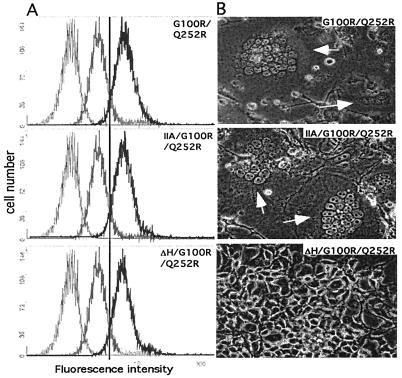
Consistent with other reports (2, 29, 32), we do not observe a role of the NTR in receptor binding. The binding of both ΔH/G100R/Q252R and IIA/G100R/Q252R viruses on NIH 3T3 cells yielded the same high level as the binding of the virus bearing G100R/Q252R Env (Fig. 4A), confirming the role of NTR in the postbinding regulatory process. However, IIA/G100R/Q252R and ΔH/G100R/Q252R Envs have distinct effects on virus-producing D17 gag-pol cells. Similar to the case with G100R/Q252R Env encoding the wild-type NTR, expressing the IIA/G100R/Q252R Env resulted in massive syncytia. In contrast, ΔH/G100R/Q252R yielded no detectable syncytium in these cells (Fig. 4B). These results indicate that the increased binding and fusion by the G100R/Q252R Env does not override the requirement for the histidine at the NTR of SU.
FIG. 4.
Effects of alteration in NTR of G100R/Q252R Env. (A) Virus binding assays. Different Envs use in the assay are indicated at the upper right corner of each panel. Basal level, shown as a thin gray line in each panel, was assayed using mock-transfected D17 gag-pol cell supernatant. The binding level of 4070A is indicated by a thick gray line. Binding of each Env construct is shown as thick black line in each panel. The reference line is drawn at the peak of G100R/Q252R. (B) Cell-cell fusion in D17 gag-pol cells transfected by the Env vectors indicated in each panel.
Of critical importance is whether the enhanced binding and fusion correlated with productive viral entry. The titer of virus bearing Env encoding G100R and Q252R in combination with NTR mutations was determined. Virus was collected from D17 gag-pol cells packaging the GFP marker gene. The titers of MuLV vectors pseudotyped with Env variants were determined by scoring for the transfer of GFP into D17 cells. Similar results were obtained using murine NIH 3T3 and human TE671 cells as target cells (data not shown), indicating that the effects do not reflect a specific adaptation to D17 cells. Results are shown in Table 2. The titer of G100R (4.1 × 105 IU/ml) was 10-fold higher than that of wild-type 4070A (4.3 × 104 IU/ml). In contrast, incorporation of Q252R in the wild-type 4070A backbone, even in the presence of G100R, reduced the titer of virus to <102 IU/ml. ΔH/G100R/Q252R Env was not capable of supporting virus entry, yielding extremely low titers (<102 IU/ml). This was consistent with the loss of cell-cell fusion by ΔH/G100R/Q252R Env in D17 gag-pol cells and confirmed the critical role of His36 in both virus-to-cell and cell-to-cell fusion (2). In contrast to G100R/Q252R and ΔH/G100R/Q252R, IIA/G100R/Q252R Env maintained a titer of 1.1 × 104 IU/ml. This was observed under conditions that induced massive syncytium formation in D17 gag-pol cells and enhanced cellular binding. Thus, the presence of the altered SU N terminus differentiates the ability of G100R/Q252R Env proteins to yield cell-cell fusion and productive infection. With equivalent levels of cellular binding, ΔH abolished cell-cell fusion of G100R/Q252R Env, while IIA restored G100R/Q252R Env to result in productive entry. Q252R was selected within a IIA backbone, indicating that the ecotropic N terminus contributes to the productive entry of the virus.
TABLE 2.
Function of Q252R-containing Env proteins
Q252R destabilizes the virus-associated SU.
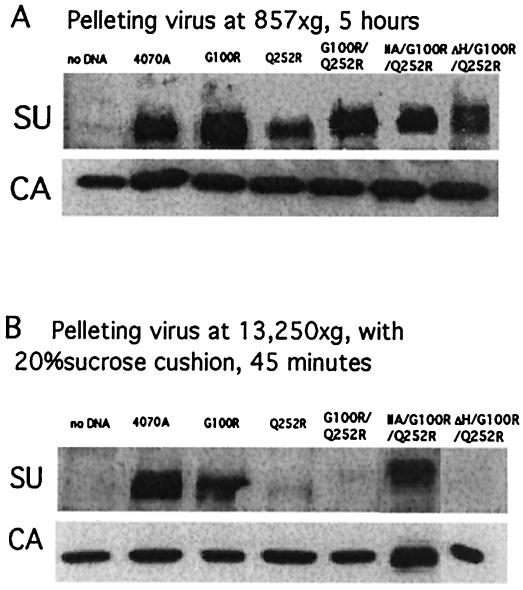
Western blot analyses were performed to examine the SU association with the virus particles. Several reports have shown that alterations in the PRR destabilized the Env complex and resulted in the loss of SU associated with viral preparations (27, 47). Virus-associated Env was therefore analyzed under two differing experimental conditions. Under lower centrifugal force (857 × g) for 5 h, SU proteins were detectable in all viral preparations, including those with Q252R, G100R/Q252R, IIA/G100R/Q252R, and ΔH/G100R/Q252R (Fig. 5A). The level of Env bearing Q252R alone was decreased; however, the levels of Env associated with G100R/Q252R and IIA/G100R/Q252R were comparable with those of the wild-type 4070A Env. The capsid protein was detected at equivalent levels in all the viral preparations, including supernatants from minus Env control, as a result of the constitutive release of virus-like particles from the retrovirus packaging cell lines. These results indicate that the Env bearing Q252R remains associated with the viral particles, and the divergent effects on binding, fusion, and titer are not simply a result of loss of Env from the viral particles.
FIG. 5.
Stability of virus-associated SU. Western blot analyses of virus-associated proteins. Viruses were prepared under two conditions: 857 × g, 5 h in the absence of sucrose (A) and 13,250 × g, 45 min plus 20% sucrose (B). The equivalent of 0.4 ml of virus medium was loaded in each lane.
Virus-associated SU was also analyzed with higher centrifugal force (13,250 × g) for 45 min and pelleted through a 20% sucrose cushion. Under these rigorous conditions, differential destabilization of the virus-associated SU (shedding) was observed. SU remains stably associated on virions with wild-type and G100R Env, whereas constructs bearing Q252R and G100R/Q252R were destabilized and dissociated from the viral particles (Fig. 5B). This destabilization was sensitive to the SU N terminus. Constructs containing the wild-type 4070A N terminus or ΔH released the SU in the presence of Q252R. In contrast, Env protein containing the ecotropic SU N terminus (IIA) remained tightly associated with the viral particles in the presence of the Q252R mutation. The level of virus-associated IIA/G100R/Q252R Env was comparable to levels on the wild-type 4070A virus under these centrifugation conditions.
Q252R Env sensitivity to shedding is revealed only by the pelleting protocol. The Q252R SU, however, does associate with virus prior to centrifugation. The stable association of SU observed in IIA indicates that the ecotropic N terminus has led to a stronger association with TM. This interaction is observed on the virion in the absence of receptor binding. This increased stability is balanced by the selection of Q252R during viral replication to activate the fusion process.
DISCUSSION
In this report, we analyzed the combined effects of mutations within SU selected during the passage of a chimeric MuLV in canine D17 cells. Our observation suggests that membrane fusion by TM is highly regulated through multiple elements within SU, including the receptor binding region, the NTR, and the PRR of SU protein. Table 2 summarizes the phenotypes of the mutations that were analyzed. The presence of G100R alone within 4070A Env enhances binding of virus to target cells, without triggering cell-cell fusion. Incorporation of the single point mutation Q252R into 4070A results in a large increase in the FACS-based viral binding assay, increased cell-cell fusion, and weakened SU stability on virions under defined conditions. The apparent increase in binding and fusion induced by Q252R was enhanced in the presence of the G100R mutation. The nature of the N-terminal region has profound effects on Env bearing G100R/Q252R. Virus bearing the 4070A NTR in the presence of G100R/Q252R was activated for cell-cell fusion, but the titer of virus was at background levels. The presence of the ecotropic N terminus (IIA) stabilized the G100R/Q252R SU on the virion and maintained viral titer in the presence of cell-cell fusion. In contrast, deletion of the NTR His abolished cell-cell fusion and detectable titer of virus and did not stabilize the SU-TM interaction. These results highlight the multiple elements within SU that regulate the onset of fusion following receptor binding.
Increased viral binding was observed with both the G100R and the Q252R mutations. These mutations act independently and are additive. The G100R mutation is within the VRA receptor binding region and displays increased binding without triggering fusion. The G100R mutation generates a heparin binding site consensus sequence, providing a potential mechanism for the increased binding observed. Q252R, though, does not create a consensus sequence for heparin binding. The binding shift induced by Q252R is distinct from that for G100R and correlates with increased fusogenicity. Several possible mechanisms can account for this observed increase. Env proteins with the Q252R mutation may generate an opened configuration, either allowing more monomers within the trimer to bind or exposing unknown secondary binding sites within a monomer. The increased binding may also reflect a tighter binding of the primary antibody to the viral SU, indicative of an altered Env conformation in the presence of Q252R. This model is greatly supported by the recent characterization of monoclonal antibody 83A25, used in the binding studies (8). The epitope for 83A25 maps to the C terminus of SU. Quite significantly, prebinding of 83A25 to MuLV virions blocks the formation of syncytia without affecting receptor binding. It is proposed that 83A25 blocks an undefined postattachment step necessary for virus-cell membrane fusion (8). The Q252R mutation within the PRR may results in an altered conformation exposing the epitope recognized by 83A25, leading to a strong shift in the fluorescent intensity scored in the binding assay. Alternatively, the conformation change may expose the fusion peptide, allowing the initial insertion within the host membrane, reminiscent of a fusion intermediate proposed for the influenza hemagglutinin (45) and for the Env protein complex of HIV (11). This would decrease the off rate of the virus from the cell surface. Increased hydrophobicity has been observed in other membrane virus Env proteins, supporting the model of exposing the fusion peptide. Liposome binding affinity can be induced by soluble receptor binding in Rous sarcoma virus (10) and avian sarcoma/leukosis virus (21); and in influenza virus by low-pH treatment (43). Without Q252R in Env, exposure of the fusion peptide would be impeded at 4°C on the cell surface and therefore not be observed in G100R alone or wild-type virus.
We do not believe that the increase binding represents receptor-independent attachment or binding of shed SU (49). The fluorescence shift detected in these binding assays is strictly dependent on the presence of the cognate viral receptor. The baseline for nonspecific attachment of MuLV viruses to cells lacking receptor is distinct from that for identically matched cells expressing the receptor (24). Moreover, Burkhart et al. has recently shown that the 83A25 antibody has a higher affinity for intact virions than monomeric SU (8). Binding due to shed SU would therefore yield a decrease in fluorescence intensity in the viral binding assay. In contrast, a large increase in fluorescence intensity was observed for Q252R Env over that for the wild-type 4070A protein. In addition, analysis of virus-associated proteins indicated that the SU remained associated with virion under mild centrifugal conditions. The susceptibility of SU to shedding was observed only under more severe conditions in which the virus was pelleted with higher centrifugal force through a sucrose cushion. Shedding was dependent on the SU N terminus. Constructs such as ΔH/G100R/Q252R and G100R/Q252R, which are sensitive to shedding, displayed the identical high level of binding as IIA/G100R/Q252R, which remained stably associated on the virion under the conditions tested. The increased binding observed for Env bearing Q252R using monoclonal antibody 83A25 is therefore more consistent with the binding of an intact virion with an altered Env conformation exposing the C-terminal SU epitope.
The Q252R Env mutant was identified as a gain-of-function variant activated for cell-to-cell fusion. Although the phenotype observed is cell-cell fusion, it cannot be ruled out that viral particles bridge cells to cause the fusion phenotype. Human T-cell leukemia viruses are proposed to enter through cell-to-cell rather than virus-cell contact (16). It is possible that Q252R-containing viruses, which share the shedding-prone phenotype with human T-cell leukemia viruses (19), propagate in tissue culture in a similar manner by creating a virological synapse (23). Quite interestingly, the fusogenic G100R/Q252R protein fails to mediate virus entry as judged by transfer of a vector by cell-free viral particles. However, within the context of an infectious provirus, active replication of the Q252R virus was observed. Unidentified mutations outside the env gene may also contribute to the viability of Q252R provirus. In influenza virus, mutations that alter entry properties can be found in capsid protein (7). Alternatively, factors to establish a virological synapse (23) may work in conjunction with the G100R/Q252R Env to support cell-cell transmission.
The PRR was initially viewed as a nonessential linker region in MuLV SU. However, it is now emerging as an active participant in relaying the receptor binding signal. Postbinding regulatory interaction between the N terminus and PRR of SU have been reported in an independent selection scheme, in which three mutations were identified simultaneously in the NTR (H41R) and the PRR (Q260R and D276Y) in ecotropic Moloney MuLV (M-MuLV) (50). Others have reported that mutations or deletions within the conserved region of the PRR altered the SU stability, decreased viral infectivity, and up-regulated cell-to-cell fusion in rat XC cells (27, 47). Q252R has these phenotypes plus the altered cellular binding, supporting an active role of the PRR during the entry process.
How can conformational changes within the PRR regulate fusion? Conformational changes within the PRR can juxtapose the SU N-terminal His (2, 5, 29) close to the SU-TM cystine bond and regulate the isomerization of cystine bonds (41). In MuLV, the covalent SU-TM interaction was mapped to a conserved CWLC motif at the C terminus of SU and a CX6CC motif of TM (39). It is reported that the interaction between the N- and C-terminal domains of SU is also important for Env fusion activation (5, 26, 32). It is intriguing that a WLC motif was also conserved in a D-peptide library shown to block HIV-induced cell-cell fusion by binding to a hydrophobic pocket formed in the HIV TM coiled-coil region (12). It is possible that CWLC in MuLV SU not only binds to TM but also prevents viral hairpin formation. Removal of the C terminus of SU from TM by the SU N terminus is therefore crucial for fusion to occur.
The independent isolation of Q252R in different chimeric Env backbones supports its role as a key regulatory position. The Q252R mutation was originally isolated in IIA. However, the defect of IIA is not visible in measuring virus replication. Q252R in the IIA Env did not result in an increase in titer. Q252R has also been identified by passage of a chimeric virus (AE6) encoding the 4070A Env through the middle of the C terminus of SU appended onto ecotropic M-MuLV sequence (35). The AE6 junction is between the conserved CWLC and a neutralizing epitope at the C-terminal cysteine loop that blocks fusion (8). Chimeric junctions within this region could uncouple these two motifs, resulting in the low titer of virus (<102 IU/ml) and the late onset of replication observed (36). The reconstruction of Q252R (Q252R/AE6) into env of a MuLV provirus resulted in improved replication of AE6 in D17 cells without triggering syncytium formation (32a) and yielded a high titer (104 IU/ml) in a vector system (data not shown). This suggests that Q252R can also assist in constructs bearing a defective SU C terminus. It is possible that IIA at the SU N terminus and AE6 at the C terminus of SU compromise a common interaction, causing the fusion to occur at a suboptimal rate.
Consistent with this model, Lavillette et al. (28) reported that a chimeric 4070A MuLV Env containing an ecotropic PRR resulted in a fusion-active conformation. Through introducing additional ecotropic motifs at either the N terminus or the C terminus of SU, fusion could be further activated for receptor-independent viral entry. It is likely that the PRR controls the interactions between the N and C termini of SU to activate fusion. However, we did not observe stabilization of SU on virions by deletion of the conserved NTR His. In the paper of Lavillette et al., ΔH stabilized mutations in which the entire PRR was replaced with ecotropic sequence (PRRMO) or a double mutation that resulted in an alteration in the number of predicted β-turns (A2) within the PRR (28). Using the same computer secondary-structure prediction algorithm (27), the number of β-turns in the presence of Q252R alone was the same as that of wild-type 4070A. However, it should be noted that in our studies, Q252R was selected as a gain-of-function mutation during IIA viral replication and may not be phenotypically equivalent to other PRR mutations.
Classically, MuLV Env-mediated cell-cell fusion was measured by coculturing Env-expressing cells with XC cells (37). Ecotropic MuLV forms syncytia with XC cells, but amphotropic MuLV does not. Amphotropic MuLV bearing Q252R Env did yield massive syncytia in XC cell cocultivation assays, consistent with the up-regulated fusogenicity. However, syncytium formation in XC cells was receptor independent and did not require expression of gag-pol (30), and therefore it bypassed the regulation of Env function required for supporting virus entry. Viral entry of Env bearing Q252R remains via the amphotropic receptor. D17 cells expressing 4070A MuLV block the infection of IIA/G100R/Q252R virus (data not shown). The cell-cell fusion by Q252R Env observed in D17 cells, therefore, maintains the regulation of the wild-type Env function and is therefore a preferred system for analysis.
Multiple elements within the Env protein ensure the temporal and spatial accuracy of membrane fusion following receptor binding. For HIV, stepwise binding of gp120 to various cell surface molecules ensures the entry of virus into the correct cell type for optimal infection (reviewed in reference 11). Influenza virus utilizes the low pH of the endosome as a mechanism for conformational changes in the Env protein following receptor binding (42). Our results described multiple elements within MuLV Env which are required to regulate membrane fusion, including VRA, PRR, the C-terminal domain, and the NTR of SU. These functional motifs are conserved among the related MuLV isolates.
The functional coordination within Env subdomains that we observed is not specific to D17 cell receptors but generally reflects Env function. Q252R-triggered cell-cell fusion was identified in D17 cells. However, the titer of virus on D17, TE671, and NIH3T3 cells was similar (data not shown). In addition, G100R/Q252R Envs showed an increase in receptor binding on both NIH 3T3 cells and D17 cells. The immediate application of these mutations on MuLV-based vector design can assist in higher titers as well as activation of fusion.
Based on the assumption that Env-mediated viral entry involves sequential steps, we propose a model for ordered conformational changes that involves each of the analyzed motifs within SU (Fig. 6). Illustrated in Fig. 6A, the SU N terminus (diagrammed as an L shape) and SU C terminus (oval) are connected by the PRR (zig-zags). The cleavage of R-peptide primes the Env for its fusion function (1). Residues encompassing the conserved His (IIA) are important for maintaining the fusion inhibitory conformation (4) of SU prior to receptor binding (Fig. 6A). Receptor binding then initiates a series of conformational changes. G100R alone enhances virus binding to target cells, without triggering cell-cell fusion (Fig. 6B). The fusion phenotype of Q252R suggests its involvement in a later stage of Env conformational change. Both virus binding and cell-cell fusion by Q252R are enhanced by incorporation of G100R, suggesting that the action of Q252R follows that of G100R (Fig. 6C). A conformational change induced via PRR could result in the repositioning of the N-terminal histidine in close proximity to the CWLC motif within the SU C terminus (32a), releasing the SU C terminus from TM by catalyzing the disulfide bond pattern switch at CWLC (Fig. 6D) (25). G100R/Q252R Env depends on the regulation of His36 at the SU N terminus to result in productive entry and syncytium formation. Deletion of His36 resulted in diminishing of cell-cell fusion with G100R/Q252R. After the dissociation of SU from TM, TM goes through spontaneous conformational changes that leads to the formation of the coiled-coil viral hairpin and eventually leads to fusion between viral and cellular membranes (Fig. 6E).
FIG. 6.
Model for sequential events in conformational changes in SU. The viral membrane containing the Env protein is shown as a light grey panel at the bottom, and target cell membrane (round) expressing receptor (linked circles indicating multiple transmembrane topology) is shown at the top. The panels show critical events in fusion activation of Env protein with mutations analyzed in the text denoted: maintaining the fusion inhibitory conformation (IIA) and Env maturation by R-peptide cleavage (A); receptor binding by VRA (G100R) (B); conformational changes within SU by PRR (Q252R) (C); release of SU from TM by the N terminus histidine (ΔH) (D); and viral hairpin formation of TM and membrane fusion (E).
Using this model, the selective pressures of IIA for the isolation of the G100R/Q252R mutations can be visualized. The presence of the ecotropic N terminus encoded within IIA stabilizes the SU-TM interactions and, thus, the fusion-inhibitory conformation within the virion. This stabilizing effect is distinct from the N- and C-terminal SU interaction which mapped downstream of the chimera II junction (32a). To counter the stability of SU-TM within IIA, Q252R is selected to activate the fusion process. This activated state would be consistent with the conformation of the entry intermediate depicted in Fig. 6C. This conformation would maintain recognition of the cognate receptor; however, it would have high affinity for antibody 83A25, which neutralizes an undefined postattachment step (8). Knowledge of the structure of the IIA/G100R/Q252R Env protein may provide unique insights into the potential intermediates formed during productive retroviral entry.
Acknowledgments
We thank Chavela Carr, Keith Bupp, and Jennifer Seamon for critical reading of the manuscript and K. Bupp and Joseph Dougherty for discussions on experimental design.
Support of this work comes from NIH grant R01 CA49932 to M.J.R.
REFERENCES
- 1.Anguliar, H. C., W. F. Anerson, and P. Cannon. 2003. Cytoplasmic tail of Moloney murine leukemia virus envelope protein influences the conformation of the extracellular domain: implications for mechanism of action of the R peptide. J. Virol. 77:1281-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae, Y., S. M. Kingsman, and A. Kingsman. 1997. Functional dissection of the Moloney murine leukemia virus envelope protein gp70. J. Virol. 71:2092-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett, A., D. Wensel, W. Li, D. Fass, and J. M. Cunningham. 2003. Structure and mechanism of a coreceptor for infection by a pathogenic feline retrovirus. J. Virol. 77:2717-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnett, A. L., and J. M. Cunningham. 2001. Receptor binding transforms the surface subunit of the mammalian C-type retrovirus envelope protein from an inhibitor to an activator of fusion. J. Virol. 75:9096-9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett, A. L., R. A. Davey, and J. M. Cunningham. 2001. Modular organization of the Friend murine leukemia virus envelope protein underlies the mechanism of infection. Proc. Natl. Acad. Sci. USA 98:4113-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battini, J.-L., J. M. Heard, and O. Danos. 1992. Receptor choice determinants in the envelope glycoproteins of amphotropic, xenotropic, and polytropic murine leukemia viruses. J. Virol. 66:1468-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, E. G., H. Liu, L. C. Kit, S. Baird, and M. Nesrallah. 2001. Patterns of mutation in the genome of influenza A virus on adaptation to increase virulence in the mouse lung: identification of functional themes. Proc. Natl. Acad. Sci. USA 98:6883-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkhart, M. D., S. C. Kayman, Y. He, and A. Pinter. 2003. Distinct mechanism of neutralization by monoclonal antibodies specific for sites in the N-terminal or C-terminal domain of murine leukemia virus SU. J. Virol. 77:3993-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, C.-C., A. Rivera, N. Ron, J. P. Dougherty, and Y. Ron. 2001. A gene therapy approach for treating T-cell-mediated autoimmune diseases. Blood 97:886-894. [DOI] [PubMed] [Google Scholar]
- 10.Damico, R. L., J. Crane, and P. Bates. 1998. Receptor-triggered membrane association of a model retroviral glycoprotein. Proc. Natl. Acad. Sci. USA 95:2580-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckert, D., and P. S. Kim. 2001. Mechanism of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 12.Eckert, D. M., V. N. Malashkevich, L. H. Hong, P. A. Carr, and P. S. Kim. 1999. Inhibiting HIV entry: discovery of D-peptide inhibitors that target the gp41 coil-coil pocket. Cell 99:103-115. [DOI] [PubMed] [Google Scholar]
- 13.Evans, L. H., R. P. Morrison, F. G. Malik, J. Portis, and W. Britt. 1990. A neutralizable epitope common to the envelope glycoprotein of ecotropic, polytropic, xenotropic, and amphotropic murine leukemia viruses. J. Virol. 64:6176-6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fass, D., R. A. Davey, C. A. Harmson, P. S. Kim, J. Cunningham, and J. M. Berger. 1997. Structure of a murine leukemia virus receptor-binding glycoprotein at 2.0 angstrom resolution. Science 277:1663-1666. [DOI] [PubMed] [Google Scholar]
- 15.Fass, D., S. C. Harrison, and P. S. Kim. 1996. Retrovirus envelope domain at 1.7 A resolution. Nat. Struct. Biol. 3:465-468. [DOI] [PubMed] [Google Scholar]
- 16.Fields, B. N., and D. M. Knipe. 1985. Virology, vol. 2. Raven Press, New York, N.Y.
- 17.Gray, K. D., and M. J. Roth. 1993. Mutational analysis of the envelope gene of Moloney murine leukemia virus. J. Virol. 67:3489-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green, N., T. M. Shinnick, O. Witte, A. Ponticelli, J. G. Sutchliffe, and R. A. Lerner. 1981. Sequence-specific antibodies show that maturation of Moloney murine leukemia virus envelope protein involves removal of a COOH-terminal peptide. Proc. Natl. Acad. Sci. USA 78:6023-6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gummuluru, S., C. M. Kinsey, and M. Emerman. 2000. An in vitro rapid turn-over assay for human immunodeficiency virus type 1 replication selections for cell-to-cell spread of virus. J. Virol. 74:10882-10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heard, J. M., and O. Danos. 1991. An amino-terminal fragment of the Friend murine leukemia virus envelope glycoprotein binds the ecotropic receptor. J. Virol. 65:4026-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandez, L. D., R. J. Peters, S. E. Delos, J. A. T. Young, D. A. Agard, and J. M. White. 1997. Activation of a retroviral membrane fusion protein: soluble receptor-induced liposome binding protein of the ASLV envelope glycoprotein. J. Cell Biol. 139:1455-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirt, B. 1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 26:365-369. [DOI] [PubMed] [Google Scholar]
- 23.Igakura, T., J. C. Stinchcombe, P. C. Goon, G. P. Taylor, J. N. Weber, G. M. Griffiths, Y. P. Tanaka, M. Osame, and C. R. M. Bangham. 2003. Spread of HTLV-1 between lymphocytes by virus-induced polarization of the cytoskeleton. Science 299:1713-1716. [DOI] [PubMed] [Google Scholar]
- 24.Kadan, M. J., S. Strum, W. F. Anderson, and M. A. Eglitis. 1992. Detection of receptor-specific murine leukemia virus binding to cells by immunofluorescence analysis. J. Virol. 66:2281-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kayman, S. C., H. Park, M. Saxon, and A. Pinter. 1999. The hypervariable domain of the murine leukemia virus surface protein tolerates large insertions and deletions, enabling development of a retroviral particle display system. J. Virol. 73:1802-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavillette, D., B. Boson, S. J. Russell, and F.-L. Cosset. 2001. Activation of membrane fusion by murine leukemia virus is controlled in cis or in trans by interactions between the receptor-binding domain and a conserved disulfide loop of the carboxy terminus of the surface glycoprotein. J. Virol. 75:3685-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavillette, D., M. Maurice, C. Roche, S. J. Russell, M. Sitbon, and F.-L. Cosset. 1998. A proline-rich motif downstream of the receptor binding domain modulates conformation and fusogenicity of murine retroviral envelopes. J. Virol. 72:9955-9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavillette, D., A. Ruggieri, B. Boson, M. Maurice, and F.-L. Cossset. 2002. Relationship between SU subdomains that regulate the receptor-mediated transition from native (fusion-inhibited) to fusion-active conformation of the murine leukemia virus glycoprotein. J. Virol. 76:9673-9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavillette, D., A. Ruggieri, S. J. Russell, and F.-L. Cosset. 2000. Activation of a cell entry pathway common to type C mammalian retrovirues by soluble envelope fragments. J. Virol. 74:295-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu, C.-W. 2003. Ph.D. thesis. University of Medicine and Dentistry of New Jersey, Piscataway, N.J.
- 31.Lu, C.-W., L. O'Reilly, and M. J. Roth. 2003. G100R mutation within 4070A murine leukemia virus Env increases virus receptor binding, kinetics of entry, and viral transduction efficiency. J. Virol. 77:739-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu, C.-W., and M. J. Roth. 2001. Functional characterization of the N termini of murine leukemia virus envelope protein. J. Virol. 75:4345-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Lu, C.-W., and M. J. Roth. 2003. Functional interaction between the N- and C-terminal domains of murine leukemia virus surface envelope protein. Virology 310:130-140. [DOI] [PubMed]
- 33.Moore, J. P., J. A. McKeating, R. A. Weiss, and Q. J. Sattentau. 1990. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science 250:1139-1142. [DOI] [PubMed] [Google Scholar]
- 34.Ng, L. V., T. G. Wood, and R. B. Arlinghaus. 1982. Processing of the env gene products of Moloney murine leukemia virus. J. Gen. Virol. 59:329-343. [DOI] [PubMed] [Google Scholar]
- 35.O'Reilly, L., and M. J. Roth. 2000. Second site changes affect viability of amphotropic/ecotropic chimeric enveloped murine leukemia viruses. J. Virol. 74:899-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peredo, C., L. O'Reilly, K. Gray, and M. J. Roth. 1996. Characterization of chimeras between the ecotropic Moloney murine leukemia virus and the amphotropic 4070A envelope proteins. J. Virol. 70:3142-3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinter, A., T.-E. Chen, A. Lowy, N. G. Cortez, and S. Silagi. 1986. Ecotropic murine leukemia virus-induced fusion of murine cells. J. Virol. 57:1048-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinter, A., W. J. Honnen, J. S. Tung, P. V. O'Donnel, and U. Hammerling. 1982. Structure domains of endogenous murine leukemia virus gp70s containing specific antigenic determinants defined by monoclonal antibodies. Virology 116:499-516. [DOI] [PubMed] [Google Scholar]
- 39.Pinter, A., R. Kopelman, L. Zhiyong, S. Kayman, and D. A. Sanders. 1997. Localization of the labile disulfide bond between SU and TM of the murine leukemia virus envelope protein complex to a highly conserved CWLC motif in SU that resembles the active site sequence of the thiol-disulfide exchange enzymes. J. Virol. 71:8073-8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rein, A. 1982. Interference grouping of murine leukemia viruses: a distinct receptor for MCF-recombinant viruses in mouse cells. Virology 120:251-257. [DOI] [PubMed] [Google Scholar]
- 41.Sanders, D. A. 2000. Sulfhydryl involvement in fusion mechanisms, p. 483-514. In H. J. Hilderson and S. Fuller (ed.), Subcellular biochemistry: fusion of biological membranes and related problems, vol. 34. Kluwer Academic/Plenum Publishers, New York, N.Y. [DOI] [PubMed]
- 42.Skehel, J., and D. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biol. Chem. 69:531-569. [DOI] [PubMed] [Google Scholar]
- 43.Skehel, J. J., P. M. Bayley, E. B. Brown, S. R. Martin, M. D. Waterfield, J. M. White, I. A. Wilson, and D. C. Wiley. 1982. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc. Natl. Acad. Sci. USA 79:968-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soneoka, Y., P. M. Cannon, E. E. Ramsdale, J. C. Griffiths, G. Romano, S. M. Kingsman, and A. J. Kingsman. 1995. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 23:629-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stegmann, T., J. M. White, and A. Helenius. 1990. Intermediates in influenza induced membrane fusion. EMBO J. 9:4231-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thali, M., J. P. Moore, C. Furman, M. Charles, D. D. Ho, J. Robinson, and J. Sodroski. 1993. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J. Virol. 67:3978-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu, B. W., P. M. Cannon, E. M. Gordon, F. L. Hall, and W. F. Anderson. 1998. Characterization of the proline-rich region of murine leukemia virus envelope protein. J. Virol. 72:5385-5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu, B. W., J. Lu, T. K. Gallaher, W. F. Anderson, and P. M. Cannon. 2000. Identification of regions in Moloney murine leukemia virus SU protein that tolerate the insertion of an integrin-binding peptide. Virology 269:7-17. [DOI] [PubMed] [Google Scholar]
- 49.Yu, H., C. E. Xia, and W. F. Anderson. 1998. Quantitation of MoMuLV envelope protein on the cell surface. Virology 243:415-422. [DOI] [PubMed] [Google Scholar]
- 50.Zavorotinskaya, T., and L. M. Abritton. 1999. Suppression of a fusion defect by second site mutations in the ecotropic murine leukemia virus surface protein. J. Virol. 73:5034-5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao, Y., L. Zhu, C. A. Benedict, D. Chen, W. F. Anderson, and P. Cannon. 1998. Functional domains in the retroviral transmembrane protein. J. Virol. 72:5392-5398. [DOI] [PMC free article] [PubMed] [Google Scholar]