Abstract
Infection with human T-cell leukemia virus type 1 (HTLV-1) is characterized by long latency periods, indicating that viral gene expression is under tight control. There is presently little information available regarding the nature of extracellular stimuli that can transactivate the regulatory elements of HTLV-1 (i.e., long terminal repeat [LTR]). To gain insight into the biological importance of externally induced activation pathways in virus gene expression, primary and established T cells were transfected with HTLV-1-based reporter gene vectors and then were treated with agents that cross-linked the T-cell receptor (TCR) or the costimulatory CD28 molecule with prostaglandin E2 (PGE2). We demonstrated that a potent induction of HTLV-1 LTR-driven reporter gene activity was seen only when the three agents were used in combination. Interestingly, similar observations were made when using C91/PL, a cell line that carries integrated HTLV-1 proviral DNA. This TCR-CD28-PGE2-mediated increase in virus transcription was dependent on protein kinase A activation and induction of the cAMP response element binding protein. Experiments with a mutated reporter construct further revealed the importance of the Tax-responsive elements in the HTLV-1 LTR in the observed up regulation of virus gene expression when TCR/CD28 engagement was combined with PGE2 treatment. The protein tyrosine kinases p56lck and the transmembrane tyrosine phosphatase CD45 were all found to be involved in TCR-CD28-PGE2-directed increase in HTLV-1 LTR activity. This study presents new information on the possible mechanisms underlying reactivation of this retrovirus.
Human T-cell leukemia virus (HTLV-1), the first isolated human pathogenic retrovirus, is the etiologic agent of diverse clinical syndromes. Indeed, this retroviral agent is responsible for an aggressive lymphoproliferative disorder termed adult T-cell leukemia (ATL), HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), and HTLV-1 uveitis (30, 60). Infection with HTLV-1 is characterized by a long latent period with minimal viral gene expression (67). Approximately 4% of infected individuals develop HTLV-1-associated diseases. Although the mechanism(s) through which this occurs is unclear, these diseases are thought to be associated with increased viral expression (23). Following infection, the HTLV-1 provirus integrates into the host genome, where it can remain latent for several decades. Transcription from the viral promoter produces unspliced and singly spliced mRNAs that encode the Gag, Pol, and Env proteins as well as other spliced mRNAs that encode Tax, Rex, and other important viral proteins. The regulation of basal transcription is thought to play an important role in viral latency.
The Tax protein is a potent transcription transactivator that regulates HTLV-1 replication and facilitates the transition from quiescent infection to high levels of virus production in T cells (22, 71, 72). Tax is also the transforming protein of HTLV-1, since it is necessary and sufficient for cell immortalization (reviewed in reference 48). The tax gene product can also affect expression of a large array of cellular and viral genes (7, 24, 69, 76). A wide variety of cellular factors interact with the promoter region of HTLV-1 localized in the long terminal repeat (LTR), such as members of the cAMP response element-binding protein (CREB)/activation transcription factor family, TIF, THP, HEBI, TFIID, SP1, and others (6, 8, 18, 39, 46, 52, 55, 77, 83). Tax does not bind to the HTLV-1 LTR region directly but rather associates via protein-protein interactions with cellular proteins associated with the viral promoter (13, 26, 45, 84). More specifically, Tax interacts with a cellular complex made of CREB and CREB-binding protein, allowing the formation of a strong, transcriptionally active complex which binds to three incomplete cyclic AMP-responsive element (CRE) sites located within 21-bp repeats, termed Tax-responsive elements (TRE) (4, 9, 25, 39, 70, 75). CREB is a 43-kDa basic-leucine zipper transcription factor whose transcriptional activity is regulated by phosphorylation of the single residue Ser 133, which is not required for Tax-mediated activation of this factor (35).
Prostaglandin E2 (PGE2), an oxygenated polyunsaturated fatty acid that contains a cyclopentane ring structure, is present in high concentrations in individuals infected with numerous pathogens (5, 20, 37, 41, 49, 58, 64, 65, 73, 79, 80). PGE2 molecules have been shown to modulate the immune response both in vitro and in vivo. For example, this hormone-like molecule has been implicated in decreasing T-cell proliferation, interleukin-2 (IL-2) production, and IL-2 receptor expression (59). PGE2 shifts the balance of the cellular immune response away from T helper type 1 (Th1), favoring a Th2 response, which drives humoral responses toward the production of immunoglobulin E (IgE) (21). Interestingly, a recent study has reported a bidirectional interaction between HTLV-1 and PGE2 (51).
Several issues concerning viral latency occurring in HTLV-1-infected cells in vivo are still poorly understood. Indeed, although the molecular mechanisms through which the replicative cycle of HTLV-1 is controlled by the two regulatory genes rex and tax are well defined (15, 48), less is known about external stimuli that can regulate viral gene expression. Given that HTLV-1 is frequently detected in T lymphocytes in infected individuals (54, 66) and that PGE2 can induce HTLV-1 LTR activity (51), the aim of this study was to define the relative importance of T-cell receptor (TCR)-, CD28-, and/or PGE2-mediated signal pathway(s) in controlling HTLV-1 gene expression. We provide evidence indicating that, when taken individually, each of these signal transduction pathways acts as a weak inducer of virus transcription. However, the combined action of these cellular activation stimuli resulted in a more significant enhancement of HTLV-1 LTR-directed transcriptional activity and virus production. These observations collectively provide new insights into the mechanisms underlying reactivation of this retrovirus.
(This work was performed by N. Dumais in partial fulfillment of the requirements for the Ph.D. degree from the Microbiology-Immunology Program, Faculty of Medicine, Laval University, Quebec, Canada.)
MATERIALS AND METHODS
Reagents.
PGE2 was purchased from Sigma (St. Louis, Mo.), and forskolin was obtained from BioMol (Plymouth Meeting, Pa.). OKT3 hybridoma produces an antibody specific for the ɛ chain of the CD3 complex and was obtained from the American Type Culture Collection (Manassas, Va.). Antibodies from this hybridoma cell line were purified with mAbTrap protein G affinity columns according to the manufacturer's instructions (Pharmacia LKB Biotechnology AB, Uppsala, Sweden). Purified anti-CD28 antibody (clone 9.3) was a generous gift from J. A. Ledbetter (Bristol-Myers Squibb Pharmaceutical Research Institute, Princeton, N.J.) (47).
Cells and culture conditions.
The established cell lines used in this work include Jurkat (clone E6.1), JCAM1.6, J45.01, and DT30. Jurkat is considered a model cell line for the study of T-cell signaling machinery (33). JCAM1.6 and J45.01 are Jurkat derivatives and are deficient in p56lck and CD45 expression, respectively (42, 74). DT30 cells are derived from the mastocytoma P815 cell line and stably express cell surface human B7.1 (i.e., CD80) (1). Such cells also express murine FcγRII receptors and are thus capable of binding and cross-linking soluble antibody. DT30 cells were fixed in 1% paraformaldehyde, washed extensively with phosphate-buffered saline (PBS), and then stored frozen at a density of 2 × 106 cells/ml in PBS. C91/PL is an HTLV-1-infected human T-cell line that proliferates independently of IL-2 (82). All described cell lines were cultured in medium made of RPMI 1640 supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan, Utah), glutamine (2 mM), penicillin G (100 U/ml), and streptomycin (100 μg/ml) and were maintained at 37°C under a 5% CO2 humidified atmosphere. Peripheral blood mononuclear cells (PBMCs) were obtained from healthy donors and purified by Ficoll-Hypaque centrifugation. Human T-helper cells (i.e., CD4+) were negatively isolated from fresh PBMCs through negative selection by using the CD4+ T-cell purification kit (Miltenyi Biotec).
Plasmids.
pHTLV-1-Luc was kindly provided by W. C. Greene (The J. Gladstone Institutes, San Francisco, Calif.) and contains the HTLV-1 LTR upstream to the luciferase reporter gene (28). A pGL2-TATA plasmid was first constructed by subcloning a synthetic double-stranded oligonucleotide containing a TATA box and extremities compatible with NheI/HindIII (produced from hybridized 5′-CTAGCGGGTATATAATGGATCCA-3′ and 5′-AGCTTGGATCCATTATATACCCG-3′) in NheI/HindIII-digested pGL2 basic vector (Promega). The SmaI/NdeI fragment of the HTLV-1 LTR from the pHTLV-Luc plasmid was blunted and subsequently cloned in the SmaI site of this vector, hence generating pGL2-HTLV-1 (−325/−57). The pGL3U3R and pGL3 21PMD/11T vectors were produced by cloning wild-type or TRE-mutated HTLV-1 LTRs (3) upstream of the luciferase reporter gene in the pGL3 basic vector (Promega). We used the commercial pCRE-Luc vector that contains five tandem repeats of a consensus CRE sequence and a TATA box placed upstream of the luciferase reporter gene (Stratagene, La Jolla, Calif.). The KCREB plasmid consists of the CREB cDNA that bears a mutation of a single amino acid in the DNA-binding domain and was cloned into a mammalian expression vector driven by the Rous sarcoma virus promoter. This dominant repressor CREB mutant can no longer bind to DNA and, in addition, can block the ability of wild-type CREB to bind to CRE (78). This plasmid was a generous gift from R. H. Goodman (Vollum Institute for Advanced Biochemical Research, Portland, Oreg.). pPKAcK73M codes for an inactive mutant form of the catalytic subunit of protein kinase A (PKA) (kindly provided by S. Ghosh, Yale University School of Medicine). This mutated form of PKAc was generated by altering lysine 73 to a methionine (K73M) and has been demonstrated to inhibit endogenous PKA activity (85).
Transient transfection and cell stimulation.
Cells (5 × 106) from Jurkat cell lines were first washed once in transfection solution (TS) buffer (137 mM NaCl, 25 mM Tris-HCl [pH 7.4], 5 mM KCl, 0.6 mM NaHPO4, 0.5 mM MgCl2, and 0.7 mM CaCl2) and resuspended in 0.5 ml of TS containing the indicated plasmids and 500 μg of DEAE-dextran per ml (final concentration). The cell-TS-plasmid-DEAE-dextran mixture was incubated for 25 min at room temperature. Thereafter, cells were diluted at a concentration of 106 cells/ml by using complete culture medium supplemented with 100 μM chloroquine (Sigma). After 45 min of incubation at 37°C, the cells were centrifuged, washed once, resuspended in complete culture medium, and incubated at 37°C for 24 h. PBMCs and CD4+ T lymphocytes were nucleofected by using Nucleofector technology according to the manufacturer's instructions (Amaxa Biosystems, Gaithersburg, Md.). Briefly, PBMCs and CD4+ T cells (4 × 106) were harvested, washed once with PBS, and then resuspended in human T-cell Nucleofector solution with the indicated plasmids. The cell-DNA mixture was put into a cuvette, inserted in the Nucleofector, and then nucleofected. To minimize variations in plasmid transfection efficiencies, transfected cells were pooled 24 h after transfection and were next separated into various treatment groups as follows. Transiently transfected cells were seeded at a density of 105 cells per well (100 μl) in 96-well flat-bottom plates. The cells were left untreated or were treated with PGE2 (100 nM), forskolin (100 μM), OKT3 (1 μg/ml), DT30 (104 cells), OKT3 plus PGE2, OKT3 plus DT30, DT30 plus PGE2, or OKT3 plus DT30 plus PGE2 in a final volume of 200 μl for a period of 8 h at 37°C. Luciferase activity was determined following a previously described protocol (2). Briefly, following the incubation period, 100 μl of cell-free supernatant was withdrawn from each well and 25 μl of cell culture lysis buffer (25 mM Tris phosphate [pH 7.8], 2 mM dithiothreitol [DTT], 1% Triton X-100, and 10% glycerol) was added before incubation at room temperature for 30 min. An aliquot of cell extract (20 μl) was analyzed in 96-well plates using a microtiter plate luminometer (MLX Dynex Technologies, Chantilly, Va.). Each well was injected with 100 μl of luciferase assay buffer [20 mM tricine, 1.07 mM (MgCO3)4 · Mg(OH)2 · 5H2O, 2.67 mM MgSO4, 0.1 mM EDTA, 270 μM coenzyme A, 470 μM luciferin, 530 μM ATP, and 33.3 mM DTT]. Light output was measured for 20 s with a 2-s delay. Values are expressed as relative light units as measured by the apparatus.
Induction of HTLV-1 expression.
C91/PL cells were first seeded at a density of 106 cells per well (1-ml final volume) in 24-well flat-bottom plates. The cells were either left untreated or were treated with OKT3 plus DT30 or OKT3 plus DT30 plus PGE2 for a period of 24 h at 37°C. Following this incubation period, 300 μl of cell-free supernatant was withdrawn from each well and measurements of HTLV-1 p19 antigen were achieved by a commercial enzyme-linked immunosorbent assay (Zeptometrix Corporation, Buffalo, N.Y.).
EMSA.
Nuclear extracts were prepared according to the microscale preparation protocol with slight modifications (68). Briefly, Jurkat cells (106) were either left untreated or were treated for 60 min at 37°C with PGE2 (100 nM), forskolin (10 μM), OKT3 plus DT30, or OKT3 plus DT30 plus PGE2. Incubation of the cells with the stimulating agents was terminated by the addition of ice-cold PBS, and nuclear extracts were then prepared. Sedimented cells were resuspended in 400 μl of a cold buffer made of 10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EGTA, 0.1 mM EDTA, 1 mM DTT, and 0.5 mM phenylmethylsulfonyl fluoride. The cells were allowed to swell on ice for 15 min, after which 25 μl of a 10% solution of Nonidet P-40 was added and the tubes were vigorously vortexed for 10 s. The homogenate was centrifuged for 10 s at 12,000 × g. The supernatant fraction was discarded, and the pellet was resuspended in 50 μl of a cold buffer composed of 20 mM HEPES-KOH (pH 7.9), 0.4 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, and 1 mM phenylmethylsulfonyl fluoride before being incubated on ice for 15 min on a shaking platform. Cellular debris were removed by centrifugation at 12,000 × g for 2 min at 4°C, and the supernatant fractions were stored at −70°C until used. Six micrograms of nuclear extracts was used to perform an electrophoretic mobility shift assay (EMSA). Protein content was determined by commercial BCA protein assay reagent (Pierce, Rockford, Ill.). Nuclear extracts were incubated for 30 min at room temperature in the binding buffer (100 mM HEPES [pH 7.9], 40% glycerol, 10% Ficoll, 250 mM KCl, 10 mM DTT, 5 mM EDTA, 250 mM NaCl, 2 μg of poly[dI-dC], and 10 μg of nuclease-free bovine serum albumin fraction V) containing 1 ng of 32P-5′-end-labeled double-stranded oligonucleotide. Double-stranded DNA (dsDNA; 100 ng) was labeled with [γ-32P]ATP and T4 polynucleotide kinase in a kinase buffer (New England Biolabs, Beverly, Mass.). This mixture was incubated for 30 min at 37°C, and the reaction was stopped with 5 μl of 0.2 M EDTA. The labeled oligonucleotide was extracted with phenol-chloroform and passed through a G-50 spin column. The following dsDNA oligonucleotides were used as probes and/or competitors: the consensus CREB-binding site corresponding to the sequence 5′-AGAGATTGCCTGACGTCAGAGAGCTAG-3′, the CRE mutant 5′-AGAGATTGCCTGTGGTCAGAGAGCTAG-3′, and the consensus NF-κB-binding sequence (5′-AGTTGAGGGGACTTTCCCAGGC-3′) (purchased from Santa Cruz Biotechnology Inc., Santa Cruz, Calif.). DNA-protein complexes were resolved from free-labeled DNA by electrophoresis in native 4% (wt/vol) polyacrylamide gels. The gels were subsequently dried and autoradiographed on Kodak Biomax MR film at −85°C. Cold competition assays were conducted by adding a 100-fold molar excess of unlabeled dsDNA oligonucleotide simultaneously with the labeled probe.
RESULTS
HTLV-1 LTR-driven activity is induced by TCR/CD28 cross-linking in combination with PGE2 treatment.
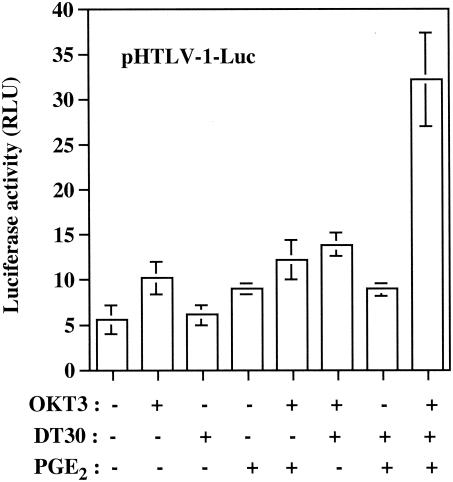
T lymphocytes that harbor HTLV-1 in the peripheral blood of infected patients are exposed to several exogenous factors, some of which might be involved in the activation of transcriptionally silent provirus. Thus, in an attempt to define whether expression of HTLV-1 could be influenced by the pleiotropic immunomodulatory molecule PGE2 and/or signaling events transduced through TCR and CD28, we transiently transfected Jurkat cells with a vector carrying the luciferase reporter gene placed under the control of the full-length HTLV-1 LTR. Such cells were next incubated with PGE2 and/or an anti-TCR antibody (i.e., OKT3). We also made use of a physiological and immunologically relevant experimental cell system based on the addition of DT30 cells. These cells express human B7.1 (CD80) and can thus cross-link cell surface CD28. Note that DT30 cells are also positive for FcγRII receptors and can present any soluble mouse IgG antibodies. When DT30 cells are used in combination with anti-TCR antibodies, this experimental cell system mimics T-cell activation (1). Under the conditions of our experiment, virus transcription was not affected by CD28-mediated signal transduction events (DT30 cells added alone), a finding in contrast to that for engagement of the T-cell receptor or treatment with PGE2 alone, which led to a minor increase in HTLV-1 LTR activity (Fig. 1). Reporter gene expression was not further increased by grouping together OKT3 and PGE2, OKT3 and DT30, or DT30 and PGE2. However, a more significant increase in HTLV-1 LTR activity was obtained when both TCR and CD28 were cross-linked in the presence of PGE2 (5.8-fold increase).
FIG. 1.
Synergistic activation of HTLV-1 LTR by TCR/CD28 cross-linking and PGE2 treatment. Jurkat cells were transiently transfected with pHTLV-1-Luc (15 μg) and were either left untreated or treated with PGE2 (100 nM), OKT3 antibody (1 μg/ml), and/or DT30 cells. Luciferase activities were assayed at 8 h after stimulation. Averages of the results for luciferase activities from quadruplicate samples are presented, and standard deviations are indicated by the error bars. The data are representative of results from three independent experiments. RLU, relative light units.
Importance of TRE sequences and CREB in TCR-CD28-PGE2-dependent induction of HTLV-1 LTR activity.
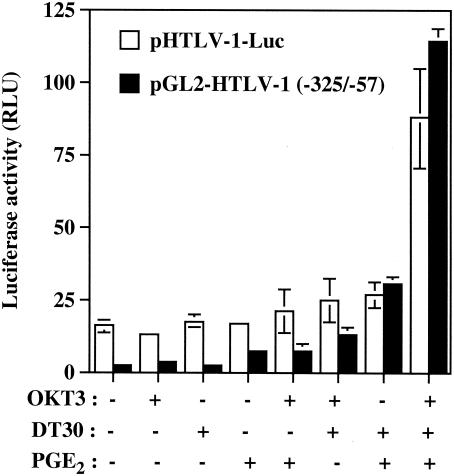
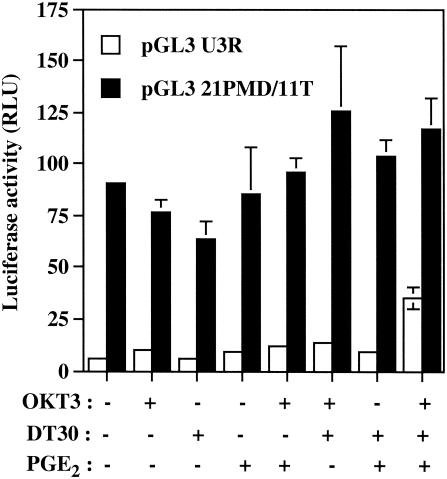
In order to examine the role of the TRE sequences in the observed phenomenon, Jurkat cells were next transiently transfected with pGL2-HTLV-1 (−325/−57). This vector contains the luciferase reporter gene positioned downstream of a TATA box and the −325 to −57 HTLV-1 LTR region harboring all three TRE sequences. The most potent activating condition for luciferase gene transcription was again observed when PGE2 was added to TCR/CD28-multimerized T cells (Fig. 2). Interestingly, a more dramatic increase in luciferase activity was seen with pGL2-HTLV-1 (−325/−57) than with pHTLV-1-Luc (a 62-fold versus a 5.6-fold increase), therefore suggesting that this region, i.e., −325 to −57, is more positively affected by these activation signals. To directly assess the involvement of TRE sequences in this positive modulation by the tested combination of agents, an HTLV-1-based reporter construct was used that bears mutations abolishing CREB binding to the three CRE-like sites (i.e., pGL3 21PMD/11T). The introduced mutations resulted in a C-to-T change at bp 11 of the 21-bp TRE sequence. This mutation has previously been shown to inhibit Tax-mediated transactivation events (32). Consistent with our previous findings, the highest up regulation of HTLV-1 LTR activity was observed following treatment of Jurkat cells with OKT3, DT30, and PGE2 (Fig. 3). However, mutations that prevent binding of CREB to TRE sequences resulted in a dramatic reduction in HTLV-1 LTR-driven gene activity mediated by OKT3-DT30-PGE2 treatment.
FIG. 2.
An HTLV-1-based vector carrying TRE sequences is more potently activated by TCR-CD28-PGE2-mediated signal pathways than a full-length HTLV-1 construct. Jurkat cells were transfected with 15 μg of pHTLV-1-Luc or pGL2-HTLV-1 (−325/−57) and were left unstimulated or were stimulated with the indicated agents. Luciferase activity of the transfected cell lysates was determined 8 h poststimulation. Averages of the results for luciferase activities from quadruplicate samples are presented, and standard deviations are indicated by the error bars. The data are representative of results from three independent experiments. RLU, relative light units.
FIG. 3.
Importance of TRE sequences in the HTLV-1 LTR in TCR-CD28-PGE2-dependent induction of virus gene expression. The pGL3 UR3 or pGL3 21PMD/11T plasmid (15 μg) was transfected into Jurkat cells, which were then either left untreated or were treated with the indicated stimuli. At 8 h after stimulation, luciferase activity of the transfected cell lysates was monitored as described in Materials and Methods. Averages of the results for luciferase activities from quadruplicate samples are presented, and standard deviations are indicated by the error bars. The data are representative of results from three independent experiments. RLU, relative light units.
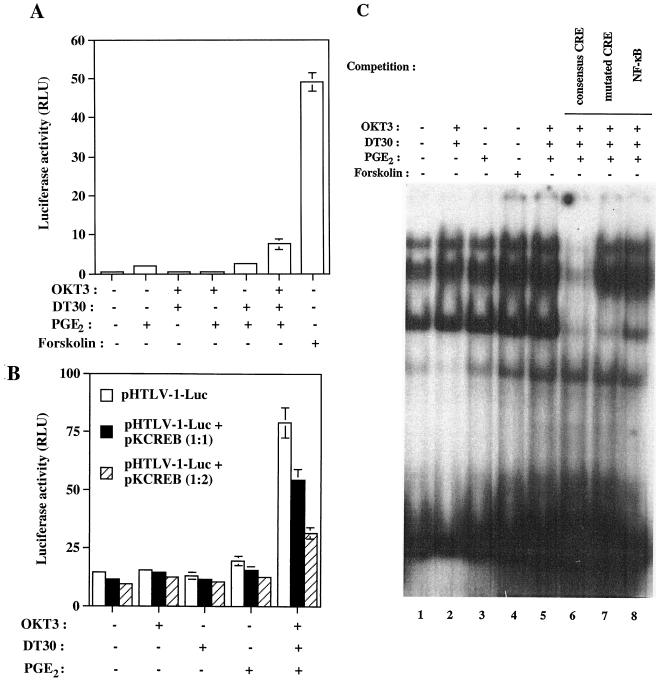
Our next set of experiments was aimed at demonstrating that CREB is indeed a key player in TCR-CD28-PGE2-mediated enhancement of HTLV-1 LTR gene expression. This goal was achieved by introducing a CRE-driven luciferase molecule into Jurkat cells before treatment with the studied activating agents. Forskolin, an agent known to lead to an increase in intracellular cAMP concentration by directly activating adenylate cyclase, was used as a positive control and resulted in an important 122-fold increase in CRE-dependent luciferase activity (Fig. 4). A marked up regulation of reporter gene activity was seen when PGE2 was used in combination with agents that mimic T-cell activation (i.e., OKT3 and DT30) (18.8-fold increase). To more directly address the importance of CREB, we made use of a vector that codes for a dominant negative mutant of CREB (i.e., pKCREB), along with the pHTLV-1-Luc vector. Expression of this CREB mutant reduced TCR-CD28-PGE2-dependent activation of HTLV-1-driven increase in reporter gene expression, further strengthening the importance of CREB in these cooperative signal transduction pathways (Fig. 4B). Next, nuclear protein extracts from Jurkat cells were subjected to mobility shift assays using a probe specific for the binding site of CREB. A more intense CRE-binding activity was detected in OKT3-DT30-PGE2-treated Jurkat cells than in untreated cells (Fig. 4C). The nature of the CRE-binding complexes was comparable to that seen in cells treated with forskolin, but the intensity was slightly weaker in cells undergoing treatment with forskolin than in OKT3-DT30-PGE2-treated cells. Little increase in the intensity of the signal was apparent with nuclear extracts from OKT3- or DT30-treated cells. These complexes were competed by a 100-fold molar excess of a specific unlabeled CRE oligonucleotide but were unaffected by an excess of unlabeled NF-κB or mutant CRE oligonucleotides. Together, these data indicate that PGE2 and agents mimicking antigenic stimulation cooperate to activate the ubiquitous transcription factor CREB, which in turn positively affects HTLV-1 transcription through the TRE sequences.
FIG. 4.
TCR-CD28-PGE2-directed up regulation of virus transcription requires the transcription factor CREB. (A) Jurkat cells were transfected with 15 μg of pCRE-Luc and then were either left unstimulated or were stimulated with the indicated agents. RLU, relative light units. (B) The pHTLV-1-Luc plasmid (15 μg) was cotransfected into Jurkat cells with a vector coding for a dominant repressor CREB mutant (pKCREB; 15 and 30 μg) or an empty vector control. Luciferase activity for the transfected cell lysates was determined 8 h poststimulation. Averages of the results for luciferase activities from quadruplicate samples are presented, and standard deviations are indicated by the error bars. The data are representative of results from three independent experiments. (C) Jurkat cells were either left untreated or were treated for 60 min with the indicated stimuli. The nuclear extracts were incubated with a CRE-labeled probe, and the complexes were resolved on a native 4% polyacrylamide gel. Competitions were performed with a 100-fold molar excess of either specific (consensus CRE, lane 6; mutated CRE, lane 7) or nonspecific (NF-κB, lane 8) oligonucleotides.
TCR-CD28-PGE2-mediated induction of CREB involves the participation of PKA.
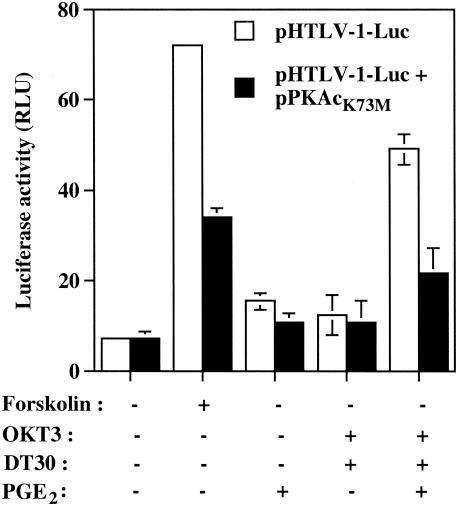
Multiple signaling pathways in different cell lineages can mediate CREB phosphorylation and activation. These pathways include (i) a PKA-dependent pathway activated by increased intracellular concentrations of cAMP, (ii) a calmodulin kinase-dependent pathway activated by increased intracytoplasmic calcium (Ca2+), and (iii) a Ras-dependent pathway in which ribosomal S6 kinase B can phosphorylate CREB on Ser 133 (10, 19, 34-36). Recent studies have demonstrated that each of these pathways is functional in T cells. To shed light on the nature of the signaling pathway that leads to CREB activation, Jurkat cells were transiently cotransfected with pHTLV-1-Luc and a plasmid expressing an inactive mutant form of the catalytic subunit of PKA (i.e., pPKAcK73M). The latter expression vector severely diminished the HTLV-1 LTR activity that is observed following TCR/CD28 cross-linking and PGE2 treatment (Fig. 5). Forskolin activation of the HTLV-1 LTR and PGE2 activation were similarly and significantly affected but to a lesser extent.
FIG. 5.
PKA activity is required for transcriptional activation of HTLV-1 by TCR-CD28-PGE2-mediated signal transduction pathways. Jurkat cells were cotransfected with pHTLV-1-Luc and pPKAcK73M or the empty vector control (15 μg of each). The cells were then either left untreated or were treated for 8 h with the listed stimuli. Averages of the results for luciferase activities from quadruplicate samples are presented, and standard deviations are indicated by the error bars. The data are representative of results from three independent experiments. RLU, relative light units.
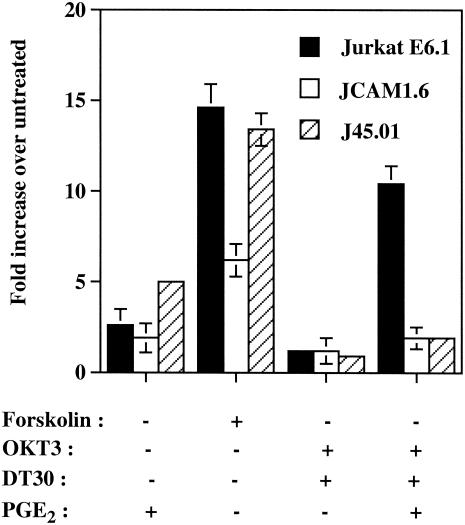
TCR-CD28-PGE2-dependent activation of HTLV-1 transcription requires p56lck and CD45.
Following TCR engagement, CREB is phosphorylated rapidly on Ser 133 by a pathway that involves activation of p56lck, protein kinase C, Ras, Raf-1, MEK, and ribosomal S6 kinase 2 (53). Some cellular constituents in these signaling cascades initiated upon TCR engagement were next tested for their roles in the studied phenomenon. One of the earliest biochemical events elicited by the TCR response remains tyrosine phosphorylation of intracellular second messengers such as p56lck (11). This protein tyrosine kinase was demonstrated to be essential, since TCR-CD28-PGE2-mediated induction of LTR activity was reduced by 82% in p56lck (JCAM1.6)-deficient cells (a 10.4-fold compared to a 1.9-fold increase) (Fig. 6). Similar observations were made when a CD45-deficient cell line was used (J45.01), therefore suggesting that this transmembrane protein tyrosine phosphatase was deeply involved in up regulation of the regulatory elements of HTLV-1 by TCR/CD28 cross-linking and PGE2. When these results were compared to the results obtained with forskolin and PGE2 alone, no such drastic effects were accounted for except for forskolin induction, which was affected by the absence of p56lck. This body of data supports the expected role played by the elements activated early on following TCR multimerization in the induction of the HTLV-1 LTR by the OKT3-DT30-PGE2 combination.
FIG. 6.
The protein tyrosine kinases p56lck as well as the phosphotyrosyl phosphatase CD45 are important in TCR-CD28-PGE2-dependent induction of HTLV-1 LTR-driven gene expression. p56lck (JCAM1.6)- and CD45 (J45.01)-deficient Jurkat derivatives and parental Jurkat cells were transiently transfected with pHTLV-1-Luc (15 μg) and were either left untreated or were treated for 8 h with the listed agents. Luciferase activity of the transfected cell lysates was finally monitored as described in Materials and Methods. Averages of the results for luciferase activities from quadruplicate samples are expressed as fold induction relative to basal reporter gene activity in untreated control cells (considered as 1). Standard deviations are indicated by the error bars, and the data are representative of results from three independent experiments.
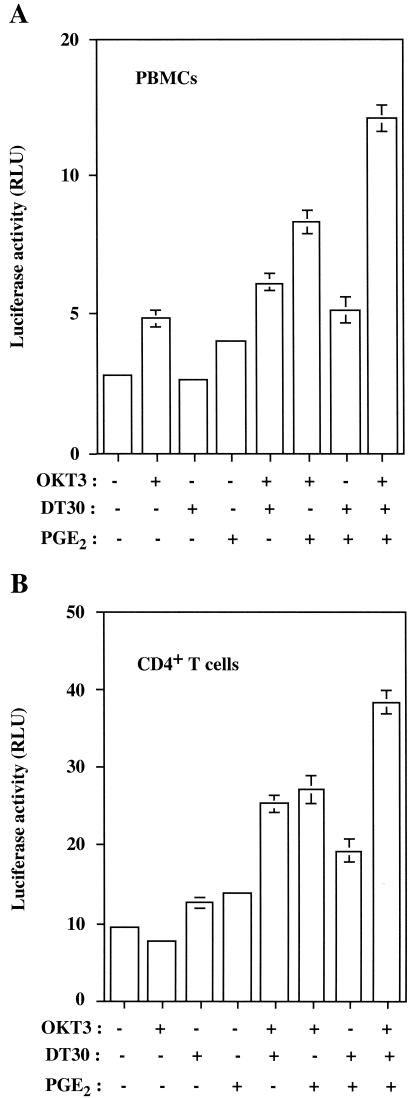
TCR/CD28 engagement and PGE2 treatment activate HTLV-1 LTR activity in primary human cells and promote virus production from an HTLV-1-infected T-cell line.
The physiological significance of our findings was assessed by defining whether the tested activation signals can modulate HTLV-1 transcription in more natural cellular microenvironments, i.e., human PBMCs and purified CD4+ T lymphocytes. In agreement with results obtained with the Jurkat T-lymphoblastoid T-cell line, transfection of either PBMCs or CD4+ T cells with the pHTLV-1-Luc plasmid indicated maximal activation upon treatment with PGE2 and multimerization of both TCR and CD28 (Fig. 7).
FIG. 7.
TCR-CD28-PGE2-directed induction of HTLV-1 transcription is still observed in primary human cells. PBMCs (A) and purified CD4+ T lymphocytes (B) were transfected with pHTLV-1-Luc (15 μg) and were either left untreated or were treated with the indicated agents. Luciferase activity of the transfected cell lysates was determined 8 h poststimulation. The results shown are presented in terms of luciferase activity from the calculated means ± standard deviations of four different lysed cell samples in the same experimental setting. These results are representative of results from three independent experiments. RLU, relative light units.
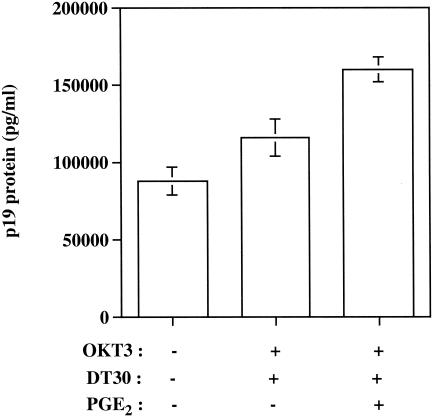
Recent findings indicate that integrated proviral DNA behaves quite differently from transfected plasmids. Indeed, expression of integrated human retroviruses such as HTLV-1 has been shown to require cellular factors different from those necessary for expression of transiently transfected retrovirus-based plasmids (57). More specifically, it has been demonstrated that transcription from integrated versus transiently introduced retroviral LTRs does not proceed via identical signal transduction pathways. This finding suggests that the signaling requirements necessary to achieve expression of an integrated proviral DNA can differ from those of a transfected viral plasmid. Since the process of integration into the host chromosome is an obligatory step in the HTLV-1 life cycle, we performed experiments with an HTLV-1-infected T-cell line (i.e., C91/PL). Virus production was induced upon TCR/CD28 signaling events and exposure to PGE2 (Fig. 8). This set of data suggests that our findings can also be seen in the context of an integrated proviral genome.
FIG. 8.
Virus production is induced following TCR/CD28 engagement and PGE2 treatment of an HTLV-1-infected human T-cell line. C91/PL cells were either left untreated or were treated with the listed agents. After 24 h, the levels of p19 in cell-free culture supernatants were measured by a commercial enzyme-linked immunosorbent assay. The results represent means ± standard errors of the means for triplicate wells.
DISCUSSION
HTLV-1 gene expression in vivo likely plays an important role in the various steps leading to diverse HTLV-1-associated diseases such as ATL, HAM/TSP, inflammatory arthritis, polymyositis, uveitis, and Sjögren's syndrome. Results from many studies indicate that the majority of HTLV-1-infected cells under natural conditions carry a provirus that is transcriptionally silent. For example, HTLV-1 RNA was expressed in only 10% of HTLV-1-infected lymphocytes, as monitored by in situ PCR experiments (56). Low levels of cells expressing HTLV-1 transcripts were detected in PBMCs by reverse transcriptase PCR in asymptomatic carriers and HAM/TSP patients (27, 31), providing further evidence that HTLV-1 induces a latent infection. An early induction of latency in HTLV-1-bearing T cells is also observed in experimentally infected squirrel monkeys (Saimiri sciureus) (40). The precise mechanisms responsible for HTLV-1 latency remain to be characterized. It has been proposed that an important common denominator in the pathogenesis of the various HTLV-1-induced clinical syndromes appears to be HTLV-1 gene expression. Although the mechanisms through which Tax can potently stimulate viral transcription are well defined, the biological importance of cellular activation signals in HTLV-1 gene expression remains to be more fully characterized. The central objective of the present work was thus to assess the abilities of some specific external stimuli to affect HTLV-1 LTR-directed gene expression.
Treatment of cells chronically infected with HTLV-1, which express low levels of HTLV-1 RNAs, with phytohemagglutinin resulted in induction of virus gene expression (44). Phytohemagglutinin is a mitogenic agent that mimics antigen stimulation and activates resting T cells through the cell surface TCR/CD3 complex (81). Optimal T-cell activation requires signaling provided by recognition of antigen-major histocompatibility complex by the TCR and a second antigen-independent signal called costimulation. CD28 is now considered the prototypic T-cell costimulatory molecule (reviewed in reference 43). Moreover, it has previously been shown that treatment of human peripheral T lymphocytes and Jurkat cells with anti-CD3 and anti-CD28 antibodies leads to stimulation of COX-2 transcription, therefore suggesting that engagement of these cell surface receptors can affect prostaglandin synthesis (38). These previous findings coupled with the recent demonstration that PGE2 can positively regulate HTLV-1 expression and that Tax can induce the production of PGE2 through an effect on the inducible COX-2 enzyme (51) prompted us to test whether cellular activation stimuli such as TCR/CD28 engagement and PGE2 can modulate HTLV-1 transcription. Our experiments were performed with human T cells and were based on previously published observations indicating that T lymphocytes constitute the major cellular reservoir for HTLV-1 in human peripheral blood (66).
In this work, we present evidence demonstrating that signaling events mediated either by PGE2, engagement of TCR, or CD28 cross-linking result in a weak induction of HTLV-1 LTR activity. However, a more significant induction of HTLV-1 LTR-directed reporter gene activity was obtained when these three ligands that bind to distinct cell surface receptors were simultaneously added. These results have been confirmed with Jurkat cells and total PBMCs or with purified CD4+ T-cell fractions. The physiological relevance of our findings is provided by the observation that the combination of these external stimuli can also promote virus production in an experimental cell system consisting of integrated proviral DNA, i.e., a human T-cell line infected with HTLV-1. After transfection of a vector harboring the −325/−57 region of the HTLV-1 LTR in Jurkat cells, a more important induction was measured upon the addition of the complete combination of agents. In fact, a stronger induction was noted than in similarly treated pHTLV-1-Luc-transfected cells. Although we have not yet fully addressed this issue, the absence of possible negative regulatory elements or the spatial reorganization of the TRE repeats in relation to the TATA box might all be plausible explanations for these differences. Transient transfection experiments conducted with a vector carrying mutations in the three TRE sequences indicated that these cis-acting motifs are also important for TCR-CD28-PGE2-mediated induction of LTR activity. The TCR-mediated weak induction of HTLV-1 LTR activation is consistent with data from Copeland et al. (14). However, our observation that PGE2 when used alone is a very weak inducer of HTLV-1 transcription in human T cells is in contrast to that of Moriuchi and coworkers (51). For example, we observed that treatment with PGE2 at 100 nM resulted in a less-than-twofold increase in HTLV-1 activity in Jurkat cells and PBMCs, while a fourfold increase was obtained with identical cell types and a similar concentration of PGE2. We did find, however, that PGE2 alone could positively modulate the transcription of the luciferase reporter gene driven by the isolated TRE sequences (pGL2-HTLV-1 [−325/−57]). Nonetheless, these discrepant data could be related to differences in the methodologies of experiments, such as the use of different HTLV-1-based molecular constructs. In addition, the time of measurement for luciferase activity was 8 h poststimulation in our study and 2 days posttransfection in the other study; possibly, later and indirect events might have contributed to their reported HTLV-1 LTR activation in T cells by PGE2 alone.
Earlier reports indicated that interaction between PGE2 and an adenylate cyclase-coupled stimulatory receptor (i.e., EP4) leads to activation of adenylate cyclase, hydrolysis of ATP, enhanced turnover of intracellular cAMP, and binding to PKA in human T cells (12). HTLV-1 LTR is known to be responsive to stimuli involving the activation of the CREB transcription factor. Several studies have indeed indicated that strong activators of CREB such as forskolin or dibutyryl cyclic AMP lead to CREB-dependent activation of HTLV-1 LTR in the context of T cells (61). Our findings are clearly supportive of this signaling cascade, since we found that TCR-CD28-PGE2-mediated enhancement of HTLV-1 LTR activity requires the participation of both PKA and CREB, as determined by transient transfection experiments and EMSA analyses. The PKA signaling pathway has already been shown to be important in activation of HTLV-1 by PGE2, as is seen in PBMCs derived from asymptomatic carriers (51). Data from our experiments indicate that p56lck and the transmembrane protein tyrosine phosphatase CD45 also participate in the TCR-CD28-PGE2-directed up regulation of LTR activity. Furthermore, unpublished results have recently indicated involvement of ZAP-70 protein tyrosine kinase in the activation of the HTLV-1 LTR by the combination of agents. The involvement of these cellular components is clearly in line with the importance of the antigenic-like stimulatory agents (i.e., OKT3 and DT30) for the observed HTLV-1 LTR activation, due to their implication in classical TCR-mediated signaling.
The formation and production of elevated levels of inflammatory mediators such as PGE2 are hallmarks of several microbial infections (5, 20, 37, 41, 49, 58, 64, 65, 73, 79, 80). Prostaglandins play a role in disease exacerbation by directly altering T-cell functions or macrophage activation. Although it was initially thought that PGE2 is primarily an immunosuppressive molecule that acts as a down-regulator of many aspects of B- and T-cell function and proliferation, recent findings support a role for PGE2 as a potentiator of immunoglobulin class switching and in regulating production of type 1 and 2 cytokines (21). The capacity of PGE2 to affect virus gene expression is not unique to HTLV-1, since expression of bovine leukemia virus, a type C retrovirus that is closely related to HTLV-1, is also enhanced by PGE2 (62). PGE2 has also been reported to influence the life cycle of human immunodeficiency virus type 1 in human T cells (16, 17). Since concentrations of PGE2 as high as 70 μM have been reported in seminal fluids (29, 63), PGE2 can possibly affect transmission of retroviruses such as HTLV-1, which is transmitted not only by breast milk but also through sexual contact. It is of interest that Moriuchi and coworkers have reported that HTLV-1 infection of PBMCs leads to secretion of PGE2 (51), an observation that was confirmed in T-cell lines infected with HTLV-1 (50). However, based on our results indicating that a more efficient enhancement of HTLV-1 LTR-directed gene expression is achieved when cell surface TCR and CD28 are also engaged, PGE2 alone might not be sufficient per se to affect virus load in seminal fluids.
In conclusion, the TCR-CD28-PGE2-mediated activation pathway described here represents a combination of external stimuli that may act in a cooperative fashion to enhance expression of integrated HTLV-1 genomes. Given that rather long latency periods before disease progression appear in most HTLV-1-infected individuals, extracellular factors that result in Tax-independent virus gene expression may be important elements in disease progression. The present study provides new information on molecular mechanisms that can relieve HTLV-1 latency in its natural target cell, i.e., human T lymphocytes. It also indicates that HTLV-1 gene expression is under tight control and that it requires a combination of events that must act in a concerted manner to initiate virus transcription. Since the probability of encountering the appropriate sequence of events in a given cell is rather low, this might help to explain why most HTLV-1-carrying cells are transcriptionally silent. Additional experiments are warranted to determine if other cellular activation stimuli can modulate HTLV-1 LTR-directed gene expression and could act in combination with TCR/CD28- and/or PGE2-mediated transcriptional modulation.
Acknowledgments
This study was supported by grants to M.J.T. from the Canadian Institutes of Health Research (CIHR) HIV/AIDS Research Program (grants HOP-14438, HOP-15575, and MOP-37781). N.D. was a fellow of the Fonds de la Recherche en Santé du Québec (FRSQ)/Fonds pour la Formation de Chercheurs et l'Aide à la Recherche-Programme Santé. B.B. holds a scholarship award (junior 1 level) from the FRSQ, and S.B. is the recipient of a CIHR doctoral research award. M.J.T. holds a tier 1 Canada Research Chair in Human Immuno-Retrovirology.
REFERENCES
- 1.Azuma, M., M. Cayabyab, D. Buck, J. H. Phillips, and L. L. Lanier. 1992. CD28 interaction with B7 costimulates primary allogeneic proliferative responses and cytotoxicity mediated by small, resting T lymphocytes. J. Exp. Med. 175:353-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbeau, B., R. Bernier, N. Dumais, G. Briand, M. Olivier, R. Faure, B. I. Posner, and M. Tremblay. 1997. Activation of HIV-1 LTR transcription and virus replication via NF-κB-dependent and -independent pathways by potent phosphotyrosine phosphatase inhibitors, the peroxovanadium compounds. J. Biol. Chem. 272:12968-12977. [DOI] [PubMed] [Google Scholar]
- 3.Barnhart, M. K., L. M. Connor, and S. J. Marriott. 1997. Function of the human T-cell leukemia virus type 1 21-base-pair repeats in basal transcription. J. Virol. 71:337-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beimling, P., and K. Moelling. 1992. Direct interaction of CREB protein with 21 bp Tax-response elements of HTLV-I LTR. Oncogene 7:257-262. [PubMed] [Google Scholar]
- 5.Ben-Hur, T., J. Rosenthal, A. Itzik, and J. Weidenfeld. 1996. Rescue of HSV-1 neurovirulence is associated with induction of brain interleukin-1 expression, prostaglandin synthesis and neuroendocrine responses. J. Neurovirol. 2:279-288. [DOI] [PubMed] [Google Scholar]
- 6.Beraud, C., G. Lombard-Platet, Y. Michal, and P. Jalinot. 1991. Binding of the HTLV-I Tax1 transactivator to the inducible 21 bp enhancer is mediated by the cellular factor HEB1. EMBO J. 10:3795-3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohnlein, E., J. W. Lowenthal, M. Siekevitz, D. W. Ballard, B. R. Franza, and W. C. Greene. 1988. The same inducible nuclear proteins regulates mitogen activation of both the interleukin-2 receptor-alpha gene and type 1 HIV. Cell 53:827-836. [DOI] [PubMed] [Google Scholar]
- 8.Bosselut, R., F. Lim, P. C. Romond, J. Frampton, J. Brady, and J. Ghysdael. 1992. Myb protein binds to multiple sites in the human T cell lymphotropic virus type 1 long terminal repeat and transactivates LTR-mediated expression. Virology 186:764-769. [DOI] [PubMed] [Google Scholar]
- 9.Brauweiler, A., P. Garl, A. A. Franklin, H. A. Giebler, and J. K. Nyborg. 1995. A molecular mechanism for human T-cell leukemia virus latency and Tax transactivation. J. Biol. Chem. 270:12814-12822. [DOI] [PubMed] [Google Scholar]
- 10.Brindle, P., S. Linke, and M. Montminy. 1993. Protein-kinase-A-dependent activator in transcription factor CREB reveals new role for CREM repressors. Nature 364:821-824. [DOI] [PubMed] [Google Scholar]
- 11.Cantrell, D. 1996. T cell antigen receptor signal transduction pathways. Annu. Rev. Immunol. 14:259-274. [DOI] [PubMed] [Google Scholar]
- 12.Coleman, R. A., W. L. Smith, and S. Narumiya. 1994. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 46:205-229. [PubMed] [Google Scholar]
- 13.Connor, L. M., M. N. Oxman, J. N. Brady, and S. J. Marriott. 1993. Twenty-one base pair repeat elements influence the ability of a Gal4-Tax fusion protein to transactivate the HTLV-I long terminal repeat. Virology 195:569-577. [DOI] [PubMed] [Google Scholar]
- 14.Copeland, K. F. T., P. J. Hendrikx, A. G. M. Haaksma, S. Fiering, R. Van Lier, J. Goudsmit, and J. L. Heeney. 1995. Comparison of the response to T-cell activation by integrated HIV-1 and HTLV-1 LTR-lacZ vectors. Virology 209:633-636. [DOI] [PubMed] [Google Scholar]
- 15.D'Agostino, D. M., V. Ciminale, L. Zotti, and L. Chieco-Bianchi. 1999. Influence of Rex and intronic sequences on expression of spliced mRNAs produced by human T cell leukemia virus type I. AIDS Res. Hum. Retrovir. 15:1351-1363. [DOI] [PubMed] [Google Scholar]
- 16.Dumais, N., B. Barbeau, M. Olivier, and M. J. Tremblay. 1998. Prostaglandin E2 upregulates HIV-1 LTR-driven gene activity in T cells via NF-κB-dependent and -independent signaling pathways. J. Biol. Chem. 273:27306-27314. [DOI] [PubMed] [Google Scholar]
- 17.Dumais, N., S. Bounou, M. Olivier, and M. J. Tremblay. 2002. Prostaglandin E2-mediated activation of HIV-1 long terminal repeat transcription in human T cells necessitates CCAAT/enhancer binding protein (C/EBP) binding sites in addition to cooperative interactions between C/EBPβ and cyclic adenosine 5′-monophosphate response element binding protein. J. Immunol. 168:274-282. [DOI] [PubMed] [Google Scholar]
- 18.Duvall, J. F., F. Kashanchi, A. Cvekl, M. F. Radonovich, G. Piras, and J. N. Brady. 1995. Transactivation of the human T-cell lymphotropic virus type 1 Tax1-responsive 21-base-pair repeats requires Holo-TFIID and TFIIA. J. Virol. 69:5077-5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enslen, H., H. Tokumitsu, and T. R. Soderling. 1995. Phosphorylation of CREB by CaM-kinase IV activated by CaM-kinase IV kinase. Biochem. Biophys. Res. Commun. 207:1038-1043. [DOI] [PubMed] [Google Scholar]
- 20.Farrell, J. P., and C. E. Kirkpatricj. 1987. Experimental cutaneous leishmaniasis. II. A possible role for prostaglandins in exacerbation of disease in Leishmania major-infected BALB/c mice. J. Immunol. 138:902-907. [PubMed] [Google Scholar]
- 21.Fedyk, E. R., and R. P. Phipps. 1996. Prostaglandin E2 receptors of the EP2 and EP4 subtypes regulate activation and differentiation of mouse B lymphocytes to IgE-secreting cells. Proc. Natl. Acad. Sci. USA 93:10978-10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felber, B. K., H. Paskalis, C. Kleinman-Ewing, F. Wong-Staal, and G. N. Pavlakis. 1985. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science 229:675-679. [DOI] [PubMed] [Google Scholar]
- 23.Franchini, G. 1995. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood 86:3619-3639. [PubMed] [Google Scholar]
- 24.Fujii, M., H. Tsuchiya, T. Chuhjo, T. Akizawa, and M. Seiki. 1992. Interaction of HTLV-1 Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 6:2066-2076. [DOI] [PubMed] [Google Scholar]
- 25.Fujisawa, J., M. Toita, and M. Yoshida. 1989. A unique enhancer element for the trans activator (p40tax) of human T-cell leukemia virus type I that is distinct from cyclic AMP- and 12-O-tetradecanoylphorbol-13-acetate-responsive elements. J. Virol. 63:3234-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujisawa, J., M. Toita, T. Yoshimura, and M. Yoshida. 1991. The indirect association of human T-cell leukemia virus tax protein with DNA results in transcriptional activation. J. Virol. 65:4525-4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furukawa, Y., M. Osame, R. Kubota, M. Tara, and M. Yoshida. 1995. Human T-cell leukemia virus type-1 (HTLV-1) Tax is expressed at the same level in infected cells of HTLV-1-associated myelopathy or tropical spastic paraparesis patients as in asymptomatic carriers but at a lower level in adult T-cell leukemia cells. Blood 85:1865-1870. [PubMed] [Google Scholar]
- 28.Geleziunas, R., S. Ferrell, X. Lin, Y. Mu, E. T. Cunningham, Jr., M. Grant, M. A. Connelly, J. E. Hambor, K. B. Marcu, and W. C. Greene. 1998. Human T-cell leukemia virus type 1 Tax induction of NF-κB involves activation of the IκB kinase α (IKKα) and IKKβ cellular kinases. Mol. Cell. Biol. 18:5157-5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerozissis, K., P. Jouannet, J. C. Soufir, and F. Dray. 1982. Origin of prostaglandins in human semen. J. Reprod. Fertil. 65:401-404. [DOI] [PubMed] [Google Scholar]
- 30.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407-410. [DOI] [PubMed] [Google Scholar]
- 31.Gessain, A., F. Saal, O. Gout, M. T. Daniel, G. Flandrin, G. de The, J. Peries, and F. Sigaux. 1990. High human T-cell lymphotropic virus type I proviral DNA load with polyclonal integration in peripheral blood mononuclear cells of French West Indian, Guianese, and African patients with tropical spastic paraparesis. Blood 75:428-433. [PubMed] [Google Scholar]
- 32.Giam, C. Z., and Y. L. Xu. 1989. HTLV-I tax gene product activates transcription via pre-existing cellular factors and cAMP responsive element. J. Biol. Chem. 264:15236-15241. [PubMed] [Google Scholar]
- 33.Gillis, S., and J. Watson. 1980. Biochemical and biological characterization of lymphocyte regulatory molecules. V. Identification of an interleukin 2-producing human leukemia T cell line. J. Exp. Med. 152:1709-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ginty, D. D., A. Bonni, and M. E. Greenberg. 1994. Nerve growth factor activates a Ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell 77:713-725. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez, G. A., and M. R. Montminy. 1989. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59:675-680. [DOI] [PubMed] [Google Scholar]
- 36.Habener, J. F. 1990. Cyclic AMP response element binding proteins: a cornucopia of transcription factors. Mol. Endocrinol. 4:1087-1094. [DOI] [PubMed] [Google Scholar]
- 37.Henke, A., H. P. Spengler, A. Stelzner, M. Nain, and D. Gemsa. 1992. Lipopolysaccharide suppresses cytokine release from coxsackie virus-infected human monocytes. Res. Immunol. 143:65-70. [DOI] [PubMed] [Google Scholar]
- 38.Iñiguez, M. A., C. Punzon, and M. Fresno. 1999. Induction of cyclooxygenase-2 on activated T lymphocytes: regulation of T cell activation by cyclooxygenase-2 inhibitors. J. Immunol. 163:111-119. [PubMed] [Google Scholar]
- 39.Jeang, K.-T., I. Boros, J. Brady, M. Radonovich, and G. Khoury. 1988. Characterization of cellular factors that interact with the human T-cell leukemia virus type 1 p40x-responsive 21-base-pair sequence. J. Virol. 62:4499-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kazanji, M., A. Ureta-Vidal, S. Ozden, F. Tangy, B. De Thoisy, L. Fiette, A. Talarmin, A. Gessain, and G. De Thé. 2000. Lymphoid organs as a major reservoir for human T-cell leukemia virus type 1 in experimentally infected squirrel monkeys (Saimiri sciureus): proviral expression, persistence, and humoral and cellular immune responses. J. Virol. 74:4860-4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kernacki, K. A., and R. S. Berk. 1994. Characterization of the inflammatory response induced by corneal infection with Pseudomonas aeruginosa. J. Ocul. Pharmacol. 10:281-288. [DOI] [PubMed] [Google Scholar]
- 42.Koretzky, G. A., J. Picus, T. Schultz, and A. Weiss. 1991. The tyrosine phosphatase CD45 is required for T-cell antigen receptor and CD2-mediated activation of a protein tyrosine kinase and interleukin 2 production. Proc. Natl. Acad. Sci. USA 88:2037-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lenschow, D. J., T. L. Walunas, and J. A. Bluestone. 1996. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14:233-258. [DOI] [PubMed] [Google Scholar]
- 44.Lin, H.-C., C. S. Dezzutti, R. B. Lal, and A. B. Rabson. 1998. Activation of human T-cell leukemia virus type 1 tax gene expression in chronically infected T cells. J. Virol. 72:6264-6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marriott, S. J., I. Boros, J. Duvall, and J. Brady. 1989. Indirect binding of human T-cell leukemia virus type I taxI to a responsive element in the viral long terminal repeat. Mol. Cell. Biol. 9:4152-4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marriott, S. J., P. F. Lindholm, K. M. Brown, S. D. Gitlin, J. F. Duvall, M. F. Radonovich, and J. N. Brady. 1990. A 36-kilodalton cellular transcription factor mediates an indirect interaction of human T-cell leukemia/lymphoma virus type I TAX1 with a responsive element in the viral long terminal repeat. Mol. Cell. Biol. 10:4192-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin, P. J., J. A. Ledbetter, Y. Morishita, C. H. June, P. G. Beatty, and J. A. Hansen. 1986. A 44 kilodalton cell surface homodimer regulates interleukin 2 production by activated human T lymphocytes. J. Immunol. 136:3282-3287. [PubMed] [Google Scholar]
- 48.Mesnard, J. M., and C. Devaux. 1999. Multiple control levels of cell proliferation by human T-cell leukemia virus type 1 Tax protein. Virology 257:277-284. [DOI] [PubMed] [Google Scholar]
- 49.Midulla, F., Y. T. Huang, I. A. Gilbert, N. M. Cirino, E. R. McFadden, Jr., and J. R. Panuska. 1989. Respiratory syncytial virus infection of human cord and adult blood monocytes and alveolar macrophages. Am. Rev. Respir. Dis. 140:771-777. [DOI] [PubMed] [Google Scholar]
- 50.Mori, N., H. Inoue, T. Yoshida, T. Tanabe, and N. Yamamoto. 2001. Constitutive expression of the cyclooxygenase-2 gene in T-cell lines infected with human T cell leukemia virus type I. Int. J. Cancer 94:813-819. [DOI] [PubMed] [Google Scholar]
- 51.Moriuchi, M., H. Inoue, and H. Moriuchi. 2001. Reciprocal interactions between human T-lymphotropic virus type 1 and prostaglandins: implications for viral transmission. J. Virol. 75:192-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muchardt, C., J. S. Seeler, A. Nirula, S. Gong, and R. Gaynor. 1992. Transcription factor AP-2 activates gene expression of HTLV-I. EMBO J. 11:2573-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muthusamy, N., and J. M. Leiden. 1998. A protein kinase C-, Ras-, and RSK2-dependent signal transduction pathway activates the cAMP-responsive element-binding protein transcription factor following T cell receptor engagement. J. Biol. Chem. 273:22841-22847. [DOI] [PubMed] [Google Scholar]
- 54.Nagai, M., M. B. Brennan, J. A. Sakai, C. A. Mora, and S. Jacobson. 2001. CD8+ T cells are an in vivo reservoir for human T-cell lymphotropic virus type I. Blood 98:1858-1861. [DOI] [PubMed] [Google Scholar]
- 55.Nyborg, J. K., and W. S. Dynan. 1990. Interaction of cellular proteins with the human T-cell leukemia virus type I transcriptional control region. Purification of cellular proteins that bind the 21-base pair repeat elements. J. Biol. Chem. 265:8230-8236. [PubMed] [Google Scholar]
- 56.Ohshima, K., K. Hashimoto, S. Izumo, J. Suzumiya, and M. Kikuchi. 1996. Detection of human T lymphotrophic virus type I (HTLV-I) DNA and mRNA in individual cells by polymerase chain reaction (PCR) in situ hybridization (ISH) and reverse transcription (RT)-PCR ISH. Hematol. Oncol. 14:91-100. [DOI] [PubMed] [Google Scholar]
- 57.Okada, M., and K. T. Jeang. 2002. Differential requirements for activation of integrated and transiently transfected human T-cell leukemia virus type 1 long terminal repeat. J. Virol. 76:12564-12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Onta, T., M. Sachida, N. Fijii, S. Sugawara, H. Rikiishi, and K. Kumagai. 1993. Induction of acute arthritis in mice by peptidoglycan derived from gram-positive bacteria and its possible role in cytokine production. Microbiol. Immunol. 37:573-582. [DOI] [PubMed] [Google Scholar]
- 59.Phipps, R. P., S. H. Stein, and R. L. Roper. 1991. A new view of prostaglandin E regulation of the immune response. Immunol. Today 12:349-352. [DOI] [PubMed] [Google Scholar]
- 60.Poiesz, B., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poteat, H., P. Kadison, K. McGuire, L. Park, R. E. Park, J. G. Sodroski, and W. A. Haseltine. 1989. Response of the human T-cell leukemia virus type 1 long terminal repeat to cyclic AMP. J. Virol. 63:1604-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pyeon, D., F. J. Diaz, and G. A. Splitter. 2000. Prostaglandin E2 increases bovine leukemia virus tax and pol mRNA levels via cyclooxygenase 2: regulation of interleukin-2, interleukin-10, and bovine leukemia virus. J. Virol. 74:5740-5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quayle, A. J., R. W. Kelly, T. B. Hargreave, and K. James. 1989. Immunosuppression by seminal prostaglandins. Clin. Exp. Immunol. 75:387-391. [PMC free article] [PubMed] [Google Scholar]
- 64.Rastogi, N., M. Bachelet, and J. P. Carvalho-de-Sousa. 1992. Intracellular growth of Mycobacterium avium in human macrophages is linked to the increased synthesis of prostaglandin E2 and inhibition of the phagosome-lysosome fusions. FEMS Microbiol. Immunol. 4:273-279. [DOI] [PubMed] [Google Scholar]
- 65.Reiner, N. E., and C. J. Malemud. 1984. Arachidonic acid metabolism in murine leishmaniasis (Donovani): ex vivo evidence for increased cyclooxygenase and 5-lipoxygenase activity in spleen cells. Cell. Immunol. 134:556-563. [DOI] [PubMed] [Google Scholar]
- 66.Richardson, J. H., A. J. Edwards, J. K. Cruickshank, P. Rudge, and A. G. Dalgleish. 1990. In vivo cellular tropism of human T-cell leukemia virus type 1. J. Virol. 64:5682-5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Richardson, J. H., P. Hollsberg, A. Windhagen, L. A. Child, D. A. Hafler, and A. M. L. Lever. 1997. Variable immortalizing potential and frequent virus latency in blood-derived T-cell clones infected with human T-cell leukemia virus type I. Blood 89:3303-3314. [PubMed] [Google Scholar]
- 68.Schreiber, E., P. Matthias, M. Müller, and W. Schaffner. 1989. Rapid detection of octamer binding proteins with ‘mini-extract’ prepared from a small number of cells. Nucleic Acids Res. 17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shannon, M. F., S. R. Himes, and L. S. Coles. 1995. GM-CSF and IL-2 share common control mechanisms in response to costimulatory signals in T cells. J. Leukoc. Biol. 57:767-773. [DOI] [PubMed] [Google Scholar]
- 70.Shimotohno, K., M. Takano, T. Teruuchi, and M. Miwa. 1986. Requirement of multiple copies of a 21-nucleotide sequence in the U3 regions of human T-cell leukemia virus type I and type II long terminal repeats for trans-acting activation of transcription. Proc. Natl. Acad. Sci. USA 83:8112-8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Slamon, D. J., K. Shimotohno, M. J. Cline, D. W. Golde, and I. S. Chen. 1984. Identification of the putative transforming protein of the human T-cell leukemia viruses HTLV-I and HTLV-II. Science 226:61-65. [DOI] [PubMed] [Google Scholar]
- 72.Sodroski, J., C. Rosen, W. C. Goh, and W. Haseltine. 1985. A transcriptional activator protein encoded by the x-lor region of the human T-cell leukemia virus. Science 228:1430-1434. [DOI] [PubMed] [Google Scholar]
- 73.Sorrell, T. C., C. P. Rochester, F. N. Breen, and M. Muller. 1989. Eicosanoids produced during interactions between Pseudomonas aeruginosa and alveolar macrophages are species-dependent. Immunol. Cell Biol. 67:169-176. [DOI] [PubMed] [Google Scholar]
- 74.Straus, D. B., and A. Weiss. 1992. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell 70:585-593. [DOI] [PubMed] [Google Scholar]
- 75.Suzuki, T., J. I. Fujisawa, M. Toita, and M. Yoshida. 1993. The transactivator tax of human T-cell leukemia virus type 1 (HTLV-1) interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21-base-pair enhancer of HTLV-1. Proc. Natl. Acad. Sci. USA 90:610-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suzuki, T., H. Hirai, J. Fujisawa, T. Fujita, and M. Yoshida. 1993. A trans-activator Tax of human T-cell leukemia virus type 1 binds to NF-kappa B p50 and serum response factor (SRF) and associates with enhancer DNAs of the NF-kappa B site and CArG box. Oncogene 8:2391-2397. [PubMed] [Google Scholar]
- 77.Tanimura, A., H. Teshima, J.-I. Fujisawa, and M. Yoshida. 1993. A new regulatory element that augments the Tax-dependent enhancer of human T-cell leukemia virus type 1 and cloning of cDNAs encoding its binding proteins. J. Virol. 67:5375-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walton, K. M., R. P. Rehfuss, J. C. Chrivia, J. E. Lochner, and R. H. Goodman. 1992. A dominant repressor of cyclic adenosine 3′, 5′-monophosphate (cAMP)-regulated enhancer-binding protein activity inhibits the cAMP-mediated induction of the somatostatin promoter in vivo. Mol. Endocrinol. 6:647-655. [DOI] [PubMed] [Google Scholar]
- 79.Wang, W., and K. Chadee. 1992. Entamoeba histolytica alters arachidonic acid metabolism in macrophages in vitro and in vivo. Immunology 76:242-250. [PMC free article] [PubMed] [Google Scholar]
- 80.Wang, W., and K. Chadee. 1995. Entamoeba histolytica suppresses gamma interferon-induced macrophage class II major histocompatibility complex Ia molecule and I-Aβ mRNA expression by a prostaglandin E2-dependent mechanism. Infect. Immun. 63:1089-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weiss, A., and J. D. Stobo. 1984. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J. Exp. Med. 160:1284-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang, D., Y. Haraguchi, H. Iwai, A. Handa, N. Shimizu, and H. Hoshino. 1994. Inhibition of adsorption of human T-cell-leukemia virus type 1 by a plant lectin, wheat-germ agglutinin. Int. J. Cancer 56:100-105. [DOI] [PubMed] [Google Scholar]
- 83.Yin, M. J., and R. B. Gaynor. 1996. Complex formation between CREB and Tax enhances the binding affinity of CREB for the human T-cell leukemia virus type 1 21-base-pair repeats. Mol. Cell. Biol. 16:3156-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoshimura, T., J. Fujisawa, and M. Yoshida. 1990. Multiple cDNA clones encoding nuclear proteins that bind to the tax-dependent enhancer of HTLV-1: all contain a leucine zipper structure and basic amino acid domain. EMBO J. 9:2537-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhong, H., H. SuYang, H. Erdjument-Bromage, P. Tempst, and S. Ghosh. 1997. The transcriptional activity of NF-κB is regulated through the IκB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89:413-424. [DOI] [PubMed] [Google Scholar]