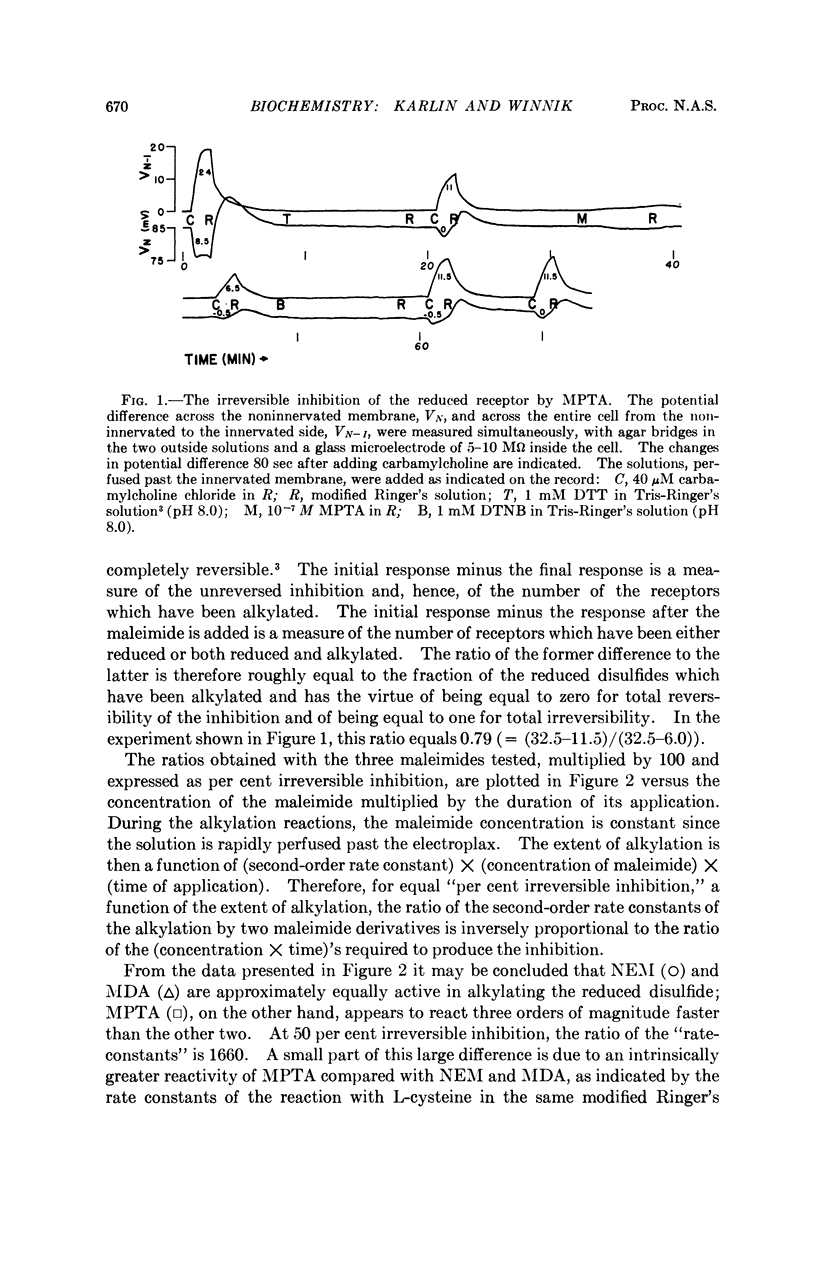
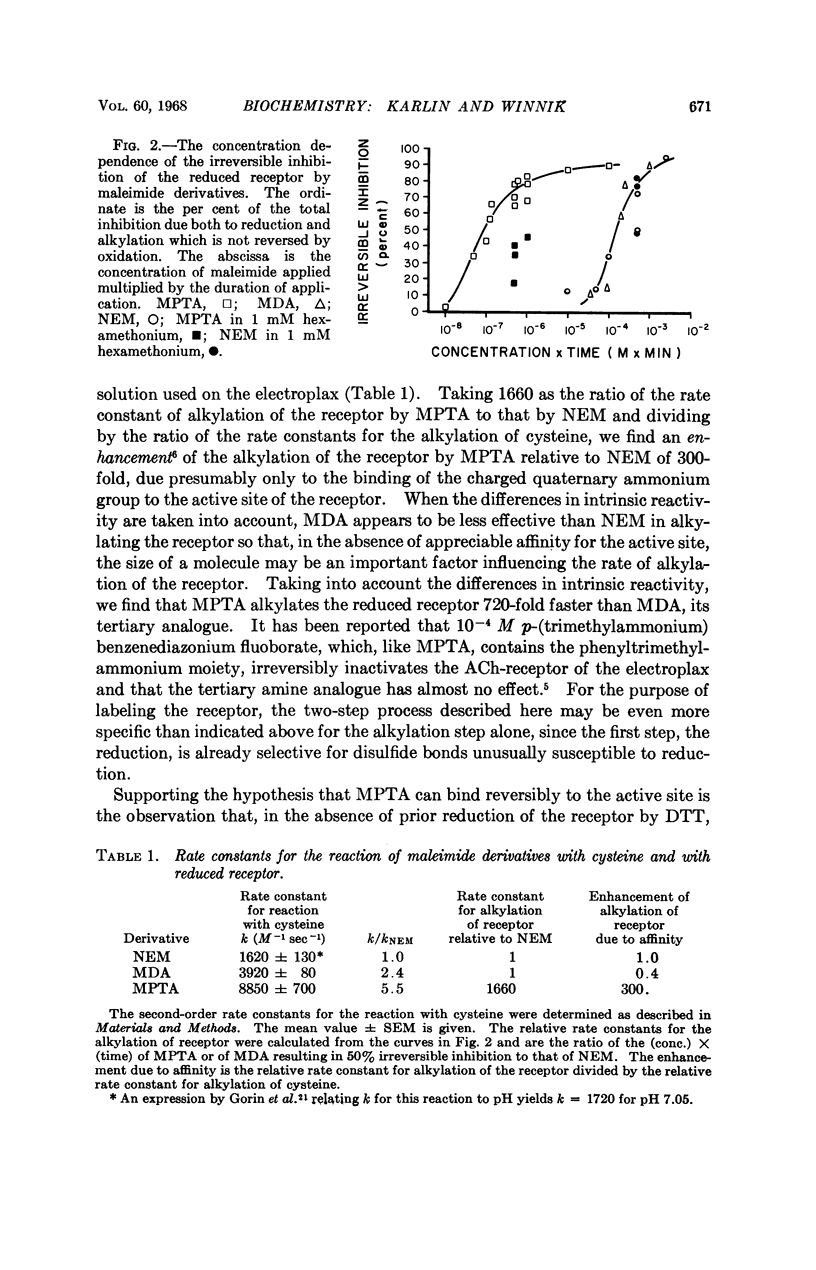
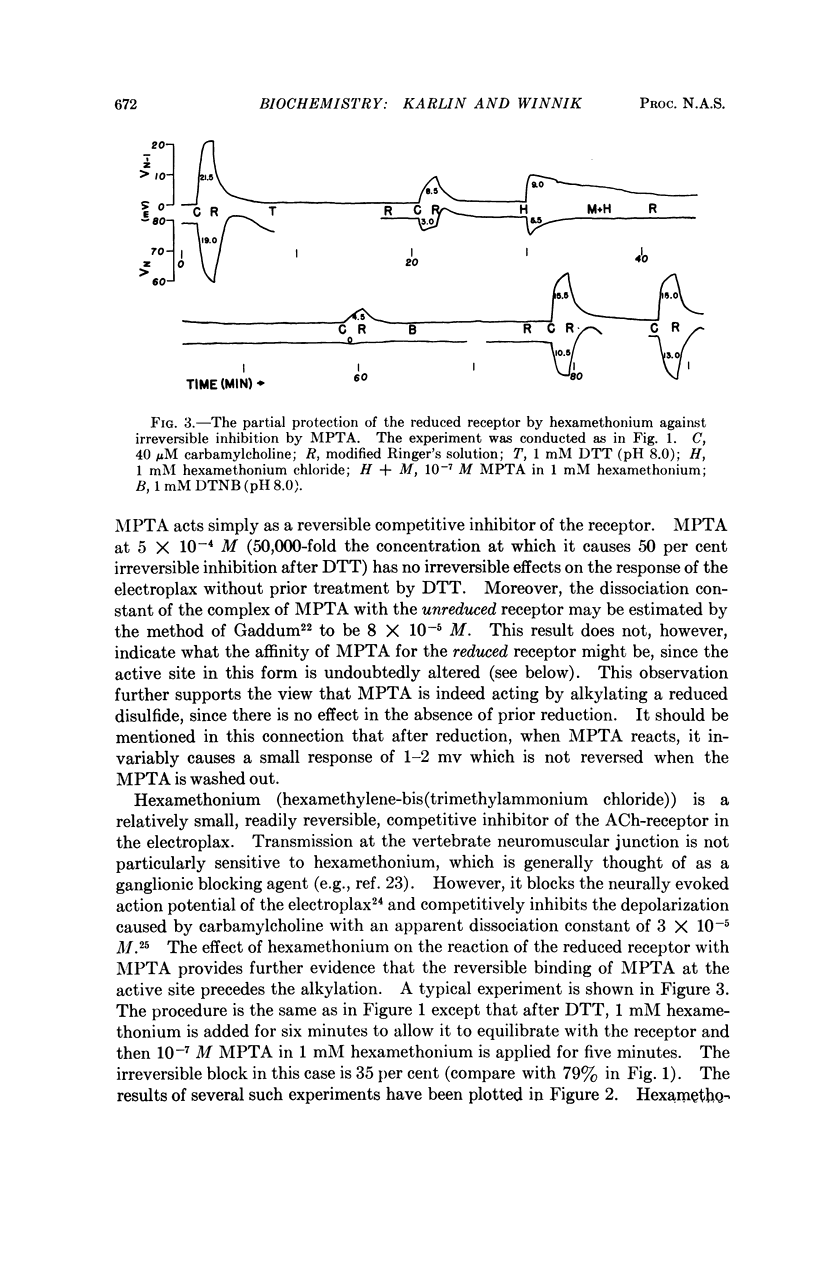
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLELAND W. W. DITHIOTHREITOL, A NEW PROTECTIVE REAGENT FOR SH GROUPS. Biochemistry. 1964 Apr;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Podleski T. R. On the excitability and cooperativity of the electroplax membrane. Proc Natl Acad Sci U S A. 1968 Mar;59(3):944–950. doi: 10.1073/pnas.59.3.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Podleski T. R., Wofsy L. Affinity labeling of the acetylcholine-receptor. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2063–2070. doi: 10.1073/pnas.58.5.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R. F. Dibenamine blockade in strips of rabbit aorta and its use in differentiating receptors. J Pharmacol Exp Ther. 1954 Jul;111(3):265–284. [PubMed] [Google Scholar]
- Gill E. W., Rang H. P. An alkylating derivative of benzilylcholine with specific and long-lasting parasympatholytic activity. Mol Pharmacol. 1966 Jul;2(4):284–297. [PubMed] [Google Scholar]
- Gorin G., Martic P. A., Doughty G. Kinetics of the reaction of N-ethylmaleimide with cysteine and some congeners. Arch Biochem Biophys. 1966 Sep 9;115(3):593–597. doi: 10.1016/0003-9861(66)90079-8. [DOI] [PubMed] [Google Scholar]
- Karlin A., Bartels E. Effects of blocking sulfhydryl groups and of reducing disulfide bonds on the acetylcholine-activated permeability system of the electroplax. Biochim Biophys Acta. 1966 Nov 8;126(3):525–535. doi: 10.1016/0926-6585(66)90010-0. [DOI] [PubMed] [Google Scholar]
- Karlin A. Chemical distinctions between acetylcholinesterase and the acetylcholine receptor. Biochim Biophys Acta. 1967 Jul 11;139(2):358–362. doi: 10.1016/0005-2744(67)90039-3. [DOI] [PubMed] [Google Scholar]
- Karlin A. On the application of "a plausible model" of allosteric proteins to the receptor for acetylcholine. J Theor Biol. 1967 Aug;16(2):306–320. doi: 10.1016/0022-5193(67)90011-2. [DOI] [PubMed] [Google Scholar]
- Karlin A. Permeability and internal concentration of ions during depolarization of the electroplax. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1162–1167. doi: 10.1073/pnas.58.3.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress L. F., Laskowski M., Sr The basic trypsin inhibitor of bovine pancreas. VII. Reduction with borohydride of disulfide bond linking half-cystine residues 14 and 38. J Biol Chem. 1967 Nov 10;242(21):4925–4929. [PubMed] [Google Scholar]
- Light A., Sinha N. K. Difference in the chemical reactivity of the disulfide bonds of trypsin and chymotrypsin. J Biol Chem. 1967 Mar 25;242(6):1358–1359. [PubMed] [Google Scholar]
- Mautner H. G., Bartels E., Webb G. D. Sulfur and selenium isologs related to acetycholine. and choline IV. Activity in the electroplax preparation. Biochem Pharmacol. 1966 Feb;15(2):187–193. doi: 10.1016/0006-2952(66)90059-1. [DOI] [PubMed] [Google Scholar]
- Neurath H., Walsh K. A., Winter W. P. Evolution of structure and function of proteases. Science. 1967 Dec 29;158(3809):1638–1644. doi: 10.1126/science.158.3809.1638. [DOI] [PubMed] [Google Scholar]
- Podleski T. R., Nachmansohn D. Similarities between active sites of acetylcholine receptor and acetylcholinesterase tested with quinolinium ions. Proc Natl Acad Sci U S A. 1966 Sep;56(3):1034–1039. doi: 10.1073/pnas.56.3.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOFFENIELS E. An isolated single electroplax preparation. II. Improved preparation for studying ion flux. Biochim Biophys Acta. 1957 Dec;26(3):585–596. doi: 10.1016/0006-3002(57)90106-3. [DOI] [PubMed] [Google Scholar]
- Singer S. J. Covalent labeling of active sites. Adv Protein Chem. 1967;22:1–54. doi: 10.1016/s0065-3233(08)60040-6. [DOI] [PubMed] [Google Scholar]
- Stryer L., Holmgren A., Reichard P. Thioredoxin. A localized conformational change accompanying reduction of the protein to the sulfhydryl form. Biochemistry. 1967 Apr;6(4):1016–1020. doi: 10.1021/bi00856a009. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. On the permeability of end-plate membrane during the action of transmitter. J Physiol. 1960 Nov;154:52–67. doi: 10.1113/jphysiol.1960.sp006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volle R. L. Blockade by hexamethonium of drug-induced neuromuscular facilitation. Arch Int Pharmacodyn Ther. 1967 May;167(1):1–9. [PubMed] [Google Scholar]


