Abstract
Developmental exposure to the organophosphorus pesticides chlorpyrifos and diazinon (DZN) alters serotonergic synaptic function at doses below the threshold for cholinesterase inhibition but there are some indications that the two agents may differ in several important attributes. Previously, we found that low-dose chlorpyrifos exposure in neonatal rats causes lasting changes in emotional response and in the current study we did a comparable evaluation for DZN. Male and female Sprague-Dawley rat pups (N=10-12 of each sex per treatment group) were given 0, 0.5 or 2 mg/kg/day of DZN s.c. daily on postnatal days (PND) 1-4. These doses bracket the threshold for barely-detectable cholinesterase inhibition. Starting on PND 52, these rats began a battery of tests to assess emotional reactivity. In the elevated plus maze, there was a slight decrease in the time spent in the open arms for DZN-exposed males, while DZN-exposed females were not different from control females. In the novelty-suppressed feeding test, DZN-exposed males had significantly shorter latencies to begin eating than did control males, reducing the values to those normally seen in females. DZN-exposed rats of either sex showed reduced preference for chocolate milk in the anhedonia test that compared the consumption of chocolate milk to water. These findings show that neonatal exposures to DZN at doses below the threshold for cholinesterase inhibition nevertheless evoke specific, later alterations in emotional behaviors, particularly in males. The effects show some similarities to those of chlorpyrifos but also some differences, in keeping with neurochemical findings comparing the two agents.
Keywords: organophosphorous pesticides, diazinon, emotion, depression, anxiety, serotonin, high-fat diet
Introduction
Organophosphorus (OP) pesticides comprise over half of all insecticide use worldwide, and human exposure is known to be virtually ubiquitous in the U.S. [12]. The mechanism for high-dose systemic toxicity of OPs is cholinesterase inhibition and subsequent cholinergic overstimulation [21,23]. All OPs have this effect at high doses, however they also have other mechanisms of toxicity that can vary from compound to compound within the OP class. For this reason we have extended our earlier work with chlorpyrifos to include other members of the OP class of pesticide including diazinon another widely used pesticide. Recent studies have found that OPs can alter brain development at doses below the threshold for cholinesterase inhibition, involving these additional mechanisms and reflecting the direct impact of these compounds on differentiation of neural cells (reviews, [29-31]).
Serotonin systems appear to be among the most vulnerable to disruption by low doses of OPs, displaying both immediate and long-term changes in neurochemical indices of synaptic integrity and function [1-5,26,34-36]. We recently published an extensive series of studies with prenatal or neonatal chlorpyrifos (CPF) exposure in rats that demonstrated permanent upregulation of 5HT receptors, accompanied by presynaptic hyperactivity emerging in adolescence, and these synaptic changes were associated with deficits in 5HT-related behaviors [1,2,5,34,35]. Given the important roles played by 5HT systems in emotional regulation, these results suggest that emotional function may be compromised [16].
The fact that OPs disrupt neurodevelopment through mechanisms other than cholinesterase inhibition raises the likelihood that the various OPs differ in their impact on specific neurotransmitter systems or developmental processes. Indeed, we compared CPF and diazinon (DZN) with regard to their thresholds for cholinesterase inhibition in the neonatal rat brain and the immediate effects on indices of neural cell differentiation as well as specific 5HT synaptic development [17,33,36,37]. Even at doses devoid of detectable cholinesterase inhibition, these agents impaired neurite outgrowth and evoked significant changes in expression of 5HT-related genes and up-regulation of 5HT receptors, all with a regional pattern indicative of damage to developing 5HT nerve terminals. Critically, many of these effects appeared to be different or worse for DZN exposure than those seen with comparable CPF exposures, again suggesting that the long-term outcomes of from exposures to these two OPs may exhibit some differences as well as similarities.
In the current study, we evaluated the neurobehavioral consequences of neonatal DZN exposure in a battery of emotional tests related to 5HT function, including several that were evaluated in our prior studies for CPF [1]. A battery of four tests of emotional function was used. The elevated plus maze is a widely used test of anxiety in rodent models [14,15]. Novelty-Suppressed Feeding provides an index of the initial fearfulness in a new environment [9-11] Chocolate Milk Anhedonia Test provides an index of the motivation for engaging in normally pleasurable activities, in this case drinking sweet liquids [1]. The Porsolt forced swim test provides a standard assessment of depressive symptoms [24]. In addition, we examined the interaction between diet and weight gain in these DZN-exposed rats. There is some preliminary evidence that development OP exposures can increase weight gain as animals age [18]. Here, we assessed the weekly weight gain after early postnatal DZN exposure, both on a normal diet and on a high-fat diet
Methods
Animal Treatments
All experiments were carried out humanely and with regard for alleviation of suffering, with protocols approved by the Duke University Institutional Animal Care and Use Committee and in accordance with all federal and state guidelines. Thirty-six timed-pregnant Sprague-Dawley rats (Charles River, Raleigh, NC) were housed in plastic breeding cages with a 12-hr light/dark cycle and ad lib access to food (Purina Rodent Chow Diet 5001; Ralston-Purina, St. Louis, MO, USA) and water. On the day of birth, all pups were randomized and redistributed to the dams to achieve a litter size of 12 (6 males and 6 females). Same-sexed rats from each litter were weighed in a group and given daily subcutaneous injections of 0, 0.5, or 2 mg/kg DZN (Chem Service, West Chester, PA) on postnatal days (PND) 1-4. Rats were randomized within treatment group and in addition, dams were rotated among litters to distribute any maternal caretaking differences randomly across litters and treatment groups. Because of its poor water solubility, DZN was dissolved in dimethylsulfoxide to provide consistent absorption [40] and was injected at a volume of 1 ml/kg once daily. Control rats received equivalent injections of the dimethylsulfoxide vehicle. These doses of DZN have been shown previously to span the threshold for barely-detectable cholinesterase inhibition [37] but nevertheless cause significant alterations in brain development, including the serotonin system [33,37]. Pups were weaned on PND21 and transferred to another animal housing room with a reverse dark-light cycle (lights on at 800). All behavioral testing was conducted during the dark phase, when the nocturnal rat is the most active, but in a lighted room. One male and one female pup from each litter were randomly selected for the battery of emotional tests reported here. Another set of 1 pup/sex/litter was used in a battery of cognitive tests reported elsewhere [39], and the remaining animals were maintained for neurochemical evaluations that will be evaluated in future studies. Weaned pups were originally housed in same-sex and same-exposure groups of 6/cage, but as they grew, they were subdivided into groups of 3/cage. At the time of behavioral testing, the females were triple-housed while the males were pair-housed because of their larger size. Rats were weighed weekly until the assessments in the elevated plus maze (PND 52-56), novelty-suppressed feeding (PND 64-67), home cage feeding (PND 78-79), chocolate milk anhedonia test (PND 73-74), and forced swim test (PND 86-87). The high-fat diet was introduced to a subset of the animals on PND 105.
Elevated Plus Maze
The maze was constructed of black-painted wood with arms 55 cm long and 10.2 cm wide, standing 50.8 cm above the floor. Two opposed arms had walls that were 15.2 cm high, and the other two opposed arms, set at 90° to walled arms, had railings 2 cm tall. Rats were placed in the center of the maze facing an open arm and allowed to roam freely for a total of 300 s. The experimenter recorded the time spent on the closed arms with a stopwatch and the number of open and closed arm entries. An arm entry or exit was defined as all four paws crossing the arm threshold.
Novelty-Suppressed Feeding
The methods for novelty-suppressed feeding were modified from previous descriptions [9-11]. Rats were food-deprived for approximately 24 hours prior to testing. A novel environment was created by placing an older style plastic rectangular cage, which was slightly different from their home cage in the middle of the test room without any bedding or cage top. Each rat was tested in a clean novel cage. Twelve food pellets same as those used in their regular diet (Purina Rodent Chow Diet 5001; Ralston-Purina, St. Louis, MO, USA) and approximately 2.5 cm in length were weighed prior to testing and spread across the floor of the cage in 4 rows of 3 pellets. Each rat was tested for 10 min in the novel cage, and the experimenter recorded the latency to begin eating, the number of eating sessions, and the total time spent eating. Eating was defined as chewing and not merely sniffing, holding or carrying the food pellet around in their mouth. Remaining food pellets were weighed after testing to determine the amount of food eaten during the test period.
Previous reports of novelty-suppressed feeding have evaluated home cage feeding to determine if the experimental condition alters appetite or motivation to eat [8,11]. Thus, home cage feeding was evaluated in rats one week following the novelty-suppressed feeding. Rats were again food deprived for approximately 24 hours and were tested with their cage mates in their home cage within the cage bank. Twelve pellets/rat were weighed and placed in the home cage in rows for the 10 min test session, and remaining pellets were weighed after to determine the amount of food eaten in the home cage.
Chocolate Milk Anhedonia Test
Methods were modified from a previous study [1]. One hour after the start of the dark cycle, rats were placed in individual clean cages in the test room. Rats were not food- or water-deprived prior to testing, but food was not available during the test session. Rats were presented with a choice of two bottles. One bottle contained tap water, and the other contained Hunter Farms Truly Chocolate Milk (High Point, NC, USA). All bottles were filled and weighed the day prior and refrigerated overnight. Bottles were weighed after the two-hour test session to determine the amount of fluid consumed, and it was assumed that 1 gram = 1 ml for both water and chocolate milk. Rats had no prior experience with drinking from bottles as their home cages had an automatic watering system.
Porsolt Forced Swim Test
This test provides a standard assessment of depressive symptoms [24]. Separate swim tanks were used for the male and female rats due to their size difference. The tank used for males was made of Plexiglas and measured 30 cm in diameter and 60 cm in height, and we used a water depth of 40 cm. The tank used for the females was made of glass (Pyrex) and measured 22 cm in diameter and 45 cm in height, and the water depth used was 30 cm. The water in the tanks was approximately 25°C. Rats were placed in the water for 5 min, and the experimenter recorded the total time the rat spent immobile with a stopwatch. Immobility was defined as at least three paws not moving.
High-Fat Diet
On postnatal day 105 after all of the behavioral tests were completed, half of the rats in each condition were put on an ad lib diet of high-fat food OpenSource Diet D12330 (Research Diets, Inc, New Brunswick, NJ, USA) with 23% protein, 35.5% carbohydrate and 35.8% fat. The other half of the rats in each group were maintained ad lib on regular chow (Purina Rodent Chow Diet 5001; Ralston-Purina, St. Louis, MO, USA). The high fat-diet was given for five weeks and then the rats were put back on regular chow for two weeks. This period was included to determine if there was a rebound effect when the diet was switched back to the standard chow.
Statistical Analysis
Two control males were excluded from the statistical analysis. One control male had to be euthanized before the conclusion of testing due to misaligned teeth, leaving the other control male in a single-housing condition that was inappropriate for behavioral testing given that all the other animals were group housed. This did not substantively alter the results. Data are presented as means and standard errors and treatment differences were analyzed by ANOVAs with sex and DZN dose as between-subjects factors. The cut-off for significance for main effects and planned treatment comparisons was p<0.05. For interactions at p<0.1, we also examined whether the lower-order main effects were detectable after subdivision of the interactive variables [38].
Results
Elevated plus maze
DZN exposure had sex-selective effects (DZN × sex interaction, F(2,66)=2.54, p<0.1). Males exposed to 2 mg/kg/day of DZN spent significantly (F(1,33)=4.63, p<0.05) less time in the open arms than did control males (Fig. 1), whereas no significant differences were seen in females. The DZN main effect for the total number of arm entries was at the margin for statistical significance (F(2,66)=2.83, p<0.07): controls, 10.5±0.7 entries; DZN 0.5 mg/kg/day, 11.9 ± 0.9 entries; DZN 2 mg/kg/day 9.8±0.8 entries. There were significant sex differences in the number of arm entries (F(1,66)=52.90, p<0.001), with females (13.3±0.5 entries) making more arm entries than males (8.1±0.5 entries).
Figure 1.
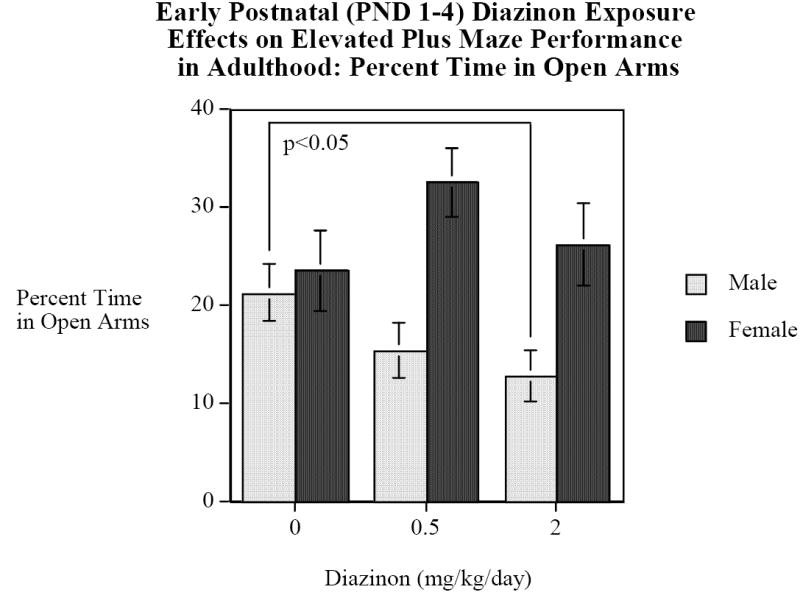
Elevated Plus Maze, percent time spent on the open arms (mean±sem). Males exposed to 2 mg/kg/d of DZN on PND 1-4 spent significantly (p<0.05) less time in the open arms than the control males
Novelty-suppressed feeding
The novelty-suppressed feeding test, like the elevated plus maze, was sensitive to sex-selective effects of DZN exposure. For latency to begin eating, there was an interaction of DZN × sex (F(2,64)=3.14, p<0.05). DZN-exposed males (both 0.5 and 2 mg/kg/day) had significantly (p<0.03) shorter latencies to begin eating than did control males (Fig. 2). There were no significant differences for females although the DZN 2 mg/kg/day group tended to have longer latencies than controls, thus again changing in the opposite direction from that of males. There were no significant DZN exposure-related differences in the number of eating bouts, total time spent eating or total amount of food eaten, but there were significant overall sex differences, with the males spending more time eating (F(1,64)=11.66, p<0.005; Males=198±13 s, Females=139±10 s) and eating more food than females (F(2,64)=51.37, p<0.0005; Males=2.8±0.1 g, Females=1.7±0.1 g). DZN had no detectable effect on feeding in the home cage, so that the effects seen in the novel environment did not simply represent an underlying difference in basal food consumption.
Figure 2.
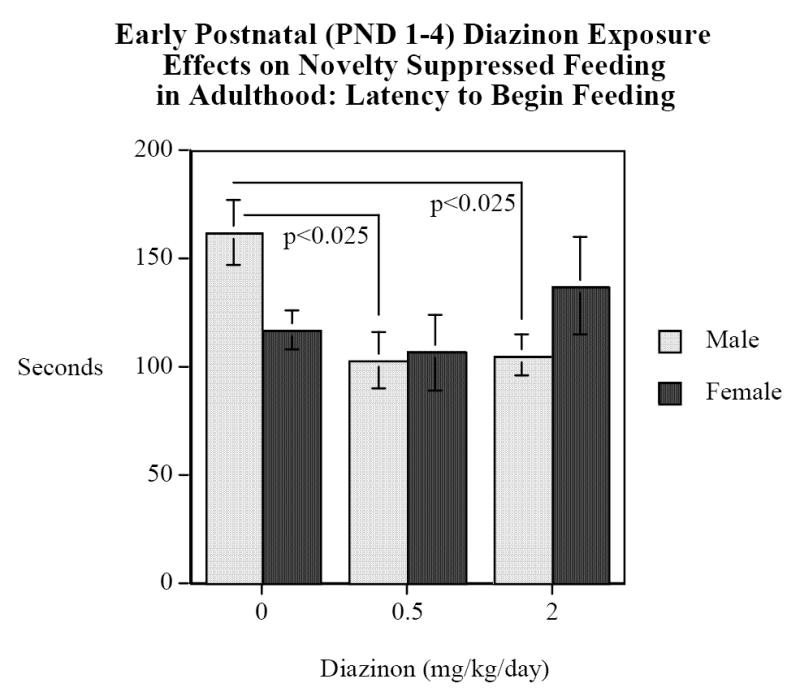
Novelty Suppressed Feeding, latency in seconds to begin eating (mean±sem). The males exposed to either 0.5 or 2 mg/kg/d DZN on PND 1-4 had significantly (p<0.03) shorter latencies than control males.
Chocolate Milk Anhedonia Test
The overall analysis of fluid consumption in this two-bottle choice test showed a significant main effect of increased chocolate milk vs. water consumption (F(1,63)=7.24, p<0.01) with the rats on the average drinking 15.5±1.3 ml of chocolate milk and 12.2±0.3 ml of water. There was a sex difference in the effects of DZN on fluid preference (fluid type × sex × DZN interaction, F(2,63)=2.97, p<0.06). In males, the 0.5 mg/kg/day DZN exposure significantly (F(1,63)=4.16, p<0.05) decreased the ratio to slightly below 1, which signifies no preference for chocolate milk. Raising the DZN dose to 2 mg/kg/day produced an effect in the males that was midway between the controls and the low dose males and was not statistically distinguishable from either of those groups. In females, there was not a significant effect toward lower chocolate milk preference at the higher DZN dose. It should be noted that the animals in this study showed a smaller preference for chocolate milk than seen in our earlier work with CPF [1], probably because the current set of animals had no prior experience with fluid bottles. The animals were housed in units with automatic watering spouts. The lower preference of controls for the chocolate milk in the current study may have made the test less sensitive.
Porsolt forced swim test
There were no significant DZN exposure-related differences in the immobility time. Control rats had an average time of 72±11 s versus 68±10 s and 73±11 s in the DZN 0.5 and 2 mg/kg/day groups, respectively. Males spent significantly (F(1,64)=41.00, p<0.0001) less time immobile than did females (males=39±5 s vs. females=101±8 s).
Body Weight on Normal and High-Fat Diets
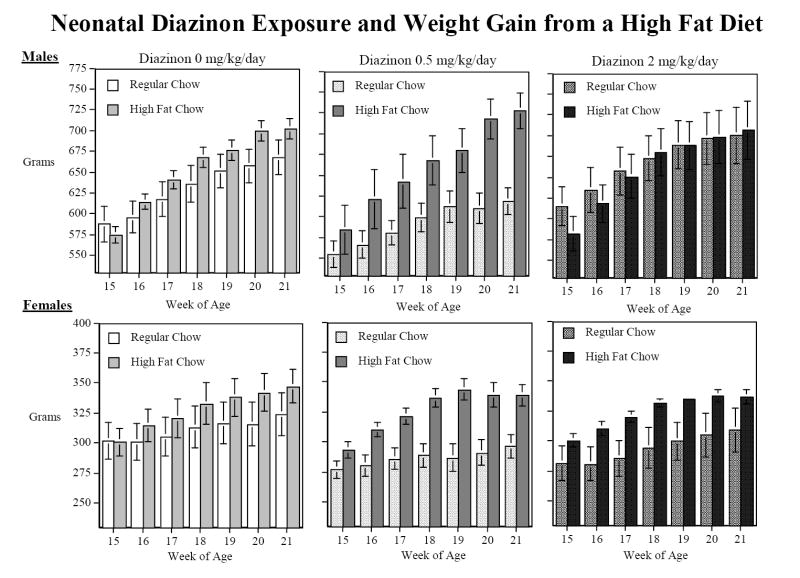
In keeping with our utilization of low doses of DZN below the threshold for any signs of systemic toxicity [33,37], there was no significant effect of neonatal DZN treatment on body weight gain (Fig. 4). After the end of the behavioral testing, approximately half of the rats in each treatment group were switched to a high-fat diet for five weeks while the other half were kept on regular chow. Then after the five weeks of high-fat diet the rats were switched back to the regular chow diet for two weeks. Interestingly, the high-fat diet increased weight gain in the rats given the low dose of DZN (DZN × diet × time, F(12,348)=2.11, p<0.05), whereas it did not do so in the controls or in animals exposed to the higher dose of DZN (Fig. 5). On the regular diet, the low dose DZN group tended to have lower body weights, albeit not significantly lower, and the high fat diet reversed this relationship so that the body weights then equaled or exceeded those in the other groups.
Figure 4.
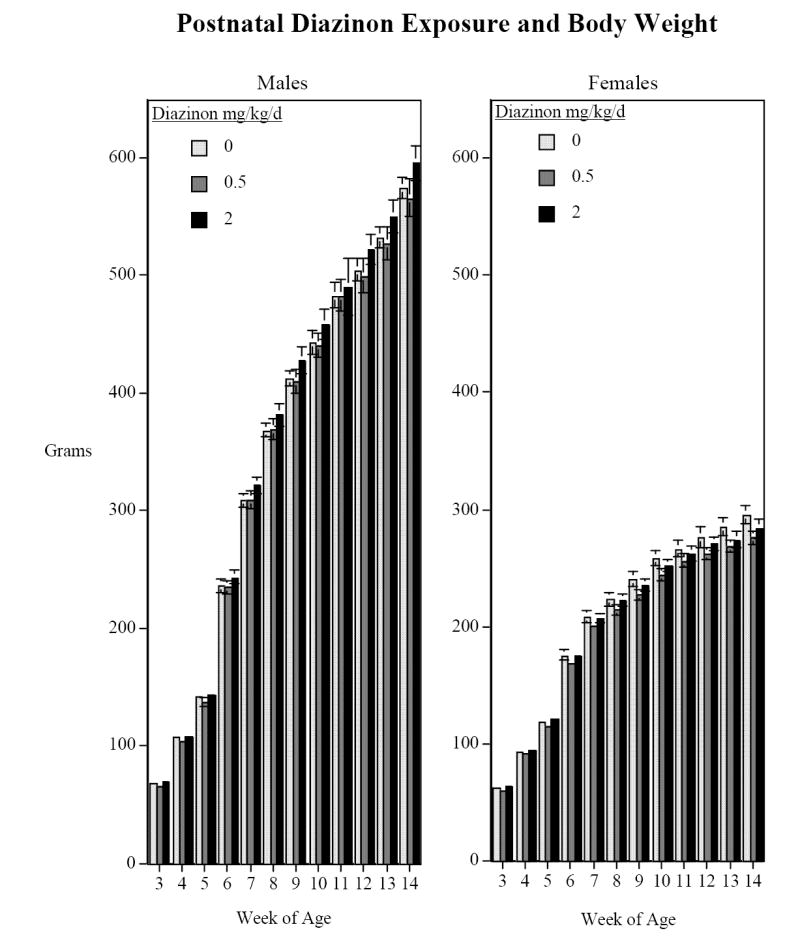
Weight Gain in grams (mean±sem). There were no significant DZN treatment effects on body weight with regular chow diet.
Figure 5.
Weight Gain in grams with a high-fat vs. regular chow diet (mean±sem). The high-fat chow diet significantly (p<0.05) increased weight in the rats exposed to 0.5 mg/kg/d DZN on PND 1-4 compared to controls with no differential effect by sex.
Discussion
Our main finding is that neonatal DZN exposures that are below the threshold for detectable cholinesterase inhibition [37], nevertheless produce lasting changes in emotional behaviors, just as they do for cognitive function [39]. A principal hallmark of the effect is that males were selectively affected, with no significant effects for females in any of the four tests. In the elevated plus maze, the 2 mg/kg/d DZN exposure evoked in the males a significant decrease in open arm exploration; in the novelty suppressed feeding test both DZN doses significantly decreased the latency to begin eating; and in the chocolate milk consumption test, the lower DZN dose abolished any preference for the chocolate milk over the water, which was a significant difference from the male controls. Each of these outcomes reflects a different aspect of emotionality. The decreased venturing into the open arms in the elevated plus maze is typically interpreted as increase in anxiety [13], and the loss of preference for sweetened liquids is a characteristic of anhedonia, a typical component of animal models of depression [25]. Paradoxically, a shortened latency to begin eating in a novel environment might seem at first to represent decreased fearfulness. However, stress reactions can also induce feeding, as seen with tail-pinching [6,19,22,27,28], so the results are not actually contradictory. In contrast to the positive findings in these challenges, the Porsolt forced swim test showed no differences, reflecting its relative insensitivity as compared to the other tests, which do not present as extreme a stressor. The lack of differences in the Porsolt forced swim test may be due to the severity of the stressor in comparison to the other tasks or may be due to the order within the testing battery. The sex difference in sensitivity to developmental DZN exposure may be due to the greater DZN induced neurotoxicity/repair response seen in males soon after the neonatal exposure period [17].
Although our study did not address the mechanisms underlying the lasting effects of DZN on emotional behaviors, it is important to note that neonatal exposure to DZN evokes immediate alterations in development of 5HT projections to the forebrain [17,33,36,37], akin to those seen with CPF, which is known to produce lasting deficits in 5HT synaptic function [1,2,5,34,36]. In turn, aberrant frontal cortical 5HT function is most closely associated with affective/anxiety disorders [15], and our continuing work on 5HT function in the DZN model should show whether this agent produces lasting defects in synaptic activity of these projections. However, we already have some indications that, although CPF and DZN both target developing 5HT systems, they differ in significant ways, including sex selectivity, patterns of gene expression and indices of neural damage [17,33,36,37]. On PN5, just after the end of the 4 days of neonatal diazinon dosing, diazinon significantly increased 5HT1A and 5HT2 receptors regardless of sex with the lower 0.5 mg/kg/day but not the higher 2 mg/kg/day causing an effect [37]. Despite this, males show greater evidence of neurotoxicity (damage/repair response) on PN5 [17], which supports the idea that cholinesterase inhibition is not the underlying cause.
Those differences are reflected in our findings for DZN in the current paper, as compared to similar studies with CPF [1]. Here, DZN decreased time spent in the open arms of the elevated plus maze in males, whereas CPF evoked an increase in the same measure, so that although they both showed sex-selective effects, the actual direction of change was not the same. The two studies differ in some design aspects that could contribute to divergent effects. The housing conditions, timing of light-cycle reversal, and interval between reversal and plus-maze testing were not the same, which could influence the degree of anxiety as measured by the apparatus; for example, the male-female differences in the control group were much more notable in the earlier study because of greater anxiety in the control males. However, the fact remains that CPF increased the open-arm time in male rats whereas DZN reduced it, effects that are hardly likely to represent minor variations in testing conditions. This reinforces one of the major consequences of OP developmental neurotoxicity unrelated to the common feature of cholinesterase inhibition: since the OPs differ in their molecular structures, the non-cholinesterase contributions to altered brain development are quite likely to be dissimilar. For the anhedonia test, the differences between DZN and CPF are less stark but are still demonstrable: DZN reduced chocolate milk preference in males, whereas in our earlier study, CPF affected both sexes. As more research reveals differences between the low dose neurobehavioral toxicity of different OP pesticides, better decisions can be made concerning continuing use of each insecticide and features desirable in the development of new insecticides that are effective in killing insects (through AChE inhibition in the case of OPs) but do not have toxic effects at low doses that do not inhibit AChE.
In our work with CPF, we found that neonatal exposure to low doses of this OP produces hyperinsulinemia and hyperlipidemia, resembling a prediabetic state [32], in accord with underlying changes in hepatic responses to gluconeogenic signals [7,20]. In the current study, we did not see statistically significant changes in body weight gain with DZN while animals were on their normal diet, so we decided to examine whether a high-fat diet might elicit changes consonant with metabolic dysregulation. Although we obtained positive evidence for such effects, the impact of neonatal DZN exposure on weight regulation was complex. When the high-fat diet was given, the 0.5 mg/kg/d group showed a significant increase in weight as compared to the same group on standard lab chow. However, this effect was superimposed on slightly (not significantly) lower body weights in the group on normal chow, so that although the high-fat diet uniquely increased the weight in the low dose DZN group, the actual body weights did not significantly exceed those of the control or high-dose DZN groups on the high-fat diet. Furthermore, the 2 mg/kg DZN group did not show a comparable increase in body weight when placed on a high-fat diet. Nevertheless, these data point to the potential for DZN effects extending beyond developmental neurotoxicity. Indeed, there are preliminary reports of long-term effects of CPF on weight gain [18] that similarly show an inverted-U dose-effect curve, with the elevation in body weight receding as the CPF dose is raised above the threshold for cholinesterase inhibition and potential systemic toxicity (Brimijoin, personal communication).
In conclusion, neonatal DZN exposure, at doses below the threshold for cholinesterase inhibition, has lasting effects on emotional responses, with preferential effects on males. These results complement previous studies on CPF in demonstrating the adverse neurodevelopmental effects of apparently subtoxic OP exposures during the neonatal period, effects that last into adulthood. Finally, although both DZN and CPF affect emotional behaviors, there are also substantial differences in their effects, an outcome likely reflecting mechanisms other than inhibition of cholinesterase and that therefore differ among the various OPs. Accordingly, evaluations of the adverse effects of a particular OP may not be predictive of the actions of all OPs.
Figure 3.
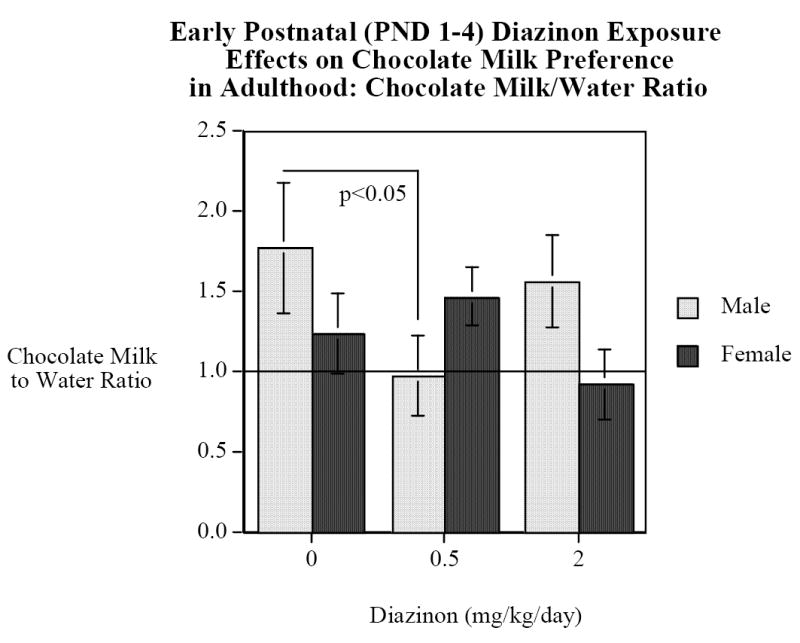
Chocolate milk preference, percent chocolate milk to water ratio (mean±sem). The males exposed to 0.5 mg/kg/d DZN on PND 1-4 had a significantly (p<0.05) decreased chocolate milk preference compared to control males.
Acknowledgments
We would like to thank J. Harrison, W.P. Blackwelder, N.C. Christopher, M. Cousins, C. Oliver, I. Ryde, and C. Tate for technical assistance. This research was supported by the Duke University Superfund Basic Research Center ES010356. The authors declare that they have no competing financial interests or other conflicts. Theodore Slotkin and Frederic Seidler have provided expert witness testimony on behalf of governmental entities, corporations and/or individuals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos leads to behavioral alterations resembling animal models of depression and involving serotonergic mechanisms. Environmental Health Perspectives. 2005;113:527–531. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ Health Perspect. 2005;113:1027–1031. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Developmental exposure to terbutaline and chlorpyrifos: pharmacotherapy of preterm labor and an environmental neurotoxicant converge on serotonergic systems in neonatal rat brain regions. Toxicol Appl Pharmacol. 2005;203:134–144. doi: 10.1016/j.taap.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Aldridge JE, Seidler FJ, Meyer A, Thillai I, Slotkin TA. Serotonergic systems targeted by developmental exposure to chlorpyrifos: effects during different critical periods. Environ Health Perspect. 2003;111:1736–1743. doi: 10.1289/ehp.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aldridge JE, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environmental Health Perspectives. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antelman SM, Szechtman H. Tail pinch induces eating in sated rats which appears to depend on nigrostriatal dopamine. Science. 1975;189:731–3. doi: 10.1126/science.1154024. [DOI] [PubMed] [Google Scholar]
- 7.Auman JT, Seidler FJ, Slotkin TA. Neonatal chlorpyrifos exposure targets multiple proteins governing the hepatic adenylyl cyclase signaling cascade: implications for neurotoxicity. Dev Brain Res. 2000;121:19–27. doi: 10.1016/s0165-3806(00)00021-3. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharya SK, Satyan KS, Chakrabarti A. Anxiogenic action of caffeine: an experimental study in rats. Journal of Psychopharmacology. 1997;11:219–24. doi: 10.1177/026988119701100304. [DOI] [PubMed] [Google Scholar]
- 9.Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology. 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- 10.Bodnoff SR, Suranyi-Cadotte BE, Quirion R, Meaney MJ. Role of the central benzodiazepine receptor system in behavioral habituation to novelty. Behavioral Neuroscience. 1989;103:209–12. doi: 10.1037//0735-7044.103.1.209. [DOI] [PubMed] [Google Scholar]
- 11.Britton DR, Britton KT. A sensitive open field measure of anxiolytic drug activity. Pharmacology, Biochemistry & Behavior. 1981;15:577–582. doi: 10.1016/0091-3057(81)90212-4. [DOI] [PubMed] [Google Scholar]
- 12.Casida JE, Quistad GB. Organophosphate toxicology: Safety aspects of nonacetylcholinesterase secondary targets. Chemical Research and Toxicology. 2004;17:983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- 13.Elliott BM, Faraday MM, Phillips JM, Grunberg NE. Effects of nicotine on elevated plus maze and locomotor activity in male and female adolescent and adult rats. Pharmacol Biochem Behav. 2004;77:21–28. doi: 10.1016/j.pbb.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 14.File SE, Cheeta S, Kenny PJ. Neurobiological mechanisms by which nicotine mediates different types of anxiety. European Journal of Pharmacology. 2000;393:231–236. doi: 10.1016/s0014-2999(99)00889-4. [DOI] [PubMed] [Google Scholar]
- 15.Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacology, Biochemistry & Behavior. 1996;54:129–41. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- 16.Heninger GR. Serotonin, sex, and psychiatric illness. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4823–4. doi: 10.1073/pnas.94.10.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jameson RR, Seidler FJ, Slotkin TA. Nonenzymatic functions of acetylcholinesterase splice variants in the developmental neurotoxicity of organophosphates: Chlorpyrifos, chlorpyrifos oxon and diazinon. Environmental Health Perspectives. 2007;115 doi: 10.1289/ehp.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lassiter TL, Brimijoin WS. Society for Neuroscience. Washington, DC: 2005. Rats gain excess weight after developmental exposure to chlorpyrifos. Program Number 106.3. [DOI] [PubMed] [Google Scholar]
- 19.Levine AS, Morley JE. Stress-induced eating in rats. American Journal of Physiology. 1981;241:R72–6. doi: 10.1152/ajpregu.1981.241.1.R72. [DOI] [PubMed] [Google Scholar]
- 20.Meyer A, Seidler FJ, Slotkin TA. Developmental effects of chlorpyrifos extend beyond neurotoxicity: critical periods for immediate and delayed-onset effects on cardiac and hepatic cell signaling. Environ Health Perspect. 2004;112:170–178. doi: 10.1289/ehp.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mileson BE, Chambers JE, Chen WL, Dettbarn W, Ehrich M, Eldefrawi AT, Gaylor DW, Hamernik K, Hodgson E, Karczmar AG, Padilla S, Pope CN, Richardson RJ, Saunders DR, Sheets LP, Sultatos LG, Wallace KB. Common mechanism of toxicity: A case study of organophosphorus pesticides. Toxicol Sci. 1998;41:8–20. doi: 10.1006/toxs.1997.2431. [DOI] [PubMed] [Google Scholar]
- 22.Morley JE, Levine AS, Rowland NE. Minireview. Stress induced eating. Life Sciences. 1983;32:2169–82. doi: 10.1016/0024-3205(83)90415-0. [DOI] [PubMed] [Google Scholar]
- 23.Pope CN. Organophosphorus pesticides: Do they all have the same mechanism of toxicity? J Toxicol Environm Hlth. 1999;2:161–181. doi: 10.1080/109374099281205. [DOI] [PubMed] [Google Scholar]
- 24.Porsolt RD, McArthur RA, Lenegre A. Psychotropic screening procedures. Meth Behav Pharmacol. 1993;10:23–51. [Google Scholar]
- 25.Pucilowski O, Overstreet DH, Rezvani AH, Janowsky DS. Chronic mild stress-induced anhedonia: greater effect in a genetic rat model of depression. Physiolgy and Behavior. 1993;54:1215–1220. doi: 10.1016/0031-9384(93)90351-f. [DOI] [PubMed] [Google Scholar]
- 26.Raines KW, Seidler FJ, Slotkin TA. Alterations in serotonin transporter expression in brain regions of rats exposed neonatally to chlorpyrifos. Dev Brain Res. 2001;130:65–72. doi: 10.1016/s0165-3806(01)00211-5. [DOI] [PubMed] [Google Scholar]
- 27.Rowland NE, Antelman SM. Stress-induced hyperphagia and obesity in rats: a possible model for understanding human obesity. Science. 1976;191:310–12. doi: 10.1126/science.1246617. [DOI] [PubMed] [Google Scholar]
- 28.Sahakian BJ, Robbins TW. Isolation-rearing enhances tail pinch-induced oral behavior in rats. Physiology & Behavior. 1977;18:53–8. doi: 10.1016/0031-9384(77)90093-2. [DOI] [PubMed] [Google Scholar]
- 29.Slotkin TA. Developmental cholinotoxicants: Nicotine and chlorpyrifos. Environmental Health Perspectives. 1999;107(Suppl 1):65–69. doi: 10.1289/ehp.99107s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: Nicotine, environmental tobacco smoke, and organophosphates. Toxicology and Applied Pharmacology. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Slotkin TA. Developmental neurotoxicity of organophosphates: A case study of chlorpyrifos. In: Gupta RC, editor. Toxicity of Organophosphate and Carbamate Pesticide. Elsevier Academic Press; San Diego: 2005. pp. 293–314. [Google Scholar]
- 32.Slotkin TA, Brown KK, Seidler FJ. Developmental exposure of rats to chlorpyrifos elicits sex-selective hyperlipidemia and hyperinsulinemia in adulthood. Environ Health Perspect. 2005;113:1291–1294. doi: 10.1289/ehp.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slotkin TA, Levin ED, Seidler FJ. Comparative developmental neurotoxicity of organophosphate insecticides: Effects on brain development are separable from systemic toxicity. Environmental Health Perspectives. 2006;114:746–751. doi: 10.1289/ehp.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slotkin TA, Seidler FJ. The alterations in CNS serotonergic mechanisms caused by neonatal chlorpyrifos exposure are permanent. Developmental Brain Research. 2005;158:115–119. doi: 10.1016/j.devbrainres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Slotkin TA, Seidler FJ. Comparative developmental neurotoxicity of organophosphates in vivo: Transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain Research Bulletin. 2007 doi: 10.1016/j.brainresbull.2007.01.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slotkin TA, Seidler FJ, Fumagalli F. Exposure to organophosphates reduces the expression of neurotrophic factors in neonatal rat brain regions: Similarities and differences in the effects of chlorpyrifos and diazinon on the fibroblast growth factor superfamily. Environmental Health Perspectives. 2007 doi: 10.1289/ehp.9901. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slotkin TA, Tate CA, Ryde IT, Levin ED, Seidler FJ. Organophosphate insecticides target the serotonergic system in developing rat brain regions: Disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environ Health Perspect. 2006;114:1542–1546. doi: 10.1289/ehp.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snedecor GW, Cochran WG. Statistical Methods. Iowa State University Press; Ames, Iowa: 1967. [Google Scholar]
- 39.Timofeeva OA, Roegge CS, Seidler FJ, Slotkin TA, Levin ED. Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide diazinon. Environmental Health Perspectives. 2007 doi: 10.1016/j.ntt.2007.10.002. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitney KD, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: Cellular mechanisms. Toxicology and Applied Pharmacology. 1995;134:53–62. doi: 10.1006/taap.1995.1168. [DOI] [PubMed] [Google Scholar]